Abstract
Background
Cystein-rich protein 61 (Cyr61/CCN1) is a member of the CCN family of matricellular proteins that has an important role in tissue development and remodeling. However, the role of CCN1 in the pathogenesis of bronchopulmonary dysplasia (BPD) is unknown. Accordingly, we have investigated the effects of CCN1 on a hyperoxia-induced lung injury model in neonatal rats.
Methods
In experiment 1, newborn rats were randomized to room air (RA) or 85% oxygen (O2) for 7 or 14 days, and we assessed the expression of CCN1. In experiment 2, rat pups were exposed to RA or O2 and received placebo or recombinant CCN1 by daily intraperitoneal injection for 10 days. The effects of CCN1 on hyperoxia-induced lung inflammation, alveolar and vascular development, vascular remodeling, and right ventricular hypertrophy (RVH) were observed.
Results
In experiment 1, hyperoxia downregulated CCN1 expression. In experiment 2, treatment with recombinant CCN1 significantly decreased macrophage and neutrophil infiltration, reduced inflammasome activation, increased alveolar and vascular development, and reduced vascular remodeling and RVH in the hyperoxic animals.
Conclusion
These results demonstrate that hyperoxia-induced lung injury is associated with downregulated basal CCN1 expression, and treatment with CCN1 can largely reverse hyperoxic injury.
Similar content being viewed by others
Main
Bronchopulmonary dysplasia (BPD) continues to be one of the most common long-term pulmonary complication associated with preterm birth (1, 2). Lung injury from antenatal/postnatal infection, oxygen toxicity, and mechanical ventilation leads to lung inflammation. The role of inflammation in the pathogenesis of BPD has been firmly established (3). Inflammation results in accumulation of inflammatory cells, activation of inflammasomes, increase in pro-inflammatory cytokines, and production of reactive oxygen species, which likely result in the pathological changes seen in BPD, characterized by alveolar simplification, reduced vascular growth, and variable interstitial fibrosis (4, 5). Severe BPD is often complicated by pulmonary hypertension (PH) that significantly increases mortality.
The CCN (cyr61, ctgf, and nov) proteins belong to an important family of matricellular regulatory factors involved in internal and external cell signaling and have a crucial role in regulation of tissue regeneration and inflammation (6). The CCN family of proteins consists of six members and, despite similar structures, CCN proteins have a diverse variety of biological functions, which are highly dependent on the cellular context (6). For example, CCN1 (Cyr61) and CCN2, also known as connective tissue growth factor (CTGF), are structurally related but functionally distinct and are expressed in many organs and tissues only during specific developmental or pathological events (7).
CCN2 has pro-inflammatory, pro-fibrotic, and anti-angiogenic activities, and its crucial role as an inducer of the pathogenesis of various forms of adult pulmonary fibrosis and vascular diseases is firmly established (8, 9). Recent studies on the role of CCN2 in BPD showed that mechanical ventilation and exposure to hyperoxia induced CCN2 overexpression in lungs of neonatal rat (10, 11), and conditional overexpression of CCN2 in airway and alveolar type II epithelial cells severely disrupted alveolarization and vascular development (12, 13). Furthermore, CCN2 overexpression has been demonstrated in the postmortem lungs of preterm BPD infants as well as in the lungs of hyperoxia-exposed neonatal rats (14). Moreover, treatment with FG-3149, a monoclonal neutralizing CCN2 antibody, prevented hyperoxia-induced alveolar damage in neonatal rats (14).
On the other hand, most studies show that CCN1 has anti-inflammatory, antifibrotic, and pro-angiogenic activities during tissue development and injury repair (15, 16, 17, 18), although some studies conversely suggest a pro-inflammatory/pro-fibrotic activity for CCN1 (19, 20). CCN1 largely exerts its antifibrotic effect by promoting cellular senescence and apoptosis and by attenuating TGF-β signaling (15, 17, 21). In addition, it has been recently demonstrated that CCN1 has an important downregulatory role in the early inflammatory phase of wound-healing by stimulating the clearance of neutrophils via the process of efferocytosis (16). In addition, CCN1 promotes angiogenesis by increasing vascular endothelial growth factor (VEGF) receptor 2 (VEGFR2) expression and by enhancing endothelial cell adhesion, migration, and survival (18, 22). However, the role of CCN1 in BPD pathogenesis is unknown.
