Abstract
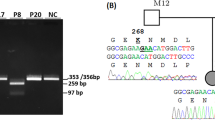
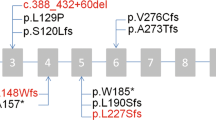
Primary ciliary dyskinesia (PCD) is a genetically heterogeneous disorder affecting ciliary structure and function. PCD exhibiting dynein regulatory complex subunit 1 (DRC1) exon 1–4 deletion has been reported in several Japanese PCD patients; however, no large scale studies have been performed. Here, we aimed to determine the prevalence and founder effect of this variant in the Korean population. Using an in-house copy number variation tool, we screened for DRC1 exon 1–4 deletion in 20 patients with PCD and exome data of 1435 patients in the Seoul National University Hospital repository. In cases of suspected DRC1 deletion, confirmatory gap-PCR was performed. In a PCD cohort, three of 20 (15%) patients were positive for DRC1 exon 1–4 deletion (NM_145038.5(DRC1): c.1‐3952_540 + 1331del27748‐bp) while pathogenic variants were found in CCDC39 (N = 1), DNAAF6 (N = 1), DNAH9 (N = 1). In the 1,435-sample exome data, seven patients (0.49%) were confirmed to have DRC1 exon 1–4 deletion. A chimeric sequence including the junction was searched from the 1000 Genomes Project data repository. One Japanese patient (0.96%) was found to have the same DRC1 exon 1–4 deletion, which was absent in other populations. This study demonstrated that the DRC1 exon 1–4 deletion is a founder mutation based on haplotype analysis. In summary, the prevalence of PCD based on DRC1 exon 1–4 deletion is particularly high in Korean and Japanese populations, which is attributed to the founder effect. Genetic testing for DRC1 exon 1–4 deletion should be considered as an initial screening tool for Korean and Japanese patients with PCD.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout


Similar content being viewed by others
References
Zariwala MA, Knowles MR, Leigh MW. Primary Ciliary Dyskinesia. In: Adam MP, Everman DB, Mirzaa GM, Pagon RA, Wallace SE, Bean LJH, et al., editors. GeneReviews((R)). Seattle (WA) 1993.
Bush A, Chodhari R, Collins N, Copeland F, Hall P, Harcourt J, et al. Primary ciliary dyskinesia: current state of the art. Arch Dis Child. 2007;92:1136–40.
Shapiro AJ, Zariwala MA, Ferkol T, Davis SD, Sagel SD, Dell SD, et al. Diagnosis, monitoring, and treatment of primary ciliary dyskinesia: PCD foundation consensus recommendations based on state of the art review. Pediatr Pulmonol. 2016;51:115–32.
Niziolek M, Bicka M, Osinka A, Samsel Z, Sekretarska J, Poprzeczko M, et al. PCD genes-from patients to model organisms and back to humans. Int J Mol Sci. 2022;23:1749.
Morimoto K, Hijikata M, Zariwala MA, Nykamp K, Inaba A, Guo TC, et al. Recurring large deletion in DRC1 (CCDC164) identified as causing primary ciliary dyskinesia in two Asian patients. Mol Genet Genom Med. 2019;7:e838.
Keicho N, Hijikata M, Morimoto K, Homma S, Taguchi Y, Azuma A, et al. Primary ciliary dyskinesia caused by a large homozygous deletion including exons 1-4 of DRC1 in Japanese patients with recurrent sinopulmonary infection. Mol Genet Genom Med. 2020;8:e1033.
Takeuchi K, Xu Y, Kitano M, Chiyonobu K, Abo M, Ikegami K, et al. Copy number variation in DRC1 is the major cause of primary ciliary dyskinesia in the Japanese population. Mol Genet Genom Med. 2020;8:e1137.
Kim YG, Sung H, Shin HS, Kim MJ, Lee JS, Park SS, et al. Intronic LINE-1 insertion in SLCO1B3 as a highly prevalent cause of rotor syndrome in East Asian population. J Hum Genet. 2022;67:71–7.
