Abstract
Both betaine homocysteine methyltransferase (BHMT) and cystathionine β-synthase (CBS) are major enzymes in the metabolism of plasma homocysteine (Hcy). Abnormal methylation levels of BHMT and CBS are positively associated with Hcy levels. The present study is performed to explore the association between the methylation levels in the promoter regions of the BHMT and CBS genes and the efficacy of folic acid therapy in patient with hyperhomocysteinemia (HHcy). A prospective cohort study recruiting HHcy (Hcy ≥ 15 μmol/L) patients was performed. The subjects were treated with oral folic acid (5 mg/d) for 90 days, and the patients were divided into the success group (Hcy < 15 μmol/L) and the failure group (Hcy ≥ 15 μmol/L) according to their Hcy levels after treatment. In the logistic regression model with adjusted covariates, the patients with lower total methylation levels in the BHMT and CBS promoter regions exhibited 1.627-fold and 1.671-fold increased risk of treatment failure compared with higher methylation individuals, respectively. Similarly, subjects who had lower methylation levels (<methylation mean) in BHMT CpG1 had 1.792 times higher risks. Stratified analysis by sex found that lower CBS methylation levels were associated with a 2.128-fold increased risk for treatment failure in males with HHcy. Lower levels of BHMT or CBS promoter total methylation might be associated with increased the risk of treatment failure. These studies suggest that lower levels of BHMT and CBS methylation are all predictors of failure in folic acid therapy for HHcy. However, due to some limitations of this study, such as the small number of the loci tested, further large-scale studies are necessary to verify our observations.
Similar content being viewed by others
Introduction
Homocysteine (Hcy), a nonessential and sulfur-containing amino acid, is produced as an important intermediary of methionine metabolism [1]. The fasting total plasma Hcy levels in healthy adults are 5–15 μmol/L, and higher than 15 μmol/L is defined as hyperhomocysteinemia (HHcy). Yang and Fan performed a systematic review of the prevalence of HHcy in China and the results of these studies suggested that the overall pooled prevalence of HHcy was 27.5% and the prevalence increased with age was significantly higher in men than in women [2]. Furthermore, numerous studies have supported that HHcy could be a strong risk factor for neural tube defects, and it also has been significantly associated with many noncommunicable diseases, including cardiovascular and cerebrovascular diseases, type 2 diabetes, and other cancers [3,4,5,6,7]. Therefore, it is significant in sanitary science to reduce the total plasma Hcy levels for decreasing the risk of stroke and other cardiovascular diseases.
In the past two decades, many studies have suggested that the total Hcy levels can be effectively reduced after folic acid supplementation [8,9,10]. A meta-analysis had also indicated that folic acid supplementation can effectively reduce the risk of stroke in primary prevention [11]. However, our previous research results showed that after 90 days of folic acid (5 mg/d) therapy for HHcy patients, the total plasma Hcy levels of 43.59% of the patients failed to recover the normal range [12]. Anderson’s research also had consistent results [13]. Currently, studies on the efficacy of folic acid therapy on HHcy mainly focus on the polymorphism of genes related to Hcy metabolic enzyme and their interaction with environmental factors, but the results can only explain part of the reasons for treatment failure [14,15,16]. Therefore, in order to further investigate the causes of this phenomenon, we were inspired by a previous review which indicated that Hcy is a byproduct of methyltransferase reaction, so DNA methylation may be one of the underlying mechanisms of HHcy-associated disorders [17]. Therefore, we need to analyze the failure of folic acid intervention more comprehensively from the perspective of epigenetics, especially the changes of DNA methylation.
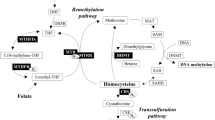
So far, there is few reports on the relationship between gene methylation and the efficacy of folic acid therapy in patients with HHcy. Recently, DNA methylation has been discovered as an important modification pathway of epigenetics. Several studies have demonstrated that DNA methylation might lead to a series of diseases related to HHcy [17,18,19]. The concentrations of Hcy are maintained by two main routes; namely: the remethylation pathway, where Hcy is converted back to methionine by betaine homocysteine methyltransferase (BHMT), and the transsulfuration pathway, where Hcy is converted to cystathionine to form cysteine by cystathionine β-synthase (CBS) [20]. However, the two key genes, which are related to Hcy metabolism, promoter regions abnormal methylation shall affect their own activity and abnormal expression, resulting in the abnormal metabolism of Hcy. Thus, the purpose of this study is to explore the association of both BHMT and CBS genes promoter methylation with the efficacy of folic acid therapy in patients with HHcy, so as to provide a new theoretical foundation for treatment of HHcy.