We hypothesized that CCN1 should have a protective role in BPD development and progression by attenuating inflammation, promoting alveolarization and angiogenesis, and decreasing PH. We thus evaluated the therapeutic potential of recombinant CCN1 protein in the prevention of hyperoxia-induced lung injury in neonatal rats—an experimental model of BPD. Given the increasingly recognized importance of the inflammasome in innate immune responses, organ injury, and BPD pathogenesis (23, 24), we also evaluated the effects of CCN1 therapy on inflammasome expression and activation. Nucleotide-binding oligomerization domain-like receptor containing pyrin domain 3 (NLRP3), NLRP1, apoptosis-associated speck-like protein containing a caspase recruitment domain (ASC), active caspase-1 and active interleukin (IL)-1β are key components of the inflammasome cascade. Our results demonstrate that hyperoxia downregulated CCN1 expression in neonatal rat lungs and treatment with recombinant CCN1 protein suppressed hyperoxia-induced activation of inflammasome, attenuated inflammation, improved alveolar and vascular development, and decreased vascular remodeling and PH in neonatal rats. These findings provide new insights into understanding the role of CCN1 in the pathogenesis of BPD, and additionally suggest that CCN1 protein may have therapeutic potential in BPD prevention or treatment in neonates.
Methods
Animal Model and Experimental Protocol
The study protocol was approved by the University of Miami Institutional Animal Care and Use Committee. Experiment 1: to evaluate the temporal and spatial effects of hyperoxia on CCN1 expression, newborn Sprague–Dawley rats were randomized on postnatal day 1 to receive room air (RA) or 85% O2 for 7 or 14 days, and animals were killed after 7 or 14 days of hyperoxia. Experiment 2: to study the efficacy of recombinant CCN1 in the prevention of hyperoxia-induced lung injury, newborn Sprague–Dawley rats were randomized on postnatal day 1 into three groups: RA+placebo (PL, normal saline), O2+PL, RA+CCN1, and O2+CCN1. Recombinant CCN1 (1 mg/kg) or PL (equal volume) was administered by intraperitoneal injection on days 1, 4, 7, and 9 during continuous RA or exposure to hyperoxia. Murine recombinant CCN1 was produced using a baculovirus expression system and chromatographically purified (16), and the dose was used as referenced previously (17). Animals were killed on day 11.
Assessments of CCN1 Protein Expression
Expression of CCN1 protein was assessed by western blot analysis as previously described (13, 14).
Assessments of Lung Inflammation
Immunostaining with Mac3, a macrophage marker, was performed, and the numbers of Mac3-positive cells in the alveolar airspaces were counted in 10 random images on each lung section for determining macrophage infiltration. To assess neutrophil infiltration, immunostaining with an anti-neutrophil elastase antibody was performed. Infiltrated neutrophils were counted from 10 random images on each lung section. Expression of inflammasome component proteins, NLRP-1, ASC, active caspase-1, and active IL-1β was determined by western blot analysis.
Lung Histology and Morphometry
Lungs were infused with 4% paraformaldehyde via a tracheal catheter at 20 cm H2O pressure for 5 min, fixed overnight, and paraffin-embedded. Hematoxylin and eosin-stained tissue sections were used to measure radial alveolar count (RAC) as previously described (13, 14).
Pulmonary Vascular Morphometry
Lung tissue sections were stained for von Willebrand factor (vWF), an endothelial marker to assess vascular density. The average number of vWF-stained vessels (<50 μm in diameter) was counted from five random images on each lung section (13, 14).
Assessment of Pulmonary Vascular Remodeling
Lung tissue sections were double immunofluorescence-stained for α-smooth muscle actin and vWF to assess the extent of muscularization. The percentage of peripheral vessels (<50 μm in diameter) that were stained with α-smooth muscle actin (>50% circumference) was determined from 10 random images on each lung section (13, 14). To assess vascular smooth muscle cell proliferation, double immunofluorescence with an anti-Ki67 antibody (nuclear proliferating antigen) and an α-smooth muscle actin antibody was performed. The percentage of vessels with at least one positive Ki67 nuclei was determined.
Assessment of Right Ventricular Hypertrophy
Right ventricular hypertrophy (RVH, Fulton’s index) was utilized as an index for PH. Hearts were dissected and the weight ratio of RV to left ventricle plus septum (13, 14) was determined.
Data Management and Statistical Analysis
Data were expressed as means±SD, and comparisons were performed by two-way ANOVA followed by post hoc analysis (Student–Newman Keuls). A P value of less than 0.05 was considered statistically significant.
Detailed descriptions of the Materials and methods are provided in Supplementary methodsonline.
Results
Hyperoxia Downregulates CCN1 Expression in Neonatal Lungs
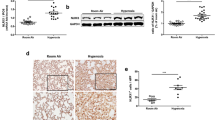
We evaluated the expression of CCN1 in lungs using western blot analysis on days 7 and 14 after continuous exposure to hyperoxia. As demonstrated in Figure 1, quantitative densitometry analysis demonstrated that hyperoxia exposure resulted in significant suppression of CCN1 expression on both day 7 (1.73±0.33 vs. 0.4±0.45, P<0.001, RA vs. O2) and day 14 (2.25±0.64 vs. 0.79±0.28, P<0.01, RA vs. O2; Figure 1a). These data suggest that CCN1 may have a role in hyperoxia-induced neonatal lung injury.
