Abstract
Recurrent cancer that spreads to distant sites is the leading cause of disease-related death among cancer patients. Cancer cells are likely to disseminate during cancer progression, and some may enter dormancy, remaining viable but not increasing. These dormant cancer cells (DCCs) are rarely detectable with current diagnostic systems. Moreover, they can interpret homoeostatic signals from the microenvironment, thereby evading immune surveillance and chemotherapy. Eventually, DCCs can reawaken in response to signals, which are not yet fully understood, resulting in recurrence and metastasis. Therefore, understanding the biology of DCC reawakening is key to preventing metastasis. Over the last decade, a growing body of literature has revealed the mechanisms involved in cancer dormancy and reawakening. The cytotoxic activity of immune cells can cause cancer cells to enter a dormant state, and chronic inflammation can reactivate cancer proliferation at distant sites. Upon the binding of circulating DCCs to extracellular molecules, various signaling cascades are activated and reinitiate cell proliferation. In the present review, we attempt to consolidate the existing literature to provide a framework for the understanding of this crucial step in cancer progression.
Similar content being viewed by others
Introduction
The primary treatment for cancer is the surgical removal of cancer cells, which is often combined with chemoradiotherapy to kill surgically inaccessible cancer cells throughout the body. However, even patients who are considered clinically free of cancer cells after initial treatment frequently relapse with distant metastasis. Such metastatic outgrowth rapidly becomes uncontrollable with chemoradiation and manages to seed additional metastatic colonies, resulting in the disruption of vital organ function. Although the clinical importance of metastasis is therefore apparent, its underlying mechanisms remain unclear.
Metastasis is considered a series of linear events, termed the invasion–metastasis cascade1. The initiation step of metastasis begins when cancer cells at the primary tumor growth site foster basement membrane degradation and enter the underlying interstitial matrix2. During this process, cancer cells usually promote vascularization in tumor tissues to sculpt a permissive microenvironment for cancer cell proliferation and gain access to the bloodstream3. Once cancer cells successfully penetrate into the blood or lymphatic circulatory system, they can disseminate throughout the body. In circulation, cancer cells are likely to exhibit mitotic arrest through reversible G0-G1 arrest, termed quiescence, in which they remain viable but do not increase. These dormant cancer cells (DCCs) are more susceptible to antiproliferative drugs. More recently, these circulating DCCs have been shown to evade immune surveillance by expressing programmed death ligand 1 (PDL-1); thus, they can persist for an extended period4,5. At some point, DCCs reach distant organs and infiltrate into the stroma, although they cannot grow into macroscopic lesions until they escape dormancy. This period is termed “metastatic cancer dormancy” and occurs between initial therapy and metastatic relapse. Eventually, in response to microenvironmental cues, DCCs gain the ability to re-enter the cell cycle and adapt to their new microenvironment, thereby progressing to metastatic outgrowth. Therefore, understanding the biology of DCC reawakening is key to preventing metastasis.
A growing body of research has provided insight into the molecular mechanisms of cellular dormancy and reactivation. Central to these mechanisms is crosstalk between cancer cells and their microenvironment, which is affected by complex interactions between cancer cells and stromal cells and surrounding extracellular matrix (ECM) components, as well as host immunity. After a long period in the bloodstream, DCCs eventually reach distant organs and encounter a new composition of ECM produced from the local stromal cells. Then, the binding of membrane receptors on DCCs activates various signaling cascades, driving cell cycle promotion and breaking dormancy. Meanwhile, the host immune system initially acts as a tumor suppressor but eventually favors cancer progression and promotes metastatic outgrowth by reactivating DCCs. In the present review, we focused on these cellular and acellular factors that reawaken DCCs and contribute to metastasis.
Primary molecular mechanisms underlying cancer cell dormancy
An overwhelming amount of evidence supports the notion that extracellular signal-regulated kinase (ERK) activation has a determinant role in whether cancer cells will proliferate or enter a state of dormancy. Persistently proliferating cancer cells exhibit constitutive ERK activation, which permits Go-G1-S phase transition and cell division6,7. During ERK-induced proliferation, a high level of p38 mitogen-activated protein kinase (p38) activity functions as an inhibitory regulator of ERK and prevents cell proliferation by inducing G0-G1 arrest or triggering senescence and apoptosis8,9,10. Indeed, a luciferase reporter system visualized the in vivo ERK and p38 MAPK activities and provided direct evidence of p38/ERK activity as an indicator of DCCs in various types of cancer, including breast cancer, prostate cancer, melanoma, and fibrosarcoma8. Cancer cells with p38low/ERKhigh activity were highly proliferative in vivo, whereas those with p38high/ERKlow activity were incapable of proliferation without increased apoptosis, suggesting that they were dormant in vivo. Meanwhile, multiple pharmacological and genetic interventions that change the balance of p38/ERK activity in favor of ERK were able to break in vivo dormancy and induce cancer growth. Thus, it seems that regulatory factors that can change the signaling balance between ERK and p38 activities have a profound influence on whether cancer cells grow or remain dormant11.
Transforming growth factor-β2 (TGF-β2) is secreted from bone marrow-derived cells and thus is relatively abundant. TGF-β2 binds to its receptors, TGF-β receptor-I (TGF-β-RI) and TGF-β-RIII, on cancer cell membranes and induces p38high/ERKlow signaling12. The subsequent activation of Smad1/5 increases the expression of DEC2/SHARP1 and p27 and downregulates cyclin-dependent kinase 4 (CDK4), which collectively facilitates the transition into cellular quiescence12,13. The production of TGF-β1/2 is increased during osteoblast differentiation, along with that of bone morphogenetic protein (BMP) family proteins. Both TGF-β1 and BMP-3b induce cancer cell quiescence. TGF-β-RIII participates in both TGF-β1- and BMP-3b-induced dormancy and activates the phosphorylation of retinoblastoma through p38 MAPK activation. On the other hand, in the lung, where stromal TGF-β2 secretion is low, ERK activation is restored, and DCCs transition into a highly proliferative state, fueling multiorgan metastasis12. Therefore, upon the exit of DCCs from bone marrow, the lack of growth factors can shift the balance of p38 MAPK and ERK activities toward ERK activation, creating a permissive microenvironment for metastatic outgrowth.
