Abstract
Biallelic mutations in IBA57 cause a mitochondrial disorder with a broad phenotypic spectrum that ranges from severe intellectual disability to adolescent-onset spastic paraplegia. Only 21 IBA57 mutations have been reported, therefore the phenotypic spectrum of IBA57-related mitochondrial disease has not yet been fully elucidated. In this study, we performed whole-exome sequencing on a Sepharadi Jewish and Japanese family with leukodystrophy. We identified four novel biallelic variants in IBA57 in the two families: one frameshift insertion and three missense variants. The three missense variants were predicted to be disease-causing by multiple in silico tools. The 29-year-old Sepharadi Jewish male had infantile-onset optic atrophy with clinically asymptomatic leukodystrophy involving periventricular white matter. The 19-year-old younger brother, with the same compound heterozygous IBA57 variants, had a similar clinical course until 7 years of age. However, he then developed a rapidly progressive spastic paraparesis following a febrile illness. A 7-year-old Japanese girl had developmental regression, spastic quadriplegia, and abnormal periventricular white matter signal on brain magnetic resonance imaging performed at 8 months of age. She had febrile convulsions at the age of 18 months and later developed epilepsy. In summary, we have identified four novel IBA57 mutations in two unrelated families. Consequently, we describe a patient with infantile-onset optic atrophy and asymptomatic white matter involvement, thus broadening the phenotypic spectrum of biallelic IBA57 mutations.
Similar content being viewed by others
Introduction
IBA57 (MIM# 615316) encodes a protein involved in the synthesis of mitochondrial [4Fe-4S] cluster proteins [1]. The [4Fe-4S] cluster is a cofactor of multiple mitochondrial enzymes: respiratory chain complexes (RCC) l and ll, mitochondrial aconitase, and lipoic acid synthetase (LIAS). As a cofactor, LIAS transfers lipoic acid to multiple enzymes for mitochondrial energy metabolism [2]. Therefore, loss-of-function mutations in IBA57 or other genes related to [4Fe-4S] cluster synthesis can lead to multiple defects in mitochondrial enzymes, termed multiple mitochondrial dysfunction syndrome (MMDS [MIM# 605711, 614299, 615330, 616370, 617613]) [3,4,5,6,7].
IBA57-mutated MMDS is associated with a broad spectrum of neurological impairments. The most severe cases demonstrate psychomotor delay or regression, hypotonia, respiratory failure, seizures, and cavitating leukoencephalopathy, leading to an early death [4, 8]. Milder cases show childhood- to adolescent-onset spastic paraplegia, optic atrophy, distal neuropathy (SPOAN [MIM# 609541]) and cavitating leukoencephalopathy [9].
Since only 21 IBA57 mutations have been reported to date [4, 8,9,10,11,12], the phenotypic spectrum of biallelic IBA57 mutations has not been fully elucidated. Here, we report two families harbouring four novel IBA57 mutations and describe new clinical and radiological features.
Materials and methods
Study participants
Three individuals from two families with leukodystrophy were included in the study: Family 1 had two affected brothers and was from Israel, while Family 2 had one affected girl and was from Japan. Clinical information was obtained from their medical records. Informed consent was obtained from the parents in both families. The study was approved by the institutional review board of Yokohama City University School of Medicine (Yokohama, Japan).
Whole-exome sequencing
Whole-exome sequencing (WES) was performed using DNA from the proband of Families 1 and 2 as previously described [13]. In brief, genomic DNA was captured with a SureSelect Human All Exon V5 Kit (Santa Clara, CA, United States, Agilent Technologies) and sequenced on an Illumina HiSeq 2500 (San Diego, CA, United States, Illumina) with 101-bp paired-end reads. Reads were aligned to the human reference genome (GRCh37.1/hg19) using Novoalign v3.02.13 (http://www.novocraft.com/). PCR (polymerase chain reaction) duplicates were removed using Picard (http://broadinstitute.github.io/picard/). Local realignments around indels and base quality score recalibration were performed with the Genome Analysis Toolkit (GATK) 3.7-0 (http://www.broadinstitute.org/gatk/). Variants were called by GATK UnifiedGenotyper and filtered according to GATK Best Practices. Common variants registered in dbSNP135 (minor allele frequency >0.01) that were not flagged as having clinical associations were excluded. Included variants were annotated using ANNOVAR (2016nov) (http://www.openbioinformatics.org/annovar/). For each proband from Families 1 and 2, mean depth of coverage against RefSeq coding sequence by WES was 84× and 69×, with 92% and 89% of coding sequence covered by 20 reads or more, respectively.