Hyperoxia downregulates CCN1 expression. Newborn rats were exposed to room air (RA, open bar) or to hyperoxia (85% O2, solid bar) for 7 (a) or 14 (b), days and CCN1 expression in lung extracts was quantitated by western blot densitometry analysis after normalization to housekeeping gene β-actin. Representative western blot photo images are shown. (c) Hyperoxia exposure downregulated CCN1 expression at both 7 days (*P<0.001) and 14 days (**P<0.01) as compared with RA. Open bar: RA. Solid bar: hyperoxia.
CCN1 Therapy Suppresses Hyperoxia-Induced Lung Inflammation and Inflammasome Activation
We assessed the effects of CCN1 therapy on lung inflammation by quantifying macrophage and neutrophil infiltration. The macrophage counts were significantly elevated in the O2+PL group in comparison with the RA+PL group (8.0±4.63 vs. 1.6±0.47, P<0.001, O2+PL vs. RA+PL; Figure 2a). Similarly, neutrophil counts were also significantly elevated with hyperoxia exposure compared with RA exposure (7.4±4.11 vs. 1.0±0.35, P<0.001, O2+PL vs. RA+PL; Figure 2c). However, administration of CCN1 resulted in significant decreases in both the macrophage and neutrophil counts induced by hyperoxia exposure (macrophage: 3.1±0.64 vs. 8.0±4.63, P<0.01, O2+CCN1 vs. O2+PL; Figure 2a; neutrophil: 2.6±1.33 vs. 7.4±4.11, P<0.001, O2+CCN1 vs. O2+PL; Figure 2c).
Treatment with CCN1 decreases hyperoxia-induced inflammation and inflammasome activation. Immunostaining for Mac3 was performed on lung tissue sections (a) and the average numbers of macrophages in alveolar airspaces were counted from 10 random images, taken under the HPV (× 200) on each lung section (b). The O2+PL lungs showed increased macrophage counts compared with RA+PL lungs, which were decreased by administration of CCN1 (*P<0.001: RA+PL vs. O2+PL; †P<0.01: O2+PL vs. O2+CCN1). n=6/group. Bar=100 μm. Immunostaining with an anti-neutrophil elastase antibody was performed on lung tissue sections (c) and the average numbers of neutrophils in alveolar airspaces were counted from 10 random images, taken under the HPV on each lung section (d). Exposure to hyperoxia in the presence of the PL increased neutrophil infiltration into the alveolar airspaces, whereas treatment with CCN1 significantly decreased neutrophil infiltration during hyperoxia (*P<0.001: RA+PL vs. O2+PL; †P<0.001: O2+PL vs. O2+CCN1; **P<0.05: RA+CCN1 vs. O2+CCN1). n=6/group. Bar=100 μm. (e) Representative western blot images for NLRP1, ASC, active caspase-1 (aCasp-1), active IL-1β (aIL-1β), and β-actin. The relative expression levels of NLRP1 (f), ASC (g), active caspase 1 (h), and active IL-1β (i) were analyzed using densitometry and were normalized to β-actin. All three inflammasome proteins and active IL-1β were increased by hyperoxia in the PL group as compared with the RA group (*P<0.001 (NLRP-1); *P<0.001 (ASC); *P<0.001 (Caspase-1); *P<0.001 (IL-1β)). However, treatment with CCN1 during hyperoxia decreased the expression of all three inflammasome proteins and active IL-1β as compared with the hyperoxia plus PL group (†P<0.001 (NLRP-1); †P<0.001 (ASC); †P<0.001 (Caspase-1); †P<0.001 (IL-β)). **P<0.05: O2+CCN1 vs. RA+CCN1. n=6/group. Open bar, RA; solid bar, hyperoxia. HPV, high-power view; IL, interleukin; PL, placebo; RA, room air.
We further evaluated the effects of recombinant CCN1 on lung inflammation by measuring expression of inflammasome component proteins and active IL-1β production. The lungs of the animals belonging to the hyperoxia+PL group had significantly increased expression of NLRP1, ASC, and active caspase-1 compared with those belonging to the RA group (Figure 2e–i). Hyperoxia-induced inflammasome protein expression appeared to be accompanied by inflammasome activation as we observed a significant elevation of active IL-1β in hyperoxia-exposed lungs compared with lungs belonging to animals in the RA group (1.27±0.37 vs. 0.46±0.21, RA+PL vs. O2+PL, P<0.001, Figure 2e). Treatment with recombinant CCN1 resulted in significant reductions in all three elevated inflammasome proteins during hyperoxia (Figure 2e–h). In addition, similarly CCN1 treatment resulted in a significant decrease in active IL-1β expression in hyperoxia-exposed lungs (0.52±0.11 vs. 1.27±0.37, P<0.001, O2+CCN1 vs. O2+PL, Figure 2e). These results suggest a crucial role of CCN1 in protecting neonatal lungs against the hyperoxia-induced inflammatory response by downregulation of the inflammasome–IL-1β cascade.