The urokinase plasminogen activator (uPA) system has been implicated in a shift from cancer dormancy to proliferation by mediating EGFR signaling14. Numerous types of cells, including epithelial cells, immune cells, and fibroblasts, produce and secrete uPA. uPA binds to its receptor (uPAR) and initiates a proteolytic cascade, resulting in the conversion of plasminogen into plasmin15. Plasmin degrades a wide range of extracellular components through its proteolytic activity and activates other enzymatic proteins, such as metalloproteinases, thereby promoting cancer invasion. Independent of catalytic activity, the uPAR–uPA interaction leads to the activation of integrin and epidermal growth factor receptor (EGFR) signaling, which consecutively activates ERK1/2 and lowers p38 activities, promoting mitotic cascades8,16,17. However, DCCs have been reported to express a low level of uPAR; thus, they exhibit a low level of integrin and EGFR activation, resulting in a p38high/ERKlow activity ratio8. Additionally, p38high/ERKlow facilitates G0-G1 arrest by regulating a variety of transcription factors (TFs), such as nuclear receptor subfamily 2 group F member 1 (NR2F1), basic helix-loop-helix protein 3 (BHLHB3 or DEC2), and cyclin-dependent kinase inhibitors (p27 and p21), and downregulates G1 exit-promoting TFs, such as FOXM1 and c-Jun18. Therefore, this combinatorial regulation of TFs by p38high/ERKlow activity is responsible for the quiescence program in DCCs11.
Additional studies have suggested that high p38 activity is linked to the survival of DCCs and related to endoplasmic reticulum (ER) stress. High p38 activity inhibits Bax activation by increasing the expression of the ER chaperone BiP/Grp78, thereby rendering DCCs highly resistant to chemotherapy19. The activating transcription factor 6α (ATF6α), which is translocated from the ER to the nucleus to serve as a TF upon ER stress, is persistently activated in DCCs in a p38-dependent manner20. ATF6α transcriptionally induces Rheb, a small GTPase, and transduces survival signals such as mTOR and downstream S6K and S6RP phosphorylation. Knockdown of ATF6α or Rheb by RNA interference was sufficient to induce apoptosis in DCCs and remove DCCs during their quiescent phase20. This suggests that high p38 activity causes growth arrest in DCCs and simultaneously may activate the dormancy-specific survival signaling pathways that enable DCCs to resist microenvironmental and genotoxic stress.
Furthermore, some kinds of stroma-derived ligands are known to induce cancer cell dormancy in multiple types of cancer. For example, growth arrest-specific protein 6 (GAS6) has been shown to induce dormancy in several kinds of cancer cells that infiltrate the bone marrow. GAS6 is known to bind to the Tyro3, Axl, and Mer (TAM) family of receptor tyrosine kinases, thereby activating multiple downstream signaling pathways, including mitogen-activated protein kinase (MAPK) and phosphoinositide 3-kinase (PI3K)/Akt pathways21. In particular, GAS6 promotes the transition of cancer cells into DCCs in the bone marrow. Mechanistically, osteoblasts secrete GAS6 upon their contact with leukemia cells, and the binding of GAS6 to Mer on the surface of leukemia cells facilitates the entry of leukemia cells into G0/G1 arrest22. Similarly, in bone marrow, GAS6 from osteoblasts activates TAM family receptors on prostate cancer cells and switches on dormancy in proliferative cancer cells23. Additionally, BMP7, produced from bone stromal cells, can induce dormancy in prostate cancer cells by activating p38 signaling24. Mechanistically, binding of BMP7 to its receptor BMP receptor 2 (BMPR2) on prostate cancer cells activates p38 signaling; in turn, it induces reversible growth arrest by increasing the expression of the cell cycle inhibitor p21 and the metastasis suppressor gene NDRG1 (N-myc downstream-regulated gene 1). These data together show that many of these dormancy-inducing cytokines from the stroma can promote the p38high/ERKlow state in the absence of proliferative signaling, resulting in G0 cell cycle arrest and cancer dormancy.
Breaking of cellular dormancy by microenvironmental cues
Integrins are transmembranous heterodimeric glycoproteins that mediate cell-to-cell and cell-to-ECM signaling cascades. Integrin signaling activates multiple intermediaries, including cytosolic tyrosine kinases, and is involved in the regulation of cell proliferation, survival, and motility in both cancer and normal healthy cells25. Numerous studies have provided evidence that integrin signaling, particularly β-1 integrin, is a critical regulator in the switch from cellular dormancy to metastatic growth in vitro and in vivo25,26,27,28,29. Loss of β-1 integrin signaling by downregulation of the uPA-uPAR interaction appears to promote the shift from a proliferative to a dormant state in cancer cells8. The inhibition of β-1 integrin signaling by antibody treatment induced the growth arrest of mammary cancer cells in a three-dimensional basement membrane assay30. The removal of the anti-β-1 integrin antibody reversed cell cycle arrest and reinitiated cancer cell growth. Focal adhesion kinase (FAK) is a downstream molecule of β-1 integrin and has been implicated in the regulation of cancer cell dormancy. In a mouse mammary tumor virus (MMTV) transgenic breast cancer mouse model, the Cre-LoxP-mediated deletion of β-integrin results in a decrease in FAK phosphorylation, reduced cell proliferation, and growth arrest of tumor burden in vivo31. Similarly, the growth ability of a highly metastatic D2A1 mammary carcinoma cell was significantly dependent on the presence of fibronectin, β-1 integrin signaling, and downstream phosphorylation of the myosin complex in three-dimensional cell culture, suggesting that the upregulation of β-1 signaling enabled DCCs to re-enter the cell cycle26.