Sanger sequencing
IBA57 variants were analyzed by Sanger sequencing using standard methods. Primer sequences were: TGCTAGGGCTGCTGACCAATGAAC (forward) and ACCCTTGAGCTTCCACCCCATC (reverse) for c.335T>C; CAGAGCTCTTTGCGTTGG (forward) and ATCGGTGCTGGTGATAATCC (reverse) for c.386A>T and c.588dup; and GGAGCTGGACGATGTTCAAT (forward) and AAGAACCGGACAGGGAAGAG (reverse) for c.731A>C. PCR products were purified by Exonuclease l and sequenced with BigDye Terminator v3.1 Cycle Sequencing kit (Foster City, CA, United States, Applied Biosystems) on a 3500 DNA Sequencing Analyzer (Carlsbad, CA, United States, Life Technologies).
In silico analysis
Pathogenicity of missense variants was predicted using the following software: SIFT (http://sift.jcvi.org), PolyPhen-2 (http://genetics.bwh.harvard.edu/pph2/), and MutationTaster (http://www.mutationtaster.org/). Flanking amino acids at missense variants were aligned between species by ClustalW (http://www.genome.jp/tools-bin/clustalw). References for amino acid sequences were: Homo sapiens: NP_001010867.1; Macaca mulatta: XP_001083460.1; Mus musculus: NP_776146.1; Gallus gallus: NP_001026129.2; Xenopus tropicalis: XP_002939357.1; and Danio rerio: NP_001070103.2.
Results
Clinical studies
Family 1 of Sepharadi Jewish origin has two affected siblings whose early life has been previously described by Vinkler et al. [14] (Fig. 1a). Briefly, Patient 1 (II-1) was a 29-year-old man at the time of this study, and showed infantile-onset optic atrophy associated with completely asymptomatic cerebral white matter signal abnormalities by magnetic resonance imaging (MRI). He developed normally. Reduced vision was first noted at the age of 20 months. Within the following 2 weeks, he completely lost his vision. Ophthalmologic examination revealed no abnormalities in the anterior segment of his eyes. Funduscopic examination revealed pale discs with a normal retina and no vascular changes. Electroretinogram showed normal findings. Visual evoked potentials (VEP) indicated a prolonged latency with low amplitudes. Brain MRI performed at 2 years of age showed high signal intensity in bilateral periventricular white matter on T2-weighted images. Follow-up brain MRI at the age of 14 years did not demonstrate any progression. Currently, he has no neurological features and works as a musician and teacher.