Materials and methods
Study design and subjects
A prospective cohort study of HHcy patients (Hcy ≥ 15 μmol/L) was performed, who had plasma Hcy levels measured in the Department of Neurology in the Fifth Affiliated Hospital of Zhengzhou University from July to December 2014 in a central Han Chinese population. The subjects included and excluded in our study met the following criteria from a previously described database. Inclusion criteria: (1) measured the plasma Hcy level, (2) diagnosed with HHcy (total plasma Hcy ≥ 15 μmol/L), (3) >18 years of age, and (4) voluntarily participated in this study and received 90 days folic acid supplementation. Exclusion criteria: (1) a history of serious infection, hepatic or kidney diseases, hematologic disorders, or cancer and (2) used vitamin B or folic acid supplements or use medications that interfere with folic acid metabolism (methotrexate, phenytoin, etc.) in the prior 2 weeks. Fasting plasma Hcy levels were measured on the first day. Then, the recruited patients were treated with oral folic acid (5 mg/d) for 90 days. The compliance with oral folic acid was assessed by telephone interview at 45 day (first compliance) and 90 day (second compliance) of follow-up. Criteria for compliance were as follows: (1) perfect compliance, following doctor’s prescription on time; (2) intermediate compliance, missing a folic acid dose no more than 3 times per week; and (3) poor compliance, missing a folic acid dose 3 times or more per week. Finally, plasma Hcy levels were obtained on the 90th day (second follow-up).
The study was approved by the Ethics Review Committee of the Life Science of Zhengzhou University. All subjects or relatives signed informed consent.
Efficacy criteria and methylation grouping
Among 1071 patients were enrolled in this study. However, 253 patients with poor compliance and 180 subjects with loss to follow-up were excluded. Thus, a total of 638 subjects were ultimately included in our study.
If the patients’ Hcy levels dropped to 15 μmol/L or less, then the treatment was effective and they were included in the success group (N = 325). If the patients’ Hcy levels were ≥15 μmol/L, then the therapy was unsuccessful and they were included in the failure group (N = 313).
There were 152 cases of patients with the best efficacy (Hcy levels decrease maximum) in the success group and 152 cases of patients with the worst efficacy (Hcy levels decrease minimum) in the failure group whose key genes promoter methylation level was detected, and five cases of patients in the success group whose methylation level was not detected for some reason were excluded. Thus, a total of 299 cases of patients in the oral folic acid therapy group were included.
Blood collection and DNA extraction
At baseline, overnight-fasting blood samples were drawn, plasma and cells were separated and stored, and DNA extraction was performed. The methods in this part are consistent with the previous research done by the research group. Please refer to this literature for details.
DNA quantification and purity determination
The methods in this part are consistent with the previous research done by the research group. Please refer to this literature for details.
PCR amplification and MethylTargetTM analysis gene promoter methylation
DNA methylation level was analysis by MethylTargetTM (Genesky Biotechnologies Inc., Shanghai, China), an NGS-based multiple Targeted CpG methylation analysis method. First, Genomic DNA (400 ng) was subjected to sodium bisulfite treatment using the EZ DNA MethylationTM kit (Zymo Research) according to manufacturer’s protocols. Multiplex PCR was performed with optimized primer sets combination. A 20 μl PCR reaction mixture was prepared for each reaction and included 1× reaction buffer (Takara), 3 mM Mg2+, 0.2 mM dNTP, 0.1 μM of each primer, 1U HotStarTaq polymerase (Takara) and 2 μl template DNA. The cycling program was 95 °C for 2 min; 11 cycles of 94 °C for 20 s, 63 °C for 40 s with a decreasing temperature step of 0.5 °C per cycle, 72 °C for 1 min; then followed by 24 cycles of 94 °C for 20 s, 65 °C for 30 s, 72 °C for 1 min; 72 °C for 2 min. Then, PCR amplicons were diluted and amplified using indexed primers. Finally, sequencing: libraries from different samples were quantified and pooled together, followed by sequencing on the Illumina MiSeq platform according to manufacturer’s protocols. Sequencing was performed with a 2 × 300 bp paired-end mode. Quality control of sequencing reads was performed by FastQC. Filtered reads were mapped to genome by Blast. After reads recalibration with USEARCH, methylation was analyzed using Perl script. The total methylation level was calculated as (CpG1 + CpG2 + CpG3,…,CpGn)/n. The primer was designed online (http://primer3.ut.ee/). The sequences of the primers were presented in Table 1.