An additional in vivo study revealed that metastatic outgrowth of the mouse mammary cancer cell lines D2.0R and D2A1 was dependent on β1-integrin signaling32. Binding of collagen to integrin receptors resulted in FAK/SRC activation and subsequent ERK phosphorylation. Integrin-mediated ERK activation induced cell proliferation, driving metastatic outgrowth. These data suggest that the interaction between β1-integrin/FAK and the MAPK pathway is essential for cancer cell growth. Meanwhile, noncanonical discoidin domain receptor 1 (DDR) signaling is also activated by binding to collagen, and it is known to activate cancer cell proliferation at metastatic sites33. Mechanistically, tetraspanin transmembrane 4 L six family member 1 (TM4SF1) couples DDR1 to syntenin 2 and then activates protein kinase C alpha (PKCα). Activated PKCα subsequently phosphorylates Janus kinase 2 (JAK2) to drive noncanonical DDR1 signaling through phosphorylation of signal transducer and activator of transcription 3 (STAT3). In cancer, constitutive activation of STAT3 increases the transcription of cell cycle regulators, such as c-Myc and cyclin D, and promotes cancer cell proliferation. Consistently, histopathologic analysis of metastatic murine breast cancer cells has identified that micrometastatic tissues are surrounded by collagen. In metastatic tissues, the majority of cancer cells apart from collagen are dormant, whereas those nearby collagen are proliferative. These findings indicate how the interaction between DCCs and the ECM microenvironment influences cancer cell behavior and metastatic reactivation.
Furthermore, Wnt signaling has been implicated as a mediator during ECM-induced DCC reactivation. Wnt signaling is known to control diverse biological processes and is a well-known proliferation inducer. Wnt activation promotes G1-to-S progression through both transcriptional and nontranscriptional regulation of cyclin D1, cyclin E1, and c-myc34. Therefore, inhibition of Wnt signaling by secretion of Dickkopf WNT signaling pathway inhibitor 1 (DKK1) is one mechanism by which cancer cells enter quiescence35. Tenascin C, initially produced by metastasis-initiating cancer cells and later secreted from stromal fibroblasts, is known to support the metastatic outgrowth of breast cancer cells by promoting Wnt signaling. Tenascin C binds to syndecan-4, a coreceptor of the Wnt receptor Frizzled-7, thereby enhancing Wnt signaling activation and facilitating metastatic colonization. Additionally, periostin has the ability to recruit Wnt ligands; thus, it can increase the presentation of Wnt ligands to cancer cells. Periostin is mainly produced from stromal fibroblasts upon TGF-β activation and can be secreted from endothelial tip cells that reside in new vascular sprouts. Thus, periostin is abundant in micrometastatic lesions undergoing neoangiogenesis and is a profound factor for a permissive microenvironment of cancer metastasis. Moreover, both tenascin C and periostin can foster integrin signaling through an indirect pathway; they coassemble with fibronectin and modulate its adhesiveness and stiffness, which collectively increase the integrin signaling capacity.
Collectively, these facts suggest that the ECM components from metastasis-initiating cancer cells and stromal cells may sculpt a permissive niche, facilitating the activation of signaling pathways that support metastatic cell proliferation.
Chronic inflammation awakens dormant cells
Growing evidence has suggested that chronic inflammation is involved in cancer development. For example, patients with inflammatory bowel disease are at higher risk of colorectal cancer development. Hepatitis and fatty liver disease correlate with the incidence of liver cancer development. Acid reflux esophagitis can cause esophageal cancer. Chronic Helicobacter infection is the leading cause of stomach cancer. During inflammation, free radicals such as reactive oxygen and nitrogen species (RONS) increase and induce double-strand breaks in DNA, which are potently mutagenic if not accurately and promptly repaired, thereby facilitating the transformation of normal healthy cells to cancer cells36. Moreover, free radicals can trigger a wide range of signaling pathways, including MAPK/ERK, PI3K/Akt, and IκB kinase/nuclear factor kappa-light-chain-enhancer of activated B cells (NFκB), that lead to cancer malignancy37. However, not all individuals who have experienced chronic inflammatory diseases eventually develop cancer in their lifetime. In situ carcinoma can be found in the lesion without any chronic inflammation. These phenomena raised the question about whether a cause-effect relationship exists between chronic inflammation and cancer. One of the possible explanations for this conflicting evidence may be that reawakening DCCs could be a key factor for cancer development from chronic inflammation. For instance, chronic inflammation supports angiogenesis, which breaks cancer dormancy by supplying sufficient oxygen and nutrients and facilitates cancer growth38. Moreover, there is a strong correlation between inflammation and recurrence of cancer, including recurrence of endometrial39, oral40, and breast cancer41,42. The escape of cancer from dormancy can be induced by the inflammatory cytokine interferon-gamma (IFN-γ)43,44,45,46. In addition, the correlation between the high levels of serum inflammatory cytokines and cancer recurrence supports this hypothesis. In a cohort consisting of 734 breast cancer patients, high levels of circulating acute-phase proteins (APPs) were positively correlated with distant recurrence47. Additionally, C-reactive protein (CRP) and interleukin 6 (IL-6), other serum inflammatory markers, have shown their possibilities as posttreatment prognostic monitoring factors for predicting the risk of cancer recurrence and patient death48,49,50. Hepatocyte CRP secretion is controlled by interleukin 6 (IL-6). The synthesis of CRP is stimulated by interleukin-1 (IL-1) and tumor necrosis factor (TNF). A rise in serum levels of CRP often reflects tissue damage. Collectively, these data support the hypothesis that inflammation can be the DCC reawakening factor and therefore can function as a cancer-promoting factor.