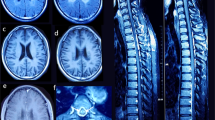
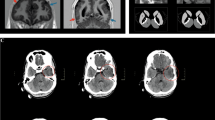
Clinical findings in Families 1 and 2. a Pedigrees of Families 1 and 2. IBA57 variants are shown below each individual. Arrows, probands; squares, males; circles, females; closed symbols, affected; and open symbols, unaffected; wt, wild-type sequence of IBA57. b–k Brain fluid-attenuated inversion recovery (FLAIR) (b, d, e, f, h, i, k, l, and m) and T1-weighted (c, g, and j) images in Patient 2 (b–d) and Patient 3 (e–m). Ages when the images were obtained are indicated at the right bottom of each image: y indicates years, m indicates months. White matter volume loss was observed (b and d). The middle blade of the corpus callosum was affected (arrow) (c, g, and j). Rarefaction, cystic degeneration, and rapid loss of white matter volume were detected (e, f, h, i, k, and l). The anterior portion of the pons was affected (m)
Patient 2 is a 19-year-old male and the younger brother (II-2) of Patient 1 (Fig. 1a). He showed a similar clinical course as Patient 1 in early childhood, with visual loss and asymptomatic white matter changes on brain MRI at the age of 18 months. Ophthalmologic examination revealed marked visual loss, a sluggish pupillary response to light, and pendular nystagmus. Funduscopic examination revealed diffuse pallor in both discs with a normal retina. VEP showed a prolonged latency with very low amplitudes. Brain MRI showed thin optic nerves and signal abnormalities in periventricular white matter and middle blade of the corpus callosum (Fig. 1b, c, and S1A). Mitochondrial dysfunction was suspected due to association of optic atrophy with white matter involvement. Consequently, a skeletal muscle biopsy was performed at 4 years of age revealing no ragged-red fibres and normal staining of oxidative enzymes, namely, nicotinamide adenine dinucleotide-tetrazolium reductase, succinate dehydrogenase, and cytochrome c oxidase staining. Biochemical studies demonstrated normal RCC activity. At the age of 5 years, he developed seizures, which were controlled with anti-epileptic drugs. At the age of 7 years, after a Shigella infection, he rapidly developed spasticity and weakness of his legs leading to a spastic gait. Brain MRI was repeated, revealing that the white matter abnormalities observed at 18 months of age had not progressed. Brain MRI at 15 years of age showed partial white matter rarefaction, a thin corpus callosum, and white matter volume loss (Fig. 1d). Currently he has borderline intelligence, learning disabilities, and anxiety. He attends a special school for children with cerebral palsy. His neurological examination has been stable since his acute deterioration.
Patient 3 (II-1 in Family 2) is a 7-year-old Japanese girl with developmental regression, spastic quadriplegia, and seizures (Fig. 1a). Her developmental milestones were initially normal. However, developmental regression was noticed at the age of 7 months: she lost head control and stopped reaching out for toys and rolling over. She frequently became drowsy with no clear cause. Neurological examination revealed spasticity with increased deep tendon reflexes in all four limbs. Lactate and pyruvate levels were normal in blood and cerebrospinal fluid. Brain MRI at the age of 8 months showed cerebral white matter involvement, periventricular rarefaction, and corpus callosum involvement at the middle blade (Fig. 1e, f, g, and S1B). She developed febrile seizures at the age of 18 months. Brain MRI performed at 2 years of age detected significant loss of white matter volume and cystic degeneration of periventricular white matter, showing progression of white matter changes (Fig. 1h, i, and j). Abnormal signal intensity in the posterior limb of the internal capsule (Fig. 1h) and an atrophic cerebellum were observed. The thalamus had also atrophied and demonstrated abnormal signal intensity (Fig. 1h). From 4 years of age, she developed complex partial seizures that are controlled by carbamazepine and levetiracetam. To date, she is bedridden with no head control or voluntary movements. She can only understand a few words. Brain MRI at 7 years of age showed further white matter loss, suggesting that the white matter lesions are still gradually progressing, albeit not as rapidly as before (Fig. 1k and l). The anterior portion of the pons showed abnormal signal intensity (Fig. 1m). There is no involvement of U fibres (Fig. 1k and l).
Genetic analysis
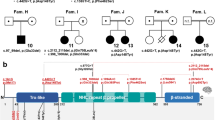
WES was performed on two patients, as previously described: Patient 1 in Family 1 and Patient 3 in Family 2. We identified two heterozygous variants in IBA57 (NM_001010867.2), namely c.335T>C (p.Leu112Ser) and c.588dup (p.Arg197Alafs*7) in Patient 1 and c.386A>T (p.Asp129Val) and c.731A>C (p.Glu244Ala) in Patient 3. In both families, Sanger sequencing of the patients and parents confirmed that the variants were compound heterozygous: c.335T>C (maternally derived) and c.588dup (paternally derived) in Patient 1; and c.386A>T (maternal) and c.731A>C (paternal) in Patient 3 (Fig. 2a and b). In Family 1, the compound heterozygous variants identified in Patient 1 were shared with his affected brother (Patient 2). All the identified variants are novel (Fig. 2c) and not in publicly available human variation databases (Table 1). The missense variants were predicted as disease-causing by two or more in silico web-based tools (Table 1). The substituted amino acids are highly conserved among species (from fish to human) (Fig. 2d and Table 1). To confirm the pathogenicity of the variants, we would like to test respiratory chain enzyme deficiency or other enzymes containing [4Fe-4S] in the biopsied muscles of Patient 2. However, those from Patient 2 were used up and not left.