Treatment with CCN1 Improves Hyperoxia-Suppressed Alveolar Development
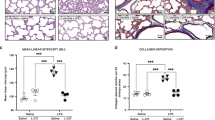
We next evaluated the effects of CCN1 on alveolar development by measuring RAC. Compared with RA-exposed rats, the lungs from hyperoxia and PL-exposed rats had significantly reduced RAC, suggesting poor alveolar development (6.06±0.4 vs. 8.93±1.03, P<0.001, O2+PL vs. RA+PL, Figure 3a). Treatment with CCN1 resulted in attenuation of the alveolar injury induced by hyperoxia as demonstrated by increased RAC (6.06±0.4 vs. 7.97±2.11; P<0.01, O2+PL vs. O2+CCN1, Figure 3a). Thus, CCN1 improves alveolarization during hyperoxia.
Treatment with CCN1 improves hyperoxia-suppressed alveolarization. (a) Histological examination of O2+PL lung sections revealed larger and simplified alveoli in comparison with RA+PL lungs, which showed more numerous and smaller alveoli. CCN1 treatment reversed the effects of hypoxia as O2+CNN1 lungs showed more alveolarization. Bar=50 μm. (b) Morphometric analysis demonstrated that exposure to hyperoxia decreased radial alveolar count (RAC) in PL-treated rats, which was significantly reversed by administration of CCN1 (*P<0.001: RA+PL vs. O2+PL; †P<0.01: O2+PL vs. O2+CCN1). n=6/group. Open bar, room air; solid bar, hyperoxia. PL, placebo; RA, room air.
Treatment with CCN1 Improves Hyperoxia-Suppressed Vascular Development
Pulmonary vascularization was assessed by measuring the vascular density of vWF-positive vessels (<50 μm in diameter) in lung tissue sections. As seen in Figure 4, hyperoxia exposure resulted in a significant reduction in vascular density compared with RA (5.30±1.03 vs. 11.10±2.87, P<0.001, O2+PL vs. RA+PL, Figure 4a). In contrast, treatment with CCN1 significantly increased hypoxia-reduced vascular density (7.88±0.57 vs. 5.30±1.03, P<0.05, O2+CCN1 vs. O2+PL, Figure 4a). These results suggest that CCN1 improves vascular development in hyperoxia-exposed neonatal rat lungs.
CCN1 administration improves hyperoxia-suppressed vascular development. (a) Immunofluorescence staining with an anti-vWF antibody (green signal) and 4′,6-diamidino-2-phenylindole (DAPI) nuclear staining (blue signal) were performed on lung tissue sections. Bar=50 μm. (b) Vascular density was determined by counting vWF-positive vessels (<50 μm) on five random images from each lung section. The vascular density was significantly decreased in O2+PL lungs compared with normoxic lungs (*P<0.001: RA+PL vs. O2+PL). Treatment with CCN1 significantly increased vascular density in hyperoxia-exposed animals (†P<0.05: O2+PL vs. O2+CCN1). **P<0.05: RA+CCN1 vs. O2+CCN1. n=6/group. Open bar, RA; solid bar, hyperoxia. PL, placebo; RA, room air; vWF, von Willebrand factor.
Administration of CCN1 Reduces Hyperoxia-Induced Pulmonary Vascular Muscularization
To assess whether CCN1 affects pulmonary vascular remodeling during hyperoxia, we measured the extent of muscularization of peripheral pulmonary vessels that are less than 50 μm in diameter and with more than 50% muscularization using double immunofluorescent vWF and α-smooth muscle actin staining of lung sections. The percentage of muscularized vessels was significantly increased in the hyperoxia group compared with that in the RA group (56% vs. 18%, P<0.001, O2+PL vs. RA+PL, Figure 5a). Moreover, the percentage of muscularized vessel was significantly decreased by treatment with CCN1 during hyperoxia (56% vs. 33%, P<0.01, O2+PL vs. O2+CCN1, Figure 5a). Thus, CCN1 treatment decreases hyperoxia-induced pulmonary vascular remodeling.