Chronic inflammation can induce epigenetic alterations and DNA mutations in tumor suppressor genes, thereby facilitating carcinogenesis. Fortunately, the immune system can recognize these mutant protein antigens of cancer cells and can attack cancer cells, serving as a critical mechanism of metastatic dormancy, so-called immunogenic cancer dormancy51,52. For instance, CD8+ T cells have a cytostatic effect on cancer cells, thereby allowing early disseminated cancer cells to stay in a dormant state at metastatic sites53. In some experimental models, removal of CD8+ T cells resulted in outgrowth of DCCs and induced cancer recurrence53. However, chronic inflammation can also facilitate other mechanisms that promote the reactivation of DCCs. For instance, studies in a pancreatic cancer mouse model demonstrated that circulating cancer cells underwent epithelial to mesenchymal transition (EMT) and seeded metastatic colonies in the liver. In this process, the rate of EMT and invasive potential were highest at the sites of inflammation. On the other hand, treatment with dexamethasone, an immunosuppressive drug, abrogated EMT and cancer invasiveness. These results imply that inflammation can be a cancer progression factor by facilitating the EMT process in cancer cells54. Similarly, localized inflammation in the lungs can trigger cancer cell escape from dormancy, which leads to the development of macroscopic metastases30. During this process, Zeb1 expression, a strong inducer of EMT, was required for cancer cells to escape dormancy. On the other hand, depletion of neutrophils via the administration of antibodies against the lymphocyte antigen 6 complex, locus G (Ly6G) abrogated the reactivation of DCCs.
The interaction between cancer cells and myeloid cells has also been implicated in cancer progression. For instance, inflammatory monocytes with Ly6C expression can facilitate the extravasation of cancer cells in the lung by secreting chemokine C-C-motif ligand 2 (CCL2)55 and vascular endothelial growth factor56. Then, macrophages bind to cancer cells and increase the survival of cancer cells. In this procedure, vascular cell adhesion protein 1 on cancer cells binds to β-1-integrin-positive macrophages, and this interaction activates Akt signaling in cancer cells and allows them to evade TNF-related apoptosis-inducing ligand (TRAIL)-induced apoptosis57. Together, these mechanistic roles of myeloid cells are related to metastasis-promoting effects. However, whether the interaction between myeloid cells and cancer cells switches on the growth of DCCs has not yet been sufficiently demonstrated.
The differentiation of monocytes into metastasis-associated macrophages (MAMs) can promote the metastatic outgrowth of cancer cells. The metastasis-promoting role of MAMs is more complicated and related to their participation in sculpting a more fibrotic metastatic microenvironment. In a genetic mouse model of pancreatic ductal adenocarcinoma (PDAC), MAMs secreted granulin in the liver, and granulin induced the transformation of resident hepatic stellate cells into myofibroblasts. In turn, myofibroblasts secreted periostin, which created a fibrotic microenvironment that was more favorable for integrin singling activation58. Then, activated integrin signaling led to DCC reactivation and promoted the proliferation of cancer cells at the metastatic lesion. Therefore, the development of a more fibrotic metastatic microenvironment by MAMs can function as a prometastatic factor by awakening DCCs.
The involvement of natural killer (NK) cells in cancer dormancy and reactivation has not yet been determined, and instead, it has been elucidated that DCCs are more resistant to the cytotoxicity of NK cells. In a “latency-competent cancer model” where dormant clones were selected from an in vivo experimental metastasis assay, DCCs were confirmed to activate the p38 and self-renewal pathways through Sox2/9. Sox2 was also shown to facilitate DKK1 secretion and thereby inhibit Wnt signaling as well as downstream proliferative pathways35. Once DCCs enter dormancy via DKK1, they are able to avoid NK cell-mediated cell death, while DCCs with low DKK1 expression are still proliferative and susceptible to NK cell cytotoxicity.
Recently, a growing body of evidence has highlighted the potential role of CD4 and CD8 T cells in cancer dormancy maintenance59,60. DCCs were far less susceptible to adaptive immunity and showed low expression of cancer antigen. Additionally, dormant leukemia cells were confirmed to express PDL-1, which allows them to avoid T cell-mediated cytotoxicity59,60. These findings indicate that DCCs can escape anticancer immunity, thereby surviving for an extended period. Additionally, an in vivo xenograft model using dormant murine breast cancer cell clones selected with constitutive treatment of doxorubicin has revealed that both CD8 and CD4 T cells are involved in chemotherapy-mediated dormancy as well61. Chemotherapy treatment activated IFN signaling in cancer cells through an autocrine and self-sustained increase in TF and interferon regulatory factor 7 (IRF7). IRF7/IFN signaling promoted the expansion of CD4 and CD8 T cells and prevented the mobilization of CD11b+Gr1+ myeloid-derived suppressor cells. Collectively, these effects facilitate immune cytotoxicity, resulting in immune-mediated cancer dormancy.
More recently, neutrophils have attracted significant attention because of their DCC-reawakening activity. Exposure to tobacco smoke or the nasal instillation of lipopolysaccharide induced chronic lung inflammation and converted DCCs to aggressively growing cancer cells, resulting in an increase in metastasis. In this process, neutrophils mediated the DCC reawakening through the formation of neutrophil extracellular traps (NETs), which are scaffolds of chromatin, including cytotoxic enzymes and proteases that are released into the extracellular space62. Mechanistically, two proteases, neutrophil elastase and matrix metalloproteinase 9 (MMP9), were secreted from NETs and sequentially cleaved and remodeled laminin. In turn, the remodeled laminin activated integrin α3β1 signaling in DCCs and promoted their proliferation. Treatment with antibodies against NET-remodeled laminin prevented the awakening of DCCs and reduced metastasis.
Summary and direction of future research
In inhospitable microenvironments, cancer cells may enter a state of dormancy to protect themselves against apoptotic and antiproliferative treatments so that the fittest may survive63,64. The existence of DCCs has led to the emergence of therapy resistance, and most importantly, the cells may resume growth, raising the risk of lethal metastatic outbreaks even after a long latency period of months to years. For these reasons, DCCs have been attracting significant interest as a therapeutic target for improving clinical outcomes. The removal of DCCs in combination with antiproliferative treatment is one therapeutic option; however, cellular and surface markers for DCCs are mostly unavailable at present. An overwhelming number of reports propose that DCC reawakening is the final step of the metastatic outbreak, so blocking the factors responsible for this process is key to preventing poor clinical outcomes. Although a variety of signaling cascades are linked to the breaking of dormancy, these signaling networks eventually lead to a change in the balance between p38 and ERK activities in favor of ERK8. Therefore, if we can finely modulate the balance of p38 and ERK, we may be able to induce permanent dormancy and prevent metastasis, which will mark a new era of cancer treatment.