Genetic findings in Families 1 and 2. Electropherograms of IBA57 variants in Families 1 (a) and 2 (b). Wild-type and mutant nucleotide and amino acid sequences are depicted below. Substituted or duplicated nucleotides are coloured red. c Schematic of IBA57 protein (NP_001010867.1). Mutated amino acids identified in this study and those reported previously are indicated: missense mutations are shown above and truncating mutations below the protein structure. Variants identified in this study are coloured red. The GCV domain region is shown as a black-filled box. The corresponding coding exons are shown below the protein structure: EX, exon; N, N-terminus; C, C-terminus; and GCV, glycine cleavage T-protein C-terminal barrel domain. d Conservation of amino acids. Variants identified in this study and those reported previously are depicted above and below the alignment, respectively
Discussion
Here, we describe four novel IBA57 variants in two families. The frameshift variant in Family 1 is located at the penultimate exon, >50-bp from the last nucleotide of this exon (Fig. 2c). Consequently, transcripts from this allele are most likely to be degraded via nonsense-mediated mRNA decay. The other three missense variants are located at possible mutation hotspots of previously reported missense mutations (Fig. 2c). Thus, the four variants are most likely to be pathogenic mutations.
Family 1 showed a milder phenotype than previously reported cases of IBA57 mutations [4, 8,9,10, 12] and intrafamilial variability. Patient 1 exhibited isolated infantile-onset optic atrophy with asymptomatic brain white matter involvement. Abnormal vision has been reported in an IBA57-related family with SPOAN [9]. This family demonstrated childhood-onset spastic paraparesis and distal neuropathy, followed by adolescent-onset optic atrophy. Blindness has also been reported in a few IBA57-related cases with severe developmental delay, abnormal VEP, and early death [8]. Compared with these families, Patient 1 has developed normally and been healthy except for optic atrophy. Interestingly, Patient 2, the younger brother of Patient 1, shares the same mutations, but has a different clinical course. In addition to infantile-onset optic atrophy, he rapidly developed spastic paraparesis. However, the disease was most likely exacerbated by a febrile illness at the age of 7 years because the disease has not progressed since the initial event.
Why was the phenotype of Patient 1 mild? Surprisingly, Patient 2 had normal RCC enzyme activity and was suspected clinically of having MMDS. All previously reported cases show low activity in both or either RCC l and ll [4, 8,9,10, 12], and residual RCC enzyme activity defines disease severity [4, 9, 12]. The relatively milder phenotype of Patient 1, compared with previously reported cases [4, 8–10, 12], can possibly be explained by retained RCC enzyme activity levels.
Concurrence of optic atrophy and white matter involvement, as seen in Patient 1, has also been described in other mitochondrial diseases such as other types of MMDS, Leigh syndrome (MIM# 256000), as well as Kearns–Sayre syndrome (MIM# 530000) [3, 15,16,17]. These diseases show a wide variety of symptoms, with a distinct clinical picture from Patient 1. The only mitochondrial disease that resembles the phenotype of Patient 1 is Leber’s hereditary optic neuropathy (LHON [MIM# 535000]). LHON is caused by mitochondrial DNA mutations and characterized by optic atrophy with onset from early childhood to late adulthood. LHON can concur with white matter involvement. Clinical symptoms and MRI of such cases usually resemble multiple sclerosis and are different from those of Patient 1 [18, 19]. A similar clinical presentation was described by Lev et al. [20], in a rare case of LHON with periventricular white matter involvement and no other symptoms. However, in this case, decreased vision was not as severe at 14 years of age. Thus, isolated infantile-onset optic atrophy with asymptomatic white matter involvement in IBA57-related disease is a distinct phenotype.