Statistical analysis
The statistical analyses were done with the SPSS software package (version 21.0 for windows). The clinical characteristics of the subjects were described as the mean ± S.D. or number (%) and the methylation data were expressed as the mean and range. The t test and χ2 test were used to compare clinical parameters between success group and failure group.
A logistic regression model was applied to estimate the association between BHMT and CBS promoter methylation and two groups with and without adjustment for different variables, including age, sex, smoking, drinking, body mass index (BMI), total cholesterol (TC), low-density lipoprotein cholesterol (LDL-c), high-density lipoprotein cholesterol (HDL-c), and vitamin B12 (VitB12), which were significantly different in baseline characteristics. The methylation level in the regression model was divided into a binary variable (≥ and <methylation mean). We performed unconditional logistic regression analyses in two groups to estimate the odds ratio (OR), with confidence interval (CI) of 95%. All reported P values were two-sides, and all associations were considered significant when P values were less than 0.05.
Results
Population characteristics
The baseline characteristics of all participants were shown in Tables 2 and 3. Compared with success group, the patients in the failure group had a higher BMI and baseline plasma Hcy levels, but lower TC, LDL-c, HDL-c, and VitB12 (Table 3). Furthermore, both smoking status and second compliance all had effect on the efficacy of folic acid therapy in patient with HHcy.
Lower methylation level in BHMT and CBS promoter were associated with an increased risk of folic acid therapy failure
A significant difference was found in the total methylation level of the BHMT promoter region between failure group and success group, whereas no significant difference was observed in BHMT CpG1 and CpG2 (Fig. 1a, c). The total methylation and CBS CpG1, CpG3 methylation in CBS promoter were not significant difference in two groups. However, CBS CpG2 methylation in the CBS promoter was statistically lower in failure group than success group (Fig. 1b, d).
In the logistic regression model with adjusted age, sex, smoking, drinking status BMI, vitB12, HDL-c, and LDL-c (Table 4), a significant difference was observed in BHMT and CBS methylation levels (both total methylation) between failure group and success group and the patients in the failure group with lower methylation levels. Furthermore, the patients with lower BHMT total methylation, BHMT CpG1 methylation level, and CBS total methylation level (<methylation mean) exhibited increased risks of therapy failure compared with those with higher methylation levels (≥methylation mean), respectively. In the CBS gene, the decrease of per standard deviation of CpG methylation was associated with increased the risk of therapy failure.
A negative correlation existed for CBS CpG1 and CpG2 methylation level and treatment effect, respectively. However, no significant difference was observed in CBS CpG3 and BHMT methylation level (both total methylation and each CpG methylation) between two groups.
Association between gender and CBS methylation level with risk of HHcy treatment failure
A highly significant interaction between gene methylation and sex was seen for CBS promoter methylation (P for interaction < 0.05, Table 5). The OR for treatment failure in relation to male with the lower levels of CBS promoter methylation was 1.950 when nothing is adjusted, 2.153 when adjusted for age, sex, smoking and drinking status, and 2.128 when further adjusted for other potentially confounding variables (see Table 5).
Discussion
The purpose of the prospective cohort study was to examine the association between BHMT and CBS gene promoter methylation with the efficacy of folic acid therapy in patients with HHcy. The results revealed that both BHMT and CBS promoter hypomethylation were predictive factors of failure in folic acid therapy for HHcy. Stratified analysis by sex also suggested that the lower levels CBS promoter methylation was a predictive factor in male subjects for treatment failure.