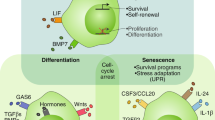
In the present review, we provide an overview of the cellular and acellular mechanisms that break the dormancy-permissive p38high/ERKlow status (Fig. 1). During their journey in the blood and lymphatic stream, DCCs do not interact with local cells or the ECM. However, once they reach an organ, they encounter a new combination of ECM, growth factors, and cytokines produced from local stromal and immune cells. The binding of fibronectin to integrins has a fundamental role in shifting the balance of p38 and ERK activities in favor of ERK. Additionally, other ECM components, such as tenascin C and periostin secreted from resident stromal cells, can foster the binding of fibronectin and integrins and can therefore act as substantial DCC-reawakening factors. In addition, chronic inflammation can initiate the regrowth of DCCs through integrin activation. Macrophages promote the secretion of fibronectin from nearby fibroblasts and sculpt a more fibrotic metastatic microenvironment, thereby fostering the binding of fibronectin to integrin on DCCs. Additionally, neutrophils participate in ECM remodeling by secreting proteinase enzymes, sequentially activating integrin signaling, and reawakening DCCs. Other immune cells, such as monocytes and myeloid cells, have functional involvement in triggering escape from dormancy in multiple experimental models, although their necessity in integrin signaling activation has not yet been tested. Several target molecules that are involved in DCC reawakening are currently under clinical investigation for cancer therapy or prevention as single or combinatory agents (Table 1). Although some of the trials have been terminated because of limited efficacy and intolerable side effects, some have shown promising clinical results, such as a significant trend toward improved disease-free survival and tumor reduction with minimal side effects. Therefore, further investigation into the microenvironmental cues that favor integrin and p38low/ERKhigh activity would broaden the current knowledge of DCC-reawakening factors.
Cancer cells often enter dormancy to evade immune attack. Once in a new location, these dormant cancer cells (DCCs) receive signals from the surrounding tissue, thereby gain the ability to re-enter the cell cycle. Also, chronic inflammation can reactivate DCCs, which can trigger tumor development. Key signaling components involved in DCC reactivation are currently being investigated and may help to fight this leading cause of death from cancer. ECM, extracellular matrix; DDR, discoidin domain receptor; FZD, frizzled.
References
Valastyan, S. & Weinberg, R. A. Tumor metastasis: molecular insights and evolving paradigms. Cell 147, 275–292 (2011).
Friedl, P. & Alexander, S. Cancer invasion and the microenvironment: plasticity and reciprocity. Cell 147, 992–1009 (2011).
De Palma, M., Biziato, D. & Petrova, T. V. Microenvironmental regulation of tumour angiogenesis. Nat. Rev. Cancer 17, 457 (2017).
Brown, J. A. et al. TGF-β-induced quiescence mediates chemoresistance of tumor-propagating cells in squamous cell carcinoma. Cell Stem Cell 21, 650–664 (2017). e658.
Gonzalez, H., Robles, I. & Werb, Z. Innate and acquired immune surveillance in the postdissemination phase of metastasis. FEBS J. 285, 654–664 (2018).
Chambard, J.-C., Lefloch, R., Pouysségur, J. & Lenormand, P. ERK implication in cell cycle regulation. Biochim. Biophys. Acta (BBA)-Mol. Cell Res. 1773, 1299–1310 (2007).
Mebratu, Y. & Tesfaigzi, Y. How ERK1/2 activation controls cell proliferation and cell death: Is subcellular localization the answer? Cell Cycle 8, 1168–1175 (2009).
Aguirre-Ghiso, J. A., Estrada, Y., Liu, D. & Ossowski, L. ERKMAPK activity as a determinant of tumor growth and dormancy; regulation by p38SAPK. Cancer Res. 63, 1684–1695 (2003).
Dhillon, A. S., Hagan, S., Rath, O. & Kolch, W. MAP kinase signalling pathways in cancer. Oncogene 26, 3279 (2007).
Zhang, W. & Liu, H. T. MAPK signal pathways in the regulation of cell proliferation in mammalian cells. Cell Res. 12, 9 (2002).
Sosa, M. S., Avivar-Valderas, A., Bragado, P., Wen, H.-C. & Aguirre-Ghiso, J. A. ERK1/2 and p38α/β signaling in tumor cell quiescence: opportunities to control dormant residual disease. Clin. Cancer Res. 17, 5850–5857 (2011).
Bragado, P. et al. TGF-β2 dictates disseminated tumour cell fate in target organs through TGF-β-RIII and p38α/β signalling. Nat. Cell Biol. 15, 1351 (2013).
Prunier, C., Baker, D., ten Dijke, P. & Ritsma, L. TGF-β family signaling pathways in cellular dormancy. Trends Cancer 5, 66–78 (2018).
Liu, D., Ghiso, J. A. A., Estrada, Y. & Ossowski, L. EGFR is a transducer of the urokinase receptor initiated signal that is required for in vivo growth of a human carcinoma. Cancer Cell 1, 445–457 (2002).
Mahmood, N., Mihalcioiu, C. & Rabbani, S. A. Multifaceted role of the urokinase-type plasminogen activator (uPA) and its receptor (uPAR): diagnostic, prognostic, and therapeutic applications. Front. Oncol. 8, 24 (2018).
Aguirre-Ghiso, J. A., Liu, D., Mignatti, A., Kovalski, K. & Ossowski, L. Urokinase receptor and fibronectin regulate the ERKMAPK to p38MAPK activity ratios that determine carcinoma cell proliferation or dormancy in vivo. Mol. Biol. cell 12, 863–879 (2001).
Heiss, M. M. et al. Individual development and uPA–receptor expression of disseminated tumour cells in bone marrow: a Reference to early systemic disease in solid cancer. Nat. Med. 1, 1035 (1995).
Adam, A. P. et al. Computational identification of a p38SAPK-regulated transcription factor network required for tumor cell quiescence. Cancer Res. 69, 5664–5672 (2009).