Kobayashi K, Nakahori Y, Miyake M, Matsumura K, Kondo-Iida E, Nomura Y, et al. An ancient retrotransposal insertion causes Fukuyama-type congenital muscular dystrophy. Nature. 1998;394:388–92.
Yau WY, Vandrovcova J, Sullivan R, Chen Z, Zecchinelli A, Cilia R, et al. Low prevalence of NOTCH2NLC GGC repeat expansion in white patients with movement disorders. Mov Disord. 2021;36:251–5.
Kusters DM, Huijgen R, Defesche JC, Vissers MN, Kindt I, Hutten BA, et al. Founder mutations in the Netherlands: geographical distribution of the most prevalent mutations in the low-density lipoprotein receptor and apolipoprotein B genes. Neth Heart J. 2011;19:175–82.
Saha B, Lessel D, Nampoothiri S, Rao AS, Hisama FM, Peter D, et al. Ethnic-specific WRN mutations in South Asian Werner syndrome patients: potential founder effect in patients with Indian or Pakistani Ancestry. Mol Genet Genom Med. 2013;1:7–14.
ElBiad O, Laraqui A, El Boukhrissi F, Mounjid C, Lamsisi M, Bajjou T, et al. Prevalence of specific and recurrent/founder pathogenic variants in BRCA genes in breast and ovarian cancer in North Africa. BMC Cancer. 2022;22:208.
Zlotogora J. High frequencies of human genetic diseases: founder effect with genetic drift or selection? Am J Med Genet. 1994;49:10–3.
Kim MJ, Lee S, Yun H, Cho SI, Kim B, Lee JS, et al. Consistent count region-copy number variation (CCR-CNV): an expandable and robust tool for clinical diagnosis of copy number variation at the exon level using next-generation sequencing data. Genet Med. 2022;24:663–72.
Stephens M, Smith NJ, Donnelly P. A new statistical method for haplotype reconstruction from population data. Am J Hum Genet. 2001;68:978–89.
Stephens M, Donnelly P. A comparison of bayesian methods for haplotype reconstruction from population genotype data. Am J Hum Genet. 2003;73:1162–9.
Stephens M, Scheet P. Accounting for decay of linkage disequilibrium in haplotype inference and missing-data imputation. Am J Hum Genet. 2005;76:449–62.
Fassad MR, Shoman WI, Morsy H, Patel MP, Radwan N, Jenkins L, et al. Clinical and genetic spectrum in 33 Egyptian families with suspected primary ciliary dyskinesia. Clin Genet. 2020;97:509–15.
Paff T, Kooi IE, Moutaouakil Y, Riesebos E, Sistermans EA, Daniels H, et al. Diagnostic yield of a targeted gene panel in primary ciliary dyskinesia patients. Hum Mutat. 2018;39:653–65.
Wirschell M, Olbrich H, Werner C, Tritschler D, Bower R, Sale WS, et al. The nexin-dynein regulatory complex subunit DRC1 is essential for motile cilia function in algae and humans. Nat Genet. 2013;45:262–8.
Lei C, Yang D, Wang R, Ding S, Wang L, Guo T, et al. DRC1 deficiency caused primary ciliary dyskinesia and MMAF in a Chinese patient. J Hum Genet. 2022;67:197–201.
Funding
This research was supported by a fund (2020-ER6904-01) by Research of Korea Centers for Disease Control and Prevention. JM was supported by the Seoul National University Hospital Research Fund (0320210170).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Kim, M.J., Kim, S., Chae, S.W. et al. Prevalence and founder effect of DRC1 exon 1–4 deletion in Korean patients with primary ciliary dyskinesia. J Hum Genet 68, 369–374 (2023). https://doi.org/10.1038/s10038-023-01122-8
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s10038-023-01122-8
This article is cited by
-
The Korean Genetic Diagnosis Program for Rare Disease Phase II: outcomes of a 6-year national project
European Journal of Human Genetics (2023)
-
The challenge of diagnosing primary ciliary dyskinesia: a commentary on various causative genes and their pathogenic variants
Journal of Human Genetics (2023)