Accumulating evidence, as well as our group’s previous research findings, indicates that HHcy may be associated with variants in genes related to Hcy metabolism. However, epigenetics, especially DNA methylation, were rarely studied [15, 21,22,23]. Both BHMT and CBS enzymes, two of three predominant enzymes, are involved in two metabolic pathways of Hcy conversion to methionine and cysteine, which contribute approximately half of the methionine necessary for AdoMet synthesis [24] and nearly 40–50% of cysteine production [25]. CBS deficiency was identified as a genetic factor that resulted in elevated levels of Hcy or HHcy [25]. However, in our study, we found that the total methylation level of the BHMT and CBS promoter in failure group was lower than that in success group. These discrepant results may be caused by the fact that this study is a study of drug efficacy. A previous study also found that HHcy might be caused by DNA Hypomethylation [26]. However, the exact molecular mechanism of the association between increased Hcy plasma concentration and changes in DNA methylation remains unclear. In addition, Hcy concentration also affects the methylation levels. Cacciapuoti [27] found that Hcy concentration was negatively correlated with the levels of genomic DNA methylation. Hcy can play an important biological role by affecting the methylation levels, thus affecting the occurrence and development of diseases. High concentration of Hcy can promote the activity of methyltransferase by inhibiting the metabolism of s-adenosine homocysteine, which result in the reduction of gene methylation levels and promote a compensatory mechanism of Hcy metabolism [28].
In addition, the risk in the treatment failure group was statistically significant only in males with the lower levels of CBS promoter methylation, and gender differences were observed in several genes [29, 30]. Sexual dimorphism in mammalian gene expression is thought to result in different sex hormone (testosterone, androgens) levels in males and females [31]. Testosterone has a distinct impact on renal CBS enzyme levels, and when female mice were injected with androgen, the renal CBS activity increased two-fold [32]. However, the specific mechanism of this gender difference is still unclear.
As far as we are aware, the present work is the first study to assess the association between BHMT and CBS gene promoter methylation with the efficacy of folic acid therapy in patients with HHcy. In addition, as this study is a prospective cohort study, it had strict population inclusion criteria, sample collection, laboratory testing, and data analysis. A face-to-face questionnaire survey was conducted to collect the data from study population, and all the investigators were trained uniformly for quality control. However, there are some limitations to this study. First, the genetic link between the ancestors of different RACES is different, and it has an important effect on DNA methylation level, leading to different susceptibility to diseases [33, 34]. Since the subjects recruited in this study were only Chinese Han individuals from Zhengzhou, the conclusion might not be suitable for other ethnic groups. Second, due to the lack of research funding, the number of methylated genes tested is limited. Therefore, further studies with larger sample size are needed to support this finding.
In conclusion, patients with HHcy had decreased BHMT and CBS promoter methylation levels. In a central Han Chinese population, BHMT and CBS promoter hypomethylation in the blood might serve as predictors of failure in folic acid therapy for HHcy. A further large-scale study in different races with gene methylation changes should be performed to verify our findings.
References
Messedi M, Frigui M, Chaabouni K, Turki M, Neifer M, Lahiyani A, et al. Methylenetetrahydrofolate reductase C677T and A1298C polymorphisms and variations of homocysteine concentrations in patients with Behcet’s disease. Gene. 2013;527:306–10.
Yang B, Fan S, Zhi X, Wang Y, Wang Y, Zheng Q, et al. Prevalence of hyperhomocysteinemia in China: a systematic review and meta-analysis. Nutrients. 2014;7:74–90.
Wald DS, Law M, Morris JK. Homocysteine and cardiovascular disease: evidence on causality from a meta-analysis. BMJ. 2002;325:1202.
Homocysteine Studies C. Homocysteine and risk of ischemic heart disease and stroke: a meta-analysis. JAMA. 2002;288:2015–22.
de Ruijter W, Westendorp RG, Assendelft WJ, den Elzen WP, de Craen AJ, le Cessie S, et al. Use of Framingham risk score and new biomarkers to predict cardiovascular mortality in older people: population based observational cohort study. BMJ. 2009;338:a3083.