Ranganathan, A. C., Zhang, L., Adam, A. P. & Aguirre-Ghiso, J. A. Functional coupling of p38-induced up-regulation of BiP and activation of RNA-dependent protein kinase–like endoplasmic reticulum kinase to drug resistance of dormant carcinoma cells. Cancer Res. 66, 1702–1711 (2006).
Schewe, D. M. & Aguirre-Ghiso, J. A. ATF6α-Rheb-mTOR signaling promotes survival of dormant tumor cells in vivo. Proc. Natl Acad. Sci. 105, 10519–10524 (2008).
Cummings, C. T., DeRyckere, D., Earp, H. S. & Graham, D. K. Molecular pathways: MERTK signaling in cancer. Clin. Cancer Res. 19, 5275–5280 (2013).
Shiozawa, Y., Pedersen, E. A. & Taichman, R. S. GAS6/Mer axis regulates the homing and survival of the E2A/PBX1-positive B-cell precursor acute lymphoblastic leukemia in the bone marrow niche. Exp. Hematol. 38, 132–140 (2010).
Yumoto, K. et al. Axl is required for TGF-β2-induced dormancy of prostate cancer cells in the bone marrow. Sci. Rep. 6, 36520 (2016).
Kobayashi, A. et al. Bone morphogenetic protein 7 in dormancy and metastasis of prostate cancer stem-like cells in bone. J. Exp. Med. 208, 2641–2655 (2011).
Barkan, D. & Chambers, A. F. β1-integrin: a potential therapeutic target in the battle against cancer recurrence. Clin. Cancer Res. 17, 7219–7223 (2011).
Barkan, D. et al. Inhibition of metastatic outgrowth from single dormant tumor cells by targeting the cytoskeleton. Cancer Res. 68, 6241–6250 (2008).
Barkan, D. et al. Metastatic growth from dormant cells induced by a col-I–enriched fibrotic environment. Cancer Res. 70, 5706–5716 (2010).
Ghiso, J. A. A., Kovalski, K. & Ossowski, L. Tumor dormancy induced by downregulation of urokinase receptor in human carcinoma involves integrin and MAPK signaling. J. Cell Biol. 147, 89–104 (1999).
Shibue, T. & Weinberg, R. A. Integrin β1-focal adhesion kinase signaling directs the proliferation of metastatic cancer cells disseminated in the lungs. Proc. Natl Acad. Sci. 106, 10290–10295 (2009).
Weaver, V. M. et al. Reversion of the malignant phenotype of human breast cells in three-dimensional culture and in vivo by integrin blocking antibodies. J. Cell Biol. 137, 231–245 (1997).
White, D. E. et al. Targeted disruption of β1-integrin in a transgenic mouse model of human breast cancer reveals an essential role in mammary tumor induction. Cancer Cell 6, 159–170 (2004).
Boyerinas, B. et al. Adhesion to osteopontin in the bone marrow niche regulates lymphoblastic leukemia cell dormancy. Blood 121, 4821–4831 (2013).
Gao, H. et al. Multi-organ site metastatic reactivation mediated by non-canonical discoidin domain receptor 1 signaling. Cell 166, 47–62 (2016).
Lecarpentier, Y., Schussler, O., HEBERT, J.-L. & VALLEE, A. Multiple targets of the canonical WNT/beta-catenin signaling in cancers. Front. Oncol. 9, 1248 (2019).
Malladi, S. et al. Metastatic latency and immune evasion through autocrine inhibition of WNT. Cell 165, 45–60 (2016).
Yang, H. et al. The role of cellular reactive oxygen species in cancer chemotherapy. J. Exp. Clin. Cancer Res. 37, 266 (2018).
Liou, G.-Y. & Storz, P. Reactive oxygen species in cancer. Free Radic. Res. 44, 479–496 (2010).
Teng, M. W., Swann, J. B., Koebel, C. M., Schreiber, R. D. & Smyth, M. J. Immune‐mediated dormancy: an equilibrium with cancer. J. Leukoc. Biol. 84, 988–993 (2008).
Machida, H. et al. Significance of monocyte counts at recurrence on survival outcome of women with endometrial cancer. Int. J. Gynecologic Cancer 27, 302–310 (2017).
Okubo, M. et al. M2-polarized macrophages contribute to neovasculogenesis, leading to relapse of oral cancer following radiation. Sci. Rep. 6, 27548 (2016).
Bowers, L. W. et al. NSAID use reduces breast cancer recurrence in overweight and obese women: role of prostaglandin–aromatase interactions. Cancer Res. 74, 4446–4457 (2014).
Hughes, R. et al. Perivascular M2 macrophages stimulate tumor relapse after chemotherapy. Cancer Res. 75, 3479–3491 (2015).
Hallermalm, K. et al. Modulation of the tumor cell phenotype by IFN-γ results in resistance of uveal melanoma cells to granule-mediated lysis by cytotoxic lymphocytes. J. Immunol. 180, 3766–3774 (2008).
Namjoshi, P., Showalter, L., Czerniecki, B. J. & Koski, G. K. T-helper 1-type cytokines induce apoptosis and loss of HER-family oncodriver expression in murine and human breast cancer cells. Oncotarget 10, 6006 (2019).
Payne, K. K. et al. Tumor‐reactive immune cells protect against metastatic tumor and induce immunoediting of indolent but not quiescent tumor cells. J. Leukoc. Biol. 100, 625–635 (2016).
Kmieciak, M., Payne, K. K., Wang, X.-Y. & Manjili, M. H. IFN-γ Rα is a key determinant of CD8+ T cell-mediated tumor elimination or tumor escape and relapse in FVB mouse. PLoS ONE 8, e82544 (2013).
Cole, S. W. Chronic inflammation and breast cancer recurrence. J. Clin. Oncol. 27, 3418 (2009).
Toiyama, Y. et al. C-reactive protein as predictor of recurrence in patients with rectal cancer undergoing chemoradiotherapy followed by surgery. Anticancer Res. 33, 5065–5074 (2013).
Shrotriya, S. et al. Serum C-reactive protein is an important and powerful prognostic biomarker in most adult solid tumors. PloS ONE 13, e0202555 (2018).