Treatment with CCN1 decreases hyperoxia-induced pulmonary vascular remodeling. (a) Double-immunofluorescence staining for vWF (green signal) and α-SMA (red signal) and DAPI nuclear staining (blue signal). Bar=50 μm. (b) The percentage of <50-μm-diameter muscularized peripheral pulmonary vessels (≥50% of circumference α-SMA-positive) was significantly increased in lungs from the O2+PL group. Administration of CCN1 significantly decreased vascular muscularization in hyperoxia-exposed animals (*P<0.001: RA+PL vs. O2+PL; **P<0.01: RA+CCN1 vs. O2+CCN1; †P<0.01: O2+PL vs. O2+CCN1). n=6/group. (c) Double immunofluorescence staining with Ki67 (red signal) and α-SMA (green signal) and DAPI nuclear staining (blue signal) were performed to assess vascular smooth muscle cell proliferation. Pink signals indicate Ki67-positive nuclei. Bar=50 μm. (d) The percentage of vessels (<50 μm in diameter) with at least one Ki67-positive nuclei on each vessel was determined. O2+PL lungs had increased proliferating vessels compared with RA lungs. Treatment with CCN1 decreased vascular proliferation (*P<0.001: RA+PL vs. O2+PL; **P<0.001: RA+CCN1 vs. O2+CCN1; †P<0.001: O2+PL vs. O2+CCN1). n=6/group. Open bar, RA; solid bar, hyperoxia. α-SMA, α-smooth muscle actin; PL, placebo; RA, room air; vWF, von Willebrand factor.
We also assessed the effects of CCN1 on vascular smooth muscle cell proliferation. With exposure to hyperoxia, there was a significant increase in peripheral vessels with proliferating smooth muscle cells (55% vs. 19%, P<0.001, O2+PL vs. RA+PL, Figures 5c and 6d). However, treatment with CCN1 protein resulted in a significant decrease in the percentage of proliferating peripheral vessels induced by hyperoxia exposure (55% vs. 36%, P<0.001, O2+PL vs. O2+CCN1, Figure 5c).
Effects of CCN1 on hyperoxia-induced RVH. Exposure to hyperoxia in the presence of the PL resulted in an increase in Fulton index (RV/LV+S), indicating RVH and PH. Administration of CCN1 significantly decreased RVH during hyperoxia (*P<0.001: RA+PL vs. O2+PL; †P<0.001: O2+PL vs. O2+CCN1). n=6/group. Open bar, RA; solid bar, hyperoxia. LV, left ventricle; PH, pulmonary hypertension; PL, placebo; RA, room air; RHV, right ventricular hypertrophy; S, septum.
Treatment with CCN1 Decreases Hyperoxia-Induced RVH
To evaluate the degree of PH, we measured RVH (Fulton index). Hyperoxia exposure resulted in a significant increase in RVH compared with RA exposure (0.41±0.04 vs. 0.31±0.02, P<0.001, O2+PL vs. RA+PL, Figure 6) and treatment with CCN1 resulted in a significant reduction in the elevated Fulton index induced by hyperoxia (0.31±0.04 vs. 0.41±0.04, P<0.001, O2+CCN1 vs. O2+PL, Figure 6). These results suggest that CCN1 can prevent hyperoxia-induced RVH in neonatal rats.
Discussion
In this study, we report that hyperoxia downregulates CCN1 in newborn rat lungs. Moreover, we found evidence for a protective role of CCN1 in hyperoxia-induced neonatal lung injury by demonstrating that treatment with recombinant CCN1 decreases lung inflammation, improves alveolarization and vascular development, and decreases pulmonary vascular remodeling and RVH, all of which are key components of BPD pathology. These findings provide new insights into understanding the role of CCN1 in the pathogenesis of BPD, and, if future studies show that CCN1 is also downregulated in BPD patients, then CCN1 has the potential to be a novel agent for the prevention or treatment of BPD in preterm infants.
Although there are many studies examining the expression pattern of CCN1, no previous studies have focused on the neonatal lung. We showed that high levels of CCN1 are expressed during normal neonatal rat lung development and that hyperoxia downregulates CCN1 expression in the neonatal rat lungs. This expression pattern is in a sharp contrast to CCN2 expression, which is low during normal lung development and is upregulated by hyperoxia (14). These results suggest that CCN1 and CCN2 may have different and/or opposing roles in lung development and injury repair in neonates.
Likewise, prior studies employing hyperoxia models in adult rodents support our finding that enhancing CCN1 levels has an anti-inflammatory protective effect against hyperoxia-induced lung injury. For example, Moon et al. (25) have reported that endogenous lung-epithelial cell-produced CCN1 exerted anti-inflammatory activity by promoting IL-10 production and by inhibiting multiple pro-inflammatory cytokines and neutrophil infiltration into the lung. Further, Jin et al. (26) demonstrated that suppressing CCN1 expression by small interfering RNA-accelerated lung-epithelial cell death after hyperoxia, and conversely that overexpressing CCN1, conferred increased resistance to hyperoxia-induced cell death. Although these reports are in agreement with our findings here that CCN1 treatment had an anti-inflammatory and protective effect on hyperoxia-induced lung inflammation and damage in neonatal rats, the work of Perkowski et al. (27) conflicts with our finding that hyperoxia decreases lung CCN1 expression as they report hyperoxia-increased lung CCN1 mRNA and protein expression. However, it is of note that the models used were significantly different from our model. They used adult mice and exposed them to >95% O2 for short period of time (24–48 h) to induce acute lung injury. On the contrary, we used a neonatal rat model, using 85% O2 hyperoxia exposure for a longer period of 7–14 days to simulate chronic lung disease. Similarly, other studies suggesting that CCN1 is pro-inflammatory/fibrotic in mouse bleomycin models of lung fibrosis were performed using 8-week- or 6-month-old mice (19). Our differing results would seem to support the hypothesis that the differential physiological function of CCN1 in cell survival/death are dependent on cell and organ types, types of cellular stimuli, and the duration of inflammation.
Our results indicate that CCN1 therapy significantly reduced the neutrophil and macrophage counts in hyperoxia-exposed rats’ lungs. Moreover, this may be partially related to the ability of CCN1 to increase efferocytosis of neutrophils, as has been described for wound tissue (16). However, to further investigate CCN’s anti-inflammatory activity we also examined lung levels of inflammasome-related proteins. Studies have shown that cyclic stretch activates NLRP3 inflammasomes and induces the release of active IL-1β in mouse alveolar macrophages (23). Studies by Liao et al. have shown that the NLRP3 inflammasome is associated with the development of BPD and that lungs of hyperoxia-exposed neonatal mouse have increased caspase-1 and IL-1β activation (24). Our recent studies have demonstrated that hyperoxia activates NLRP1 inflammasome and inhibition of Rac1 signaling downregulates NLRP1 inflammasome and decreases lung injury (28). In this study, we did not find significant changes in NLRP3 expression; however, we did find that hyperoxia upregulated expression of NLRP1, ASC, and active caspase-1, and production of active IL-1β, and that recombinant CCN1 treatment resulted in a significant downregulation of all four hyperoxia-elevated proteins, suggesting that CCN1’s anti-inflammatory activity may be mediated via attenuated inflammasome expression. Whether this attenuated inflammasome expression is due to decreased protein synthesis in lung resident macrophages and neutrophils or the result of CCN1 decreasing infiltrating neutrophil and macrophages counts, awaits further investigation. These results suggest a crucial role for inflammasomes in hyperoxia-induced neonatal lung injury and possibly also in BPD pathogenesis.
This study also demonstrated that CCN1 markedly improved alveolarization in hyperoxia-exposed neonatal lungs. This could be secondary to the decreased inflammation induced by CCN1 treatment. Previous in vitro studies have shown that CCN1 prevents hyperoxia-induced lung-epithelial cell death by activating cytoprotective signaling pathways (26, 29, 30). Thus, additional future studies are needed to investigate the potential mechanisms that are responsible for CCN1 protection of alveolar structure. Angiogenesis has a crucial role in the pathogenesis of BPD, and it has been hypothesized that disruption of angiogenesis during critical periods of lung growth can impair alveolarization and contribute to lung hypoplasia in BPD (31). Previous studies have demonstrated that treatment with recombinant VEGF, an important angiogenic factor, promotes angiogenesis and alveolarization in hyperoxia-exposed neonatal rats (32). CCN1 has been shown to have a role in inducing angiogenesis and CCN1 knockout mice display severe defects in angiogenesis during embryo development and commonly die from placental vascular inefficiency due to compromised blood vessels (18, 33, 34, 35). In agreement with these prior studies, we have demonstrated here that hyperoxia resulted in poor vascular development, which was associated with low CCN1 expression, and that treatment of hyperoxic animals with CCN1 resulted in improved vascular density. These results suggest that CCN1 might also have a critical role in vascular development in hyperoxia-induced neonatal lung injury.
We have shown that CCN1 therapy was associated with a reduction of pulmonary vascular remodeling induced by hyperoxia exposure, characterized by a decreased percentage of peripheral muscularized and proliferating vessels in the CCN1-treated hyperoxia group compared with the group exposed to hyperoxia+PL. Although the cellular mechanisms responsible for our observed reduction in pulmonary vascular remodeling by CCN1 treatment were not examined, previous studies on the role of CCN1 in cutaneous wound healing suggest that CCN1 dampens and resolves fibrosis during wound-healing by inducing myofibroblast senescence and upregulates the expression of antifibrotic genes to restrict fibrosis during tissue repair (36). Such mechanisms might explain the decrease in vascular remodeling we observed with CCN1 treatment. We also demonstrated that CCN1 therapy resulted in decreased RVH in hyperoxia-exposed rat pups, which likely is a direct reflection of improved vascular development and reduced pulmonary vascular remodeling. Lee et al. have shown that CCN1 suppresses hypoxia-induced pulmonary vascular smooth muscle contraction in vitro and it also decreases right ventricular pressure in hypoxia- as well as monocrotaline-induced PH in mice (37). These results highlight an important role of CCN1 in regulating vascular remodeling and PH.
There are potential limitations of this study. BPD is a multifactorial disease with risk factors including lung immaturity, prenatal/postnatal infection, traumatic ventilation, and oxygen toxicity. Although the current study focuses on oxygen-induced lung injury, which has phenotypic features similar to BPD, future studies are needed to investigate the role of CCN1 in the pathogenesis of BPD induced by other risk factors. In addition, more advanced stereological and three-dimensional approaches to assess lung alveolar structure have been recently reported (38), and these techniques will provide new insights into architectural changes in experimental models of BPD.
In conclusion, this study demonstrates the beneficial effects of CCN1 therapy on preventing lung inflammation and inflammasome activation, improving alveolarization and vascularization, and reducing pulmonary vascular remodeling and RVH, all of which are key components of BPD pathology. These findings provide new insights into understanding the role of CCN1 in the pathogenesis of BPD and additionally identify CCN1 as a potential novel therapeutic target for this disease.
References
Van Marter LJ . Epidemiology of bronchopulmonary dysplasia. Semin Fetal Neonatal Med 2009;14:358–66.
Bhandari A, Bhandari V . Pitfalls, problems, and progress in bronchopulmonary dysplasia. Pediatrics 2009;123:1562–73.
Wright CJ, Kirpalani H . Targeting inflammation to prevent bronchopulmonary dysplasia: can new insights be translated into therapies? Pediatrics 2011;128:111–26.
Jobe AJ, Bancalari E . Bronchopulmonary dysplasia. Am J Resp Crit Care Med 2001;163:1723–9.
Coalson JJ, Winter V, deLemos RA . Decreased alveolarization in baboon survivors with bronchopulmonary dysplasia. Am J Resp Crit Care Med 1995;152:640–6.
Perbal B . CCN proteins: multifunctional signalling regulators. Lancet 2004;363:62–4.
Chaqour B, Goppelt-Struebe M . Mechanical regulation of the Cyr61/CCN1 and CTGF/CCN2 proteins. FEBS J 2006;273:3639–49.
Allen JT, Knight RA, Bloor CA et al. Enhanced insulin-like growth factor binding protein-related protein 2 (Connective tissue growth factor) expression in patients with idiopathic pulmonary fibrosis and pulmonary sarcoidosis. Am Respir Cell Mol Biol 1999;21:693–700.
Sato S, Nagaoka T, Hasegawa M et al. Serum levels of connective tissue growth factor are elevated in patients with systemic sclerosis: association with extent of skin sclerosis and severity of pulmonary fibrosis. J Rheumatol 2000;27:149–54.
Wu S, Capasso L, Lessa A et al. High tidal volume ventilation activates Smad2 and upregulates expression of connective tissue growth factor in newborn rat lung. Pediatr Res 2008;63:245–50.
Chen CM, Wang LF, Chou HC et al. Up-regulation of connective tissue growth factor in hyperoxia-induced lung fibrosis. Pediatr Res 2007;62:128–33.
Wu S, Platteau A, Chen S et al. Conditional overexpression of connective tissue growth factor disrupts postnatal lung development. Am Respir Cell Mol Biol 2010;42:552–63.
Chen S, Rong M, Platteau A et al. CTGF disrupts alveolarization and induces pulmonary hypertension in neonatal mice: implication in the pathogenesis of severe bronchopulmonary dysplasia. Am J Physiol Lung Cell Mol Physiol 2011;300:L330–40.
Alapati D, Rong M, Chen S et al. Connective tissue growth factor antibody therapy attenuates hyperoxia-induced lung injury in neonatal rats. Am Respir Cell Mol Biol 2011;45:1169–77.
Jun J-I, Lau LF . Taking aim at the extracellular matrix: CCN proteins as emerging therapeutic targets. Nat Rev Drug Discov 2011;10:945–63.
Jun JI, Kim KH, Lau LF . The matricellular protein CCN1 mediates neutrophil efferocytosis in cutaneous wound healing. Nat Commun 2015;6:7386.
Kim KH, Chen CC, Monzon RI et al. Matricellular protein CCN1 promotes regression of liver fibrosis through induction of cellular senescence in hepatic myofibroblasts. Mol Cell Biol 2013;33:2078–90.
Leu SJ, Lam SC, Lau LF . Pro-angiogenic activities of CYR61 (CCN1) mediated through integrins alphavbeta3 and alpha6beta1 in human umbilical vein endothelial cells. J Biol Chem 2002;277:46248–55.
Grazioli S, Gil S, An D et al. CYP61 (CCN1) overexpression induces lung injury in mice. Am J Physiol Lung Cell Mol Physiol 2015;308:L759–65.
Kurundkar AR, Kurundkar D, Rangarajan S et al. The matricellular protein CCN1 enhances TGF-β1/SMAD3-dependent profibrotic signaling in fibroblasts and contributes to fibrogenic responses to lung injury. FASEB J. 2016;30:2135–50.