Brustolin S, Giugliani R, Felix TM. Genetics of homocysteine metabolism and associated disorders. Brazilian journal of medical and biological research = Revista brasileira de pesquisas medicas e biologicas. Braz J Med Biol Res. 2010;43:1–7.
Yakub M, Schulze KJ, Khatry SK, Stewart CP, Christian P, West KP. High plasma homocysteine increases risk of metabolic syndrome in 6 to 8 year old children in rural Nepal. Nutrients. 2014;6:1649–61.
Tighe P, Ward M, McNulty H, Finnegan O, Dunne A, Strain J, et al. A dose-finding trial of the effect of long-term folic acid intervention: implications for food fortification policy. Am J Clin Nutr. 2011;93:11–18.
Wald DS, Bishop L, Wald NJ, Law M, Hennessy E, Weir D, et al. Randomized trial of folic acid supplementation and serum homocysteine levels. Arch Intern Med. 2001;161:695–700.
Zappacosta B, Mastroiacovo P, Persichilli S, Pounis G, Ruggeri S, Minucci A, et al. Homocysteine lowering by folate-rich diet or pharmacological supplementations in subjects with moderate hyperhomocysteinemia. Nutrients. 2013;5:1531–43.
Wang X, Qin X, Demirtas H, Li J, Mao G, Huo Y, et al. Efficacy of folic acid supplementation in stroke prevention: a meta-analysis. Lancet. 2007;369:1876–82.
Tian H, Tian D, Zhang C, Wang W, Wang L, Ge M, et al. Efficacy of folic acid therapy in patients with hyperhomocysteinemia. J Am Coll Nutr. 2017;36:528–32.
Anderson JL, Jensen KR, Carlquist JF, Bair TL, Horne BD, Muhlestein JB. Effect of folic acid fortification of food on homocysteine-related mortality. Am J Med. 2004;116:158–64.
Qin X, Li J, Cui Y, Liu Z, Zhao Z, Ge J, et al. MTHFR C677T and MTR A2756G polymorphisms and the homocysteine lowering efficacy of different doses of folic acid in hypertensive Chinese adults. Nutr J. 2012;11:2.
Du B, Tian H, Tian D, Zhang C, Wang W, Wang L, et al. Genetic polymorphisms of key enzymes in folate metabolism affect the efficacy of folate therapy in patients with hyperhomocysteinaemia. Br J Nutr. 2018;119:887–95.
Liang S, Zhou Y, Wang H, Qian Y, Ma D, Tian W, et al. The effect of multiple single nucleotide polymorphisms in the folic acid pathway genes on homocysteine metabolism. BioMed Res Int. 2014;2014:560183.
Mandaviya PR, Stolk L, Heil SG. Homocysteine and DNA methylation: a review of animal and human literature. Mol Genet Metab. 2014;113:243–52.
Elmasry K, Mohamed R, Sharma I, Elsherbiny NM, Liu Y, Al-Shabrawey M, et al. Epigenetic modifications in hyperhomocysteinemia: potential role in diabetic retinopathy and age-related macular degeneration. Oncotarget. 2018;9:12562–90.
Wang C, Xu G, Wen Q, Peng X, Chen H, Zhang J, et al. CBS promoter hypermethylation increases the risk of hypertension and stroke. Clinics. 2019;74:e630.
Selhub J. Homocysteine metabolism. Annu Rev Nutr. 1999;19:217–46.
Li WX, Cheng F, Zhang AJ, Dai SX, Li GH, Lv WW, et al. Folate deficiency and gene polymorphisms of MTHFR, MTR and MTRR elevate the hyperhomocysteinemia risk. Clin Lab. 2017;63:523–33.
Clifford AJ, Chen K, McWade L, Rincon G, Kim SH, Holstege DM, et al. Gender and single nucleotide polymorphisms in MTHFR, BHMT, SPTLC1, CRBP2, CETP, and SCARB1 are significant predictors of plasma homocysteine normalized by RBC folate in healthy adults. J Nutr. 2012;142:1764–71.