Duffy, S. A. et al. Interleukin‐6 predicts recurrence and survival among head and neck cancer patients. Cancer: Interdisciplinary International. J. Am. Cancer Soc. 113, 750–757 (2008).
Manjili, M. H. The inherent premise of immunotherapy for cancer dormancy. Cancer Res. 74, 6745–6749 (2014).
Baxevanis, C. N. & Perez, S. A. Cancer dormancy: a regulatory role for endogenous immunity in establishing and maintaining the tumor dormant state. Vaccines 3, 597–619 (2015).
Eyles, J. et al. Tumor cells disseminate early, but immunosurveillance limits metastatic outgrowth, in a mouse model of melanoma. J. Clin. Investig. 120, 2030–2039 (2010).
Rhim, A. D. et al. EMT and dissemination precede pancreatic tumor formation. Cell 148, 349–361 (2012).
Qian, B.-Z. et al. CCL2 recruits inflammatory monocytes to facilitate breast-tumour metastasis. Nature 475, 222 (2011).
Doak, G. R., Schwertfeger, K. L. & Wood, D. K. Distant relations: macrophage functions in the metastatic niche. Trends Cancer 4, 445–459 (2018).
Chen, Q., Zhang, X. H.-F. & Massagué, J. Macrophage binding to receptor VCAM-1 transmits survival signals in breast cancer cells that invade the lungs. Cancer Cell 20, 538–549 (2011).
Nielsen, S. R. et al. Macrophage-secreted granulin supports pancreatic cancer metastasis by inducing liver fibrosis. Nat. Cell Biol. 18, 549 (2016).
Linde, N., Fluegen, G. & Aguirre-Ghiso, J. The relationship between dormant cancer cells and their microenvironment. Adv. Cancer Res. 132, 45–71 (2016).
Wang, H. et al. Immune checkpoint blockade and CAR-T cell therapy in hematologic malignancies. J. Hematol. Oncol. 12, 59 (2019).
Lan, Q. et al. Type I interferon/IRF7 axis instigates chemotherapy-induced immunological dormancy in breast cancer. Oncogene 38, 2814 (2019).
Albrengues, J. et al. Neutrophil extracellular traps produced during inflammation awaken dormant cancer cells in mice. Science 361, eaao4227 (2018).
Ranganathan, A. C., Adam, A. P., Zhang, L. & Aguirre-Ghiso, J. A. Tumor cell dormancy induced by p38SAPK and ER-stress signaling: an adaptive advantage for metastatic cells? Cancer Biol. Ther. 5, 729–735 (2006).
Senft, D. & Ze’ev, A. R. Adaptive stress responses during tumor metastasis and dormancy. Trends Cancer 2, 429–442 (2016).
Cianfrocca, M. et al. Phase 1 trial of the antiangiogenic peptide ATN-161 (Ac-PHSCN-NH 2), a beta integrin antagonist, in patients with solid tumours. Br. J. Cancer 94, 1621–1626 (2006).
Ricart, A. D. et al. Volociximab, a chimeric monoclonal antibody that specifically binds α5β1 integrin: a phase I, pharmacokinetic, and biological correlative study. Clin. Cancer Res. 14, 7924–7929 (2008).
Bell-McGuinn, K. M. et al. A phase II, single-arm study of the anti-α5β1 integrin antibody volociximab as monotherapy in patients with platinum-resistant advanced epithelial ovarian or primary peritoneal cancer. Gynecologic Oncol. 121, 273–279 (2011).
Mita, M. et al. Phase I study of E7820, an oral inhibitor of integrin α-2 expression with antiangiogenic properties, in patients with advanced malignancies. Clin. Cancer Res. 17, 193–200 (2011).
Kerklaan, B. M. et al. A phase I, dose escalation, pharmacodynamic, pharmacokinetic, and food-effect study of α 2 integrin inhibitor E7820 in patients with advanced solid tumors. Investigational N. Drugs 34, 329–337 (2016).
Sawyer, M. et al. Phase II study of E7820 in combination with cetuximab in subjects (pts) with metastatic and refractory colorectal cancer (CRC). J. Clin. Oncol. 28, 3537–3537 (2010).
Heidenreich, A. et al. A randomized, double-blind, multicenter, phase 2 study of a human monoclonal antibody to human αν integrins (intetumumab) in combination with docetaxel and prednisone for the first-line treatment of patients with metastatic castration-resistant prostate cancer. Ann. Oncol. 24, 329–336 (2013).
O’day, S. et al. A randomised, phase II study of intetumumab, an anti-α v-integrin mAb, alone and with dacarbazine in stage IV melanoma. Br. J. cancer 105, 346–352 (2011).
Tabernero, J. et al. Abstract C119: Investigation of the anti-angiogenic effects of abituzumab in patients with colorectal or ovarian cancer and liver metastases using dynamic contrast-enhanced magnetic resonance imaging (DCE-MRI), AACR-NCI-EORTC. Int. Conf.: Mol. Targets Cancer Therapeut. 14, C119 (2015).
Elez, E. et al. Abituzumab combined with cetuximab plus irinotecan versus cetuximab plus irinotecan alone for patients with KRAS wild-type metastatic colorectal cancer: the randomised phase I/II POSEIDON trial. Ann. Oncol. 26, 132–140 (2015).
Hussain, M. et al. Differential effect on bone lesions of targeting integrins: randomized phase II trial of abituzumab in patients with metastatic castration-resistant prostate cancer. Clin. Cancer Res. 22, 3192–3200 (2016).
Hersey, P. et al. A phase II, randomized, open-label study evaluating the antitumor activity of MEDI-522, a humanized monoclonal antibody directed against the human alpha v beta 3 (avb3) integrin,±dacarbazine (DTIC) in patients with metastatic melanoma (MM). J. Clin. Oncol. 23, 7507–7507 (2005).
Rosenthal, M. A. et al. Evaluation of the safety, pharmacokinetics and treatment effects of an ανβ3 integrin inhibitor on bone turnover and disease activity in men with hormone‐refractory prostate cancer and bone metastases. Asia‐Pac. J. Clin. Oncol. 6, 42–48 (2010).