Patient 3 had a severe phenotype compared with Patients 1 and 2. Patient 3 showed psychomotor regression and spastic quadriplegia within 1 year after birth, while epilepsy developed after a febrile illness. Brain MRI showed progressive, significant loss of white matter volume. Although white matter abnormalities by MRI might have been augmented by febrile illness, the overall phenotype is consistent with the most severe cases previously reported [8, 12].
Patient 3 was initially diagnosed with vanishing white matter disease (VWM [MIM# 603896]) because of shared features: chronic progressive neurological symptoms, rapid deterioration after febrile illness, and cystic degeneration of periventricular white matter [21]. However, Patient 3 has profound intellectual disability, which is not described in VWM disease. MRI images demonstrate white matter rarefaction and cystic degeneration, which are reminiscent of VWM disease (Fig. 1f and i) [21]. However, several findings are atypical and suggestive of MMDS. First, the rim of patchy multifocal white matter abnormalities is well delineated and remain patchy over time (Fig. 1e, h, and k). In contrast, they are typically more diffuse in VWM [21]. Second, severe white matter atrophy occurred from an early disease stage (Fig. 1f, i, and l). Such atrophy can be noticed in VWM with late, but not infantile, onset [22]. Third, thalamic atrophy occurred from an early disease stage (Fig. 1h and k). This finding has previously been observed in an IBA57-mutated case [8] and is not typically seen in VWM [23]. Fourth, the middle blade of the corpus callosum is preferentially affected (Fig. 1g and j). This feature may be suggestive of MMDS [3, 10,11,12, 15, 24], while the inner blade is preferentially affected in VWM. MMDS can present with MRI findings similar to VWM, making it difficult for clinicians to accurately diagnose. Our case demonstrates that the well delineated rim of patchy white matter lesions and preferential involvement of the middle blade of the corpus callosum may be useful features to distinguish the two entities.
Patients 2 and 3 showed rapid progression following a febrile illness. In patients with IBA57 mutations, it has been repeatedly reported that neurological symptoms rapidly progress following stress such as vaccination or head trauma [8, 9]. Similarly, stress-induced rapid neurological deterioration is reported in cases of MMDS caused by mutations in other genes essential for [4Fe-4S] cluster synthesis [25,26,27]. Our cases and these reports suggest that exogenous stress to the brain can have a significant deleterious effect on white matter.
In conclusion, we describe two families with four novel IBA57 mutations. Consequently, we have broadened the clinical spectrum of IBA57 mutations by presenting a previously undescribed phenotype of infantile-onset isolated optic atrophy associated with asymptomatic white matter involvement. Furthermore, we illustrate that brain MRI images of MMDS can be similar to VWM, but have unique features that can be used for differentiation.
References
Sheftel AD, Wilbrecht C, Stehling O, Niggemeyer B, Elsasser HP, Muhlenhoff U, et al. The human mitochondrial ISCA1, ISCA2, and IBA57 proteins are required for [4Fe-4S] protein maturation. Mol Biol Cell. 2012;23:1157–66.
Tort F, Ferrer-Cortes X, Ribes A. Differential diagnosis of lipoic acid synthesis defects. J Inherit Metab Dis. 2016;39:781–93.
Al-Hassnan ZN, Al-Dosary M, Alfadhel M, Faqeih EA, Alsagob M, Kenana R, et al. ISCA2 mutation causes infantile neurodegenerative mitochondrial disorder. J Med Genet. 2015;52:186–94.
Ajit Bolar N, Vanlander AV, Wilbrecht C, Van der Aa N, Smet J, De Paepe B, et al. Mutation of the iron-sulfur cluster assembly gene IBA57 causes severe myopathy and encephalopathy. Hum Mol Genet. 2013;22:2590–602.
Navarro-Sastre A, Tort F, Stehling O, Uzarska MA, Arranz JA, Del Toro M, et al. A fatal mitochondrial disease is associated with defective NFU1 function in the maturation of a subset of mitochondrial Fe-S proteins. Am J Hum Genet. 2011;89:656–67.