Borkham-Kamphorst E, Schaffrath C, Van de Leur E et al. The anti-fibrotic effects of CCN1/CYR61 in primary portal myofibroblasts are mediated through induction of reactive oxygen species resulting in cellular senescence, apoptosis and attenuated TGF-β signaling. Biochim Biophys Acta 2014;1843:902–14.
Chintala H, Krupska I, Yan L et al. The matricellular CCN1 controls retinal angiogenesis by targeting VEGF, Src homology 2 domain phosphatase-1 and Notch signaling. Development 2015;142:2364–74.
Wu J, Yan Z, Schwartz DE et al. Activation of NLRP3 inflammasome in alveolar macrophages contributes to mechanical stretch-induced lung inflammation and injury. J Immunol 2013;190:3590–9.
Liao J, Kapadia VS, Brown LS et al. The NLRP3 inflammasome is critically involved in the development of bronchopulmonary dysplasia. Nat Commun 2015: 6 1–12.
Moon HG, Qin Z, Quan T et al. Matrix protein CCN1 induced by bacterial DNA and CpG ODN limits lung inflammation and contributes to innate immune homeostasis. Mucosal Immunol 2015;8:243–53.
Jin Y, Kim HP, Ifedigbo E et al. Cyr61 protects against hyperoxia-induced cell death via Akt pathway in pulmonary epithelial cells. Am J Respir cell Mol Biol 2005;33:297–302.
Perkowski S, Sun J, Singhal S et al. Gene expression profiling of the early pulmonary response to hyperoxia in mice. Am J Respir cell Mol Biol 2003;28:682–96.
Hummler JK, Dapaa-siakwan F, Vaidya R et al. Inhibition of Rac1 signaling down-regulates inflammasome activation and attenuates lung injury in neonatal rats exposed to hyperoxia. Neonatology 2016;111:280–8.
Lu Y, Parkyn L, Otterbein LE et al. Activated Akt protects the lung from oxidant-induced injury and delays death of mice. J Exp Med 2001;193:545–9.
Truong SV, Monick MM, Yarovinsky TO et al. Extracellular signal-regulated kinase activation delays hyperoxia-induced epithelial cell death in conditions of Akt downregulation. Am J Respir Cell Mol Biol 2004;31:611–8.
Stenmark KR, Abman SH . Lung vascular development: implications for the pathogenesis of bronchopulmonary dysplasia. Ann Rev Physiol 2005;67:623–61.
Kunig AM, Balasubramaniam V, Markham NE et al. Recombinant human VEGF treatment transiently increases lung edema but enhances lung structure after neonatal hyperoxia. Am J Physiol Lung Cell Mol Physiol 2006;291:L1068–78.
Mo FE, Muntean AG, Chen CC et al. CYR61 (CCN1) is essential for placental development and vascular integrity. Mol Cell Biol 2002;22:8709–20.
Yan L, Chaqour B . Cysteine-rich protein 61 (CCN1) and connective tissue growth factor (CCN2) at the crosshairs of occular neovascular and fibrovascular disease therapy. J Cell Commun Signal 2013;7:253–63.
Babic AM, Kireeva ML, Kolesnikova TV et al. CYR61, a product of a growth factor-inducible immediate early gene, promotes angiogenesis and tumor growth. Proc Natl Acad Sci USA 1998;95:6355–60.
Jun JI, Lau LF . The matricellular protein CCN1 induces fibroblast senescence and restricts fibrosis in cutaneous wound healing. Nat Cell Biol 2010;12:676–85.
Lee SJ, Zhang M, Hu K et al. CCN1 supresses pumonary vascular smooth muscle contraction in response to hyperoxia. Pulm Circul 2015;5:716–722.
Nardiello C, Mižíková I, Morty RE . Looking ahead: where to next for animal models of bronchopulmonary dysplasia? Cell Tissue Res 2017;367:457–68.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Additional information
Statement of Financial Support
S.W. was financially supported by Project Newborn from the University of Miami and Micah Batchelor Award from the Batchelor Foundation.
Supplementary material is linked to the online version of the paper at
Supplementary information
Rights and permissions
About this article
Cite this article
Vaidya, R., Zambrano, R., Hummler, J. et al. Recombinant CCN1 prevents hyperoxia-induced lung injury in neonatal rats. Pediatr Res 82, 863–871 (2017). https://doi.org/10.1038/pr.2017.160
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/pr.2017.160
This article is cited by
-
Caffeine prevents hyperoxia-induced lung injury in neonatal mice through NLRP3 inflammasome and NF-κB pathway
Respiratory Research (2020)
-
Effects of Klotho supplementation on hyperoxia-induced renal injury in a rodent model of postnatal nephrogenesis
Pediatric Research (2020)
-
The matricellular protein CCN1 in tissue injury repair
Journal of Cell Communication and Signaling (2018)
-
CCN5 in alveolar epithelial proliferation and differentiation during neonatal lung oxygen injury
Journal of Cell Communication and Signaling (2018)