Alva, A. et al. Phase II study of cilengitide (EMD 121974, NSC 707544) in patients with non-metastatic castration resistant prostate cancer, NCI-6735. A study by the DOD/PCF prostate cancer clinical trials consortium. Investigational N. Drugs 30, 749–757 (2012).
Heinemann, V. et al. Phase II randomised proof-of-concept study of the urokinase inhibitor upamostat (WX-671) in combination with gemcitabine compared with gemcitabine alone in patients with non-resectable, locally advanced pancreatic cancer. Br. J. Cancer 108, 766–770 (2013).
Mak, G. et al. A phase Ib dose-finding, pharmacokinetic study of the focal adhesion kinase inhibitor GSK2256098 and trametinib in patients with advanced solid tumours. Br. J. Cancer 120, 975–981 (2019).
Soria, J.-C. et al. A phase I, pharmacokinetic and pharmacodynamic study of GSK2256098, a focal adhesion kinase inhibitor, in patients with advanced solid tumors. Ann. Oncol. 27, 2268–2274 (2016).
Aung, K. L. et al. A phase II trial of GSK2256098 and trametinib in patients with advanced pancreatic ductal adenocarcinoma (PDAC) (MOBILITY-002 Trial, NCT02428270). J. Clin. Oncol. 36, 409 (2018).
Jones, S. F. et al. A phase I study of VS-6063, a second-generation focal adhesion kinase inhibitor, in patients with advanced solid tumors. Investigational N. drugs 33, 1100–1107 (2015).
Patel, M. R. et al. Phase 1/1b study of the FAK inhibitor defactinib (VS-6063) in combination with weekly paclitaxel for advanced ovarian cancer. J. Clin. Oncol. 32, 5521 (2017).
Gerber, D. E. et al. Phase 2 study of the focal adhesion kinase inhibitor defactinib (VS-6063) in previously treated advanced KRAS mutant non-small cell lung cancer. Lung Cancer 139, 60–67 (2020).
Verstovsek, S. et al. Phase 1/2 study of pacritinib, a next generation JAK2/FLT3 inhibitor, in myelofibrosis or other myeloid malignancies. J. Hematol. Oncol. 9, 137 (2016).
Hurwitz, H. I. et al. Randomized, double-blind, phase II study of ruxolitinib or placebo in combination with capecitabine in patients with metastatic pancreatic cancer for whom therapy with gemcitabine has failed. J. Clin. Oncol. 33, 4039 (2015).
Sweet, K. et al. A phase I clinical trial of ruxolitinib in combination with nilotinib in chronic myeloid leukemia patients with molecular evidence of disease. Leuk. Res. 74, 89–96 (2018).
Guerra, V. A. et al. A phase I-II study of ruxolitinib (INCB18424) for patients with chronic myeloid leukemia with minimal residual disease while on therapy with imatinib. Blood 134, 5906 (2019).
Reilley, M. J. et al. STAT3 antisense oligonucleotide AZD9150 in a subset of patients with heavily pretreated lymphoma: results of a phase 1b trial. J. Immunother. Cancer 6, 1–10 (2018).
Ogura, M. et al. Phase I study of OPB‐51602, an oral inhibitor of signal transducer and activator of transcription 3, in patients with relapsed/refractory hematological malignancies. Cancer Sci. 106, 896–901 (2015).
Wong, A. et al. Phase I and biomarker study of OPB-51602, a novel signal transducer and activator of transcription (STAT) 3 inhibitor, in patients with refractory solid malignancies. Ann. Oncol. 26, 998–1005 (2015).
Limburg, P. J. et al. Randomized phase II trial of sulindac for lung cancer chemoprevention. Lung Cancer 79, 254–261 (2013).
Chen, E. Y. et al. A phase II study of celecoxib with irinotecan, 5-fluorouracil, and leucovorin in patients with previously untreated advanced or metastatic colorectal cancer. Am. J. Clin. Oncol. 41, 1193–1198 (2018).
Edelman, M. J. et al. Phase III randomized, placebo-controlled, double-blind trial of celecoxib in addition to standard chemotherapy for advanced non–small-cell lung cancer with cyclooxygenase-2 overexpression: CALGB 30801 (Alliance). J. Clin. Oncol. 35, 2184 (2017).
Coombes, R. et al. A phase III, multicenter, double-blind, randomized trial of celecoxib versus placebo in primary breast cancer patients: Randomized European Celecoxib Trial (REACT). J. Clin. Oncol. 29, TPS115–TPS115 (2011).
Coombes, R. et al. Abstract GS3-03: A phase III multicentre double blind randomised trial of celecoxib versus placebo in primary breast cancer patients (REACT – Randomised EuropeAn celecoxib trial). Cancer Res. 78, GS3-03 (2018).
Acknowledgements
This work was supported by the National Research Foundation of Korea (NRF) through a grant funded by the Korean government (MSIP: Ministry of Science, ICT and Future Planning) (No. NRF-2017R1E1A1A01075125). Additionally, this work was supported by a grant from the Cell Logistics Research Center of the National Research Foundation of Korea (NRF-2016R1A5A1007318) and by a Gwangju Institute of Science and Technology (GIST) Research Institute (GRI) grant funded by the GIST in 2020.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Park, SY., Nam, JS. The force awakens: metastatic dormant cancer cells. Exp Mol Med 52, 569–581 (2020). https://doi.org/10.1038/s12276-020-0423-z
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s12276-020-0423-z
This article is cited by
-
Revealing role of epigenetic modifiers and DNA oxidation in cell-autonomous regulation of Cancer stem cells
Cell Communication and Signaling (2024)
-
The senescence journey in cancer immunoediting
Molecular Cancer (2024)
-
Tumor heterogeneity and clinically invisible micrometastases in metastatic breast cancer—a call for enhanced surveillance strategies
npj Precision Oncology (2024)
-
Extracellular vesicle-mediated transfer of miRNA-1 from primary tumors represses the growth of distant metastases
Experimental & Molecular Medicine (2024)
-
A cell cycle centric view of tumour dormancy
British Journal of Cancer (2023)