Cameron JM, Janer A, Levandovskiy V, Mackay N, Rouault TA, Tong WH, et al. Mutations in iron-sulfur cluster scaffold genes NFU1 and BOLA3 cause a fatal deficiency of multiple respiratory chain and 2-oxoacid dehydrogenase enzymes. Am J Hum Genet. 2011;89:486–95.
Shukla A, Hebbar M, Srivastava A, Kadavigere R, Upadhyai P, Kanthi A, et al. Homozygous p.(Glu87Lys) variant in ISCA1 is associated with a multiple mitochondrial dysfunctions syndrome. J Hum Genet. 2017;62:723–7.
Torraco A, Ardissone A, Invernizzi F, Rizza T, Fiermonte G, Niceta M, et al. Novel mutations in IBA57 are associated with leukodystrophy and variable clinical phenotypes. J Neurol. 2017;264:102–11.
Lossos A, Stumpfig C, Stevanin G, Gaussen M, Zimmerman BE, Mundwiller E, et al. Fe/S protein assembly gene IBA57 mutation causes hereditary spastic paraplegia. Neurology. 2015;84:659–67.
Liu M, Zhang J, Zhang Z, Zhou L, Jiang Y, Wang J, et al. Phenotypic spectrum of mutations inIBA57, a candidate gene for cavitating leukoencephalopathy. Clin Genet. 2017;93:235–41.
Ishiyama A, Sakai C, Matsushima Y, Noguchi S, Mitsuhashi S, Endo Y, et al. IBA57 mutations abrogate iron-sulfur cluster assembly leading to cavitating leukoencephalopathy. Neurol Genet. 2017;3:e184.
Debray FG, Stumpfig C, Vanlander AV, Dideberg V, Josse C, Caberg JH, et al. Mutation of the iron-sulfur cluster assembly gene IBA57 causes fatal infantile leukodystrophy. J Inherit Metab Dis. 2015;38:1147–53.
Fujita A, Isidor B, Piloquet H, Corre P, Okamoto N, Nakashima M, et al. De novo MEIS2 mutation causes syndromic developmental delay with persistent gastro-esophageal reflux. J Hum Genet. 2016;61:835–8.
Vinkler C, Lev D, Kalish H, Watemberg N, Yanoov-Sharav M, Leshinsky-Silver E, et al. Familial optic atrophy with white matter changes. Am J Med Genet A. 2003;121a:263–5.
Ahting U, Mayr JA, Vanlander AV, Hardy SA, Santra S, Makowski C, et al. Clinical, biochemical, and genetic spectrum of seven patients with NFU1 deficiency. Front Genet. 2015;6:123.
Baker PR 2nd, Friederich MW, Swanson MA, Shaikh T, Bhattacharya K, et al. Variant non ketotic hyperglycinemia is caused by mutations in LIAS, BOLA3 and the novel gene GLRX5. Brain. 2014;137 Pt 2:366–79.
Lerman-Sagie T, Leshinsky-Silver E, Watemberg N, Luckman Y, Lev D. White matter involvement in mitochondrial diseases. Mol Genet Metab. 2005;84:127–36.
Bargiela D, Chinnery PF. Mitochondria in neuroinflammation—multiple sclerosis (MS), leber hereditary optic neuropathy (LHON) and LHON-MS. Neurosci Lett. 2017. [Epub ahead of print]
Matthews L, Enzinger C, Fazekas F, Rovira A, Ciccarelli O, Dotti MT, et al. MRI in Leber’s hereditary optic neuropathy: the relationship to multiple sclerosis. J Neurol Neurosurg Psychiatry. 2015;86:537–42.
Lev D, Yanoov-Sharav M, Watemberg N, Leshinsky-Silver E, Lerman-Sagie T. White matter abnormalities in Leber’s hereditary optic neuropathy due to the 3460 mitochondrial DNA mutation. European journal of paediatric neurology. Eur J Paediatr Neurol. 2002;6:121–3.
van der Knaap MS, Pronk JC, Scheper GC. Vanishing white matter disease. Lancet Neurol. 2006;5:413–23.
Fogli A, Rodriguez D, Eymard-Pierre E, Bouhour F, Labauge P, Meaney BF, et al. Ovarian failure related to eukaryotic initiation factor 2B mutations. Am J Hum Genet. 2003;72:1544–50.
van der Knaap MS, Barth PG, Gabreels FJ, Franzoni E, Begeer JH, Stroink H, et al. A new leukoencephalopathy with vanishing white matter. Neurology. 1997;48:845–55.
Nizon M, Boutron A, Boddaert N, Slama A, Delpech H, Sardet C, et al. Leukoencephalopathy with cysts and hyperglycinemia may result from NFU1 deficiency. Mitochondrion. 2014;15:59–64.
Ferrer-Cortes X, Narbona J, Bujan N, Matalonga L, Del Toro M, Arranz JA, et al. A leaky splicing mutation in NFU1 is associated with a particular biochemical phenotype. Consequences for the diagnosis. Mitochondrion. 2016;26:72–80.
Tonduti D, Dorboz I, Imbard A, Slama A, Boutron A, Pichard S, et al. New spastic paraplegia phenotype associated to mutation of NFU1. Orphanet J Rare Dis. 2015;10:13.
Invernizzi F, Ardissone A, Lamantea E, Garavaglia B, Zeviani M, Farina L, et al. Cavitating leukoencephalopathy with multiple mitochondrial dysfunction syndrome and NFU1 mutations. Front Genet. 2014;5:412.
Acknowledgements
We thank all the participants for their cooperation in this research. We also thank Ms. K. Takabe, Mr. T. Miyama, Ms. N. Watanabe, Ms. M. Sato, Mr. S. Nakamura, and Ms. S. Sugimoto at the Department of Human Genetics, Yokohama City University Graduate School of Medicine, for their technical assistance. This work was supported by grants from Research on Measures for Intractable Diseases (NMa), Comprehensive Research on Disability Health and Welfare (NMa), the Strategic Research Program for Brain Science (NMa), the Initiative on Rare and Undiagnosed Diseases in Pediatrics (NMa), the Initiative on Rare and Undiagnosed Diseases for Adults (NMa) from the Japanese Agency for Medical Research and Development, a Grant-in-Aid for Scientific Research on Innovative Areas (Transcription Cycle) from the Ministry of Education, Culture, Sports, Science and Technology of Japan, Grants-in-Aid for Scientific Research [A (NMa), B (NMi, HS), and C (SMiy)], the fund for Creation of Innovation Centers for Advanced Interdisciplinary Research Areas Program in the Project for Developing Innovation Systems (NMa) from the Japanese Science and Technology Agency, grants from the Ministry of Health, Labour and Welfare (NMa), the Takeda Science Foundation (NMi, HS, and NMa), and the Ichiro Kanehara Foundation for the Promotion of Medical Science & Medical Care (SMiy). We thank Rachel James, Ph.D., from Edanz Group (www.edanzediting.com/ac) for editing a draft of this manuscript.
Author contributions
KH: literature review, collection of data, and drafting the manuscript; AZS, LB, KY, AF, EI, KI, S.Mit, MN, TM, AT, NMi, HS: data collection; SMiy, DL, TL-S, MSvdK, and NMa: supervision of all aspects, including study design, data interpretation, and manuscript preparation.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Electronic supplementary material
Rights and permissions
About this article
Cite this article
Hamanaka, K., Miyatake, S., Zerem, A. et al. Expanding the phenotype of IBA57 mutations: related leukodystrophy can remain asymptomatic. J Hum Genet 63, 1223–1229 (2018). https://doi.org/10.1038/s10038-018-0516-x
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s10038-018-0516-x
This article is cited by
-
Pharmacological Aspects of the Use of Lipoic Acid (Review)
Pharmaceutical Chemistry Journal (2022)
-
Novel IBA57 mutations in two chinese patients and literature review of multiple mitochondrial dysfunction syndrome
Metabolic Brain Disease (2022)
-
Large-scale discovery of novel neurodevelopmental disorder-related genes through a unified analysis of single-nucleotide and copy number variants
Genome Medicine (2022)
-
Structural properties of [2Fe-2S] ISCA2-IBA57: a complex of the mitochondrial iron-sulfur cluster assembly machinery
Scientific Reports (2019)