Cui X, Navneet S, Wang J, Roon P, Chen W, Xian M, et al. Analysis of MTHFR, CBS, glutathione, taurine, and hydrogen sulfide levels in retinas of hyperhomocysteinemic mice. Investig Ophthalmol Vis Sci. 2017;58:1954–63.
Finkelstein JD, Martin JJ. Methionine metabolism in mammals. Distribution of homocysteine between competing pathways. J Biol Chem. 1984;259:9508–13.
Clarke R, Daly L, Robinson K, Naughten E, Cahalane S, Fowler B, et al. Hyperhomocysteinemia: an independent risk factor for vascular disease. New Engl J Med. 1991;324:1149–55.
Castro R, Rivera I, Struys EA, Jansen EE, Ravasco P, Camilo ME, et al. Increased homocysteine and S-adenosylhomocysteine concentrations and DNA hypomethylation in vascular disease. Clin Chem. 2003;49:1292–6.
Cacciapuoti F. Hyper-homocysteinemia: a novel risk factor or a powerful marker for cardiovascular diseases? Pathogenetic and therapeutical uncertainties. J Thrombosis Thrombolysis. 2011;32:82–88.
Jiang Y, Sun T, Xiong J, Cao J, Li G, Wang S. Hyperhomocysteinemia-mediated DNA hypomethylation and its potential epigenetic role in rats. Acta Biochimica et Biophysica Sin. 2007;39:657–67.
Cheng J, Wang Y, Zhou K, Wang L, Li J, Zhuang Q, et al. Male-specific association between dopamine receptor D4 gene methylation and schizophrenia. PloS ONE. 2014;9:e89128.
Gao S, Cheng J, Li G, Sun T, Xu Y, Wang Y, et al. Catechol-O-methyltransferase gene promoter methylation as a peripheral biomarker in male schizophrenia. Eur Psychiatry. 2017;44:39–46.
Rinn JL, Snyder M. Sexual dimorphism in mammalian gene expression. Trends Genet. 2005;21:298–305.
Manteuffel-Cymborowska M, Chmurzynska W, Grzelakowska-Sztabert B. Tissue-specific effects of testosterone on S-adenosylmethionine formation and utilization in the mouse. Biochimica et Biophysica Acta. 1992;1116:166–72.
Fraser HB, Lam LL, Neumann SM, Kobor MS. Population-specificity of human DNA methylation. Genome Biol. 2012;13:R8.
Doi T, Puri P, McCann A, Bannigan J, Thompson J. Epigenetic effect of cadmium on global de novo DNA hypomethylation in the cadmium-induced ventral body wall defect (VBWD) in the chick model. Toxicol Sci. 2011;120:475–80.
Acknowledgements
This work was funded by the Department of Science and Technology of Henan Province (no. 132102310431). The funder did not participate in the design, analysis or writing, or approval of the paper. The authors thank all staff from the Department of Neurology, the Fifth Affiliated Hospital of Zhengzhou University, for their assistance and support.
Author information
Authors and Affiliations
Contributions
The authors’ responsibilities were as follows: Xiaowen Huang contributed equally to the writing of this paper. Binghui Du and Opolot Godfrey were involved in the study concept and design. Bingnan Ren, Dankang Li, and Qinglin Zhao acquired data of this study. Xiliang Wang, Chengda Zhang, and Limin Yue carried out data analysis and interpretation. Weidong Zhang was in charge of study supervision. All authors read and approved the final paper.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Huang, X., Li, D., Zhao, Q. et al. Association between BHMT and CBS gene promoter methylation with the efficacy of folic acid therapy in patients with hyperhomocysteinemia. J Hum Genet 64, 1227–1235 (2019). https://doi.org/10.1038/s10038-019-0672-7
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s10038-019-0672-7
This article is cited by
-
Using the optimal method—explained variance weighted genetic risk score to predict the efficacy of folic acid therapy to hyperhomocysteinemia
European Journal of Clinical Nutrition (2022)
-
Combining genetic risk score with artificial neural network to predict the efficacy of folic acid therapy to hyperhomocysteinemia
Scientific Reports (2021)