Abstract
Background:
Experimental evidence exists indicating that maternal thyroid hormones during pregnancy may affect the metabolic set point and cardio-vascular function in the offspring. The objective of this study was to investigate the association between maternal thyroid function in week 30 of gestation and offspring adiposity and blood pressure at 20 y.
Methods:
The study was based on the follow up of a Danish birth cohort from 1988 to 1989 (n = 965). A blood sample was drawn from the pregnant women in week 30 of gestation (N = 877). In 2008–2009, the offspring were followed up with self-reported anthropometrics (N = 645) and a clinically measured blood pressure (N = 425). Multiple linear regressions were used to estimate the association between maternal thyroid function and offspring BMI, waist circumference, and blood pressure.
Results:
Offspring of subclinical hypothyroid women had higher systolic blood pressure (adjusted difference = 3.6, 95% confidence interval: 0.2, 7.0 mmHg) and a tendency toward higher diastolic blood pressure (adjusted difference = 2.3, 95% confidence interval: −0.2, 4.9 mmHg) compared to offspring of euthyroid women. No association was found with offspring BMI and waist circumference.
Conclusion:
Maternal thyroid function during third trimester of pregnancy may affect long-term blood pressure in the offspring.
Similar content being viewed by others
Main
Thyroid disease is a relatively common disorder in women of reproductive age (1,2,3) and overt as well as subclinical disease has been shown to be associated with increased risk of adverse pregnancy outcomes such as miscarriage, preterm birth, small for gestational age, and stillbirth (1,4,5,6,7,8,9,10,11).
The development of many diseases takes years and factors affecting disease risk may operate throughout life. Hence, the programming theory proposes that an unsuitable environment at sensitive periods of development might result in long-term changes in the structure and function of the organism and thereby increase the risk of developing diseases later in life (12).
The fetal thyroid gland is not capable of producing hormones during the first half of pregnancy, making the fetus entirely dependent on maternal supply. Thyroid hormones are important for proper regulation of fetal brain development (13,14) and overt maternal hypothyroidism during pregnancy has been found to be associated with impaired neurological development in the offspring, (11,15,16) whereas the association with subclinical hypothyroidism is less clear (17,18,19,20).
Generally, little is known about the possible long-term consequences in offspring exposed to maternal thyroid disease during fetal life. However, recent evidence points toward a programming effect on neurodevelopmental disorders such as seizure disorders, autism, and attention deficit hyperactivity disorder (ADHD) (21,22,23). Also, experimental studies in mice have shown that thyroid hormones may be important for the development of specific hypothalamic centres governing cardio-vascular function (24), indicating that maternal thyroid function during pregnancy may have the potential to permanently program offspring cardiovascular function. Likewise, experimental studies have shown that thyroid hormone signaling during fetal life is involved in determining the metabolic set point in the offspring and thereby potentially affecting future adiposity (25).
Only one study has previously investigated the association between maternal thyroid dysfunction and offspring cardiometabolic risk factors (26). In this study, no association was found with either subclinical or overt maternal thyroid disease. However, the results indicated that both low maternal thyroid-stimulating hormone (TSH) and high free T4 were associated with a lower BMI in the 6-y-old offspring and that low TSH was associated with a lower diastolic blood pressure. The offspring in this study were, however, relatively young and more research is needed on the subject.
Hence, the objective of this study was to investigate the association between maternal subclinical hypo- and hyperthyroidism as well as quintiles of TSH and free T4 (fT4) in week 30 of gestation and offspring adiposity and blood pressure at age 19–20 y.
Results
Offspring of mothers with no exposure information were more often girls and tended to have a lower birth weight compared to offspring of mothers with exposure information ( Table 1 ). Among offspring of mothers with exposure information, nonparticipants had a lower birth weight and their mothers tended to have a higher prepregnancy BMI and were more likely to smoke during pregnancy compared to participants. In addition, participants who only filled out the questionnaire but did not participate in the clinical examination were more likely to be male and the females had a higher self-reported BMI compared to those who participated in the clinical examination.
None of the mothers fulfilled the criteria for overt hypothyroidism, whereas two women could be classified as hyperthyroid. The distribution of both maternal and offspring covariates were similar among euthyroid, subclinical hypothyroid, and subclinical hyperthyroid mothers ( Table 2 ). However, there was a tendency toward a lower birth weight in offspring of hypothyroid mothers and in this group offspring also reported parental overweight less frequently at follow-up.
No association was found between maternal subclinical thyroid disease and offspring adiposity measured as BMI and waist circumference at 20 y ( Table 3 ). However, maternal subclinical hypothyroidsm was associated with a higher systolic and diastolic blood pressure in the 20-y-old offspring compared to offspring of euthyroid women in the minimally adjusted model (model 1) ( Table 4 ). Further adjustment for maternal age, smoking, and dietary iodine intake during pregnancy, education, and parity tended to slightly attenuate the associations (model 2) which however remained statistically significant for systolic blood pressure. The same tendency was seen in offspring of women with subclinical hyperthyroidism. However, the very low number of participants in this exposure group (N = 9–13, depending on the outcome) resulted in wide confidence intervals and high vulnerability to adjustments.
Examining maternal levels of TSH and T4 separately, no overall association was observed ( Tables 5 and 6 ).
Sensitivity analyses using multiple imputations to impute missing values tended to attenuate the estimated differences for the anthropometric measures, particularly for the offspring of subclinical hyperthyroid mothers. With regard to blood pressure, the sensitivity analysis further strengthened the association between maternal subclinical hypothyroidism and offspring blood pressure (difference in systolic blood pressure: 3.7, 95% confidence interval: 0.6, 6.8 mmHg and difference in diastolic blood pressure: 2.5, 95% confidence interval: 0.2, 4.9 mmHg compared to offspring of euthyroid mothers) whereas the association between maternal subclinical hyperthyroidism and blood pressure was completely abolished.
In the sensitivity analyses using clinically measured anthropometric measures, estimates changed to some degree compared to the analyses using self-reported measures, but still no statistically significant association was found with maternal thyroid function (Supplementary Tables S1–S3 online).
The estimated differences for the anthropometric measured showed some sensitivity toward adjustments for offspring TSH or T4, but adjustments did not change the overall conclusion of no associations. Adjustments for offspring TSH and free T4 did not change the estimated associations between maternal thyroid function and offspring blood pressure.
A total of two women with offspring participating in the follow-up and normal TSH levels had a free T4 concentration above the reference level and another two had a free T4 concentration below the reference level. According to the applied definition, these women were categorized as euthyroid. Excluding them from the analyses did not change the results.
Discussion
Results from the present study indicate an association between maternal subclinical thyroid disease during pregnancy and blood pressure in the 20-y-old offspring. Hence, maternal subclinical hypothyroidism during pregnancy was associated with higher diastolic and systolic blood pressure. The same tendency was observed in offspring of women with subclinical hyperthyroidism, but the number of women in this category was relatively low. No overall association was found between maternal thyroid function and offspring adiposity and between maternal TSH and free T4 in quintiles and offspring blood pressure or adiposity.
A major strength of this study is the long-term follow-up into young adulthood. The unique personal identification number in Denmark enabled us to identify and contact a large proportion of the offspring from the DaFO88 cohort 19–20 y after their birth. As is often the problem in these long-term follow-up studies, however, we experienced problems with attrition. Hence, 76% gave information about self-reported anthropometry and 48% participated in the clinical examination. We cannot exclude the possibility that participation was associated with adiposity and blood pressure. This is also indicated by the data showing that nonparticipation was associated with lower birth weight, higher maternal prepregnancy BMI, maternal smoking during pregnancy, offspring sex and for the clinical examination also higher self-reported BMI and a tendency towards less engagement in physical activity. However, TSH and free T4 concentrations were not different for mothers of offspring participating in the follow-up and those who did not, indicating that the risk of selection bias was limited.
The association between maternal thyroid function and offspring adiposity, was estimated using self-reported information, which might have led to some degree of underreporting. However, whereas validation of the self-reported information using clinically measured BMI (n = 423) and waist circumference (n = 407) did indicate some underreporting, this underreporting did not depend on maternal TSH or free T4. We cannot exclude that this has biased the association between maternal thyroid function and offspring adiposity towards no association. However, repeating the adiposity related analyses using clinically measured BMI and waist circumference did not change the overall conclusion of no association.
In the study, we adjusted for a number of potential confounders. We can, however, not exclude the possibility of residual and unmeasured confounding. The size of the study and in particular the small number of women with subclinical thyroid disease also limited the possibility of adjustments. It was decided not to include offspring life style habits in the analyses, since this information was collected cross-sectionally with the outcome. Hence, the direction of an association between, e.g., exercise and BMI was uncertain and adjustments could lead to collider bias. Also, birth weight and gestational age were not adjusted for in the main analysis since they could be part of the causal pathway. Adjusting for these factors in secondary analyses, however, did not change estimates materially. We tried to adjust for dietary intake of iodine during pregnancy. The intake of iodine was estimated based on self-reported diet, and a considerable amount of residual confounding is assumed to be present. It is, however, a strength that all women were living in the same geographical area indicating that the iodine intake through the drinking water was similar for all women.
The maternal concentration of free T4 and TSH was measured from blood samples collected in week 30 of gestation. At this time of gestation, the fetal thyroid gland is functioning and the fetus is probably less sensitive to maternal concentrations. It is however likely, that women categorized as having subclinical thyroid disease in week 30 of gestation also were affected in early pregnancy. Also, maternal supply of thyroid hormones still could influence the regulation of fetal hormone production in late gestation.
The association between subclinical hypothyroidism and offspring blood pressure is in line with experimental evidence (24). Hence, studies in mice have indicated that maternal hypothyroidism during pregnancy may be important in regulating the development of specific fetal hypothalamic centers involved in the autonomic control of blood pressure. It is possible that deviation from normal thyroid function, independently of whether it is hyper- or hypothyroidism, is affecting the development of these hypothalamic cells. This could potentially explain why we in this study also found an adverse association between subclinical hyperthyroidism and offspring blood pressure. However, in our study, very few women were categorized as having subclinical hyperthyroidism and the sensitivity analysis using multiple imputations completely abolished the association with blood pressure. Hence, larger studies are needed to explore the potential association between subclinical hyperthyroidism and blood pressure.
The lack of overall association between maternal TSH and free T4 when analyzed separately could indicate that only thyroid hormone levels outside the normal range affect the development of blood pressure in the offspring. Hence, in the highest and lowest quintiles of TSH, only 38 and 11% had levels above 3 mIU/l or below 0.36 mIU/l respectively, indicating that a possible association between extreme values of, e.g., TSH may have been diluted by women within the reference range.
Only one previous study has investigated the association between maternal thyroid function during pregnancy and offspring cardio-metabolic health in humans. In this study, maternal hypothyroidism or hyperthyroidism was not associated with offspring blood pressure or adiposity at 6 y. However, the study did see an association between both a low maternal TSH and a high free T4 and a lower BMI in the 6-y-old offspring. Also, a low maternal TSH was associated with a lower diastolic blood pressure. The discrepancies between the two studies could be related to the different age of the offspring. Hence, the consequences of being exposed to subclinical thyroid disease during fetal life may not have manifested in the young offspring yet.
In conclusion, our data support the hypothesis that maternal subclinical thyroid dysfunction during pregnancy is associated with increased blood pressure in the offspring. An association that could be the result of maternal thyroid hormone levels interfering with fetal hypothalamic development. If indeed maternal thyroid disease is associated with offspring long-term cardiovascular function, screening, and treatment of pregnant women for modest thyroid dysfunction could be a relatively cheap and easily implemented strategy for the prevention of cardiovascluar disease in the offspring. However, limited evidence still exists and the present study should be seen as hypothesis generating. Therefore, larger studies are needed before firm conclusions can be drawn.
Methods
The study followed-up a birth cohort established in Aarhus, Denmark from April 1988 until January 1989 (DaFO88 cohort). A total of 965 pregnant women, representing 80% of a consecutive sample of 1,212 women attending routine antenatal care at a specific antenatal care centre in the city, were included in the study. The women filled out a self-administered dietary questionnaire 1 wk prior to the scheduled antenatal visit in week 30 of gestation, and provided consent was given, a supplementary face to face interview was undertaken after the visit. Also in connection with the visit, a blood sample was obtained, processed, and stored at −20 °C. The mean age of the participating women was 29 y.
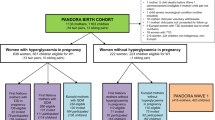
A total of 915 (95%) mother and child dyads were alive, living in Denmark and could be identified in the central registration registry in 2008–2009 where the offspring from DaFO88 were invited for follow-up at age 19–20 y. The offspring were contacted and asked to complete a self-administered web-based questionnaire concerning anthropometric measures, health, and lifestyle. Additionally, they were invited to take part in a physical examination. A sufficient amount of serum for thyroid hormone analyses was available for 877 mothers in 2015. Of the 877 offspring of mothers with exposure information, a total of 663 (76%) filled out the questionnaire and 425 (48%) participated in the clinical examination ( Figure 1 ).
Flowchart for the DaFO88 Cohort, Aarhus, Denmark 1988–2009.
Exposure Assessment
Maternal concentrations of TSH and free T4 were quantified in 2014 from serum collected in gestational week 30 of pregnancy on a Dimension Vista automated immunoassay (Siemens Healthcare Diagnostics, Germany) using luminescent detection and LOCI technique. For TSH, the manufacturer reference range was 0.358–3.74 mIU/l, functional sensitivity 0.005 mIU/l, analytical measurement range 0.005–100 mIU/l, and interassay CV 2.30–3.06% for five concentrations in the range from 0.39–76.5 mIU/l. For free T4, the manufacturer reference range was 9.8–18.8 pmol/l, analytical measurement range 1.3–103 pmol/l, and interassay CV 1.57–2.81% for five concentrations in the range from 9.7–82.2 pmol/l. For both, TSH and free T4 internal and external controls were included. Offspring TSH and free T4 was analyzed from fasting blood samples collected at the time of follow-up using the same method.
The thyrotropic activity of human gonadotropin causes a decrease in serum TSH most pronounced in the first trimester of pregnancy and it has been recommended to use trimester-specific reference ranges in pregnancy. Therefore, according to guidelines from the American Thyroid Association (27), mothers were categorized as being either hypothyroid/subclinical hypothyroid (TSH > 3.00 mIU/l and free T4 within or below reference levels), hyperthyroid/subclinical hyperthyroid (TSH < 0.36 and free T4 within or above reference levels) or euthyroid (the remaining women).
Outcome Assessment
The offspring gave self-reported information about height, weight, and waist circumference in the web-based questionnaire. Blood pressure was measured at the physical examination which was performed by trained personal. Following 7 min rest, blood pressure was measured three times (2 min apart) with an automatic blood pressure device (OMRON M6 Comfort (HEM-7000-E), Omron Healthcare Europe, the Netherlands). The mean of the last two measurements was used in the analyses.
Statistics
Multiple linear regression analyses were used to estimate the association between maternal thyroid function and self-reported BMI and waist circumference as well as clinically measured systolic and diastolic blood pressure. In the primary analyses, maternal thyroid function was categorized as subclinical hypothyroidism, euthyroidism, or subclinical hyperthyroidism. In additional analyses, maternal thyroid function was categorized according to quintiles of either TSH or T4 based on the entire study population with the third quintile as reference.
Potential confounders were identified a priori and the following covariates were included in the fully adjusted model: Maternal prepregnancy BMI (< 5% missing), age (<2 % missing), parity (<2% missing), education (7–9% missing), smoking during pregnancy (< 6% missing), and dietary iodine intake during pregnancy, estimated from the dietary questionnaire in week 30 of gestation (< 2% missing). In addition, sex of the child was considered a strong predictor for the outcomes and was also included in the model (0% missing). 10–12% of participants had missing information on at least one covariate. Since the subclinical hyperthyroid and hypothyroid exposure groups were relatively small, overadjustments could cause unstable estimates and an additional model only adjusting for sex and maternal prepregnancy BMI was included. Only participants with complete information on covariates were included in the main analyses.
Due to the relatively high loss to follow-up, sensitivity analyses were performed using multiple imputation. Assuming the values missing were missing at random, values were imputed based on sex, maternal prepregnancy BMI, parity, maternal smoking during pregnancy, maternal education, and offspring birth weight. In addition, the imputation of blood pressure was also based on self-reported height and BMI. Additionally, sensitivity analyses were run using clinically measured anthropometrics.
To investigate whether a potential association between maternal thyroid function and offspring adiposity and blood pressure could be mediated through offspring thyroid function, additional analyses were run adjusting for offspring TSH or T4.
Ethics
All procedures involving human subjects were approved by the local ethics committee for Region Midtjylland (journal no. 20070157) and the Danish Data Protection Agency (journal no. 2006-41-6257). Written informed consent was obtained from all participants.
Statement of Financial Support
This work was supported by The Danish Council for Strategic Research (grants no: 09-067124, 2101-07-0025 and 2101-06-0005).
Disclosure
The authors have no disclosures.
References
Allan WC, Haddow JE, Palomaki GE, et al. Maternal thyroid deficiency and pregnancy complications: implications for population screening. J Med Screen 2000;7:127–30.
Carlé A, Laurberg P, Pedersen IB, et al. Epidemiology of subtypes of hypothyroidism in Denmark. Eur J Endocrinol 2006;154:21–8.
Carlé A, Pedersen IB, Knudsen N, et al. Epidemiology of subtypes of hyperthyroidism in Denmark: a population-based study. Eur J Endocrinol 2011;164:801–9.
Andersen SL, Olsen J, Wu CS, Laurberg P. Spontaneous abortion, stillbirth and hyperthyroidism: a danish population-based study. Eur Thyroid J 2014;3:164–72.
Andersen SL, Olsen J, Wu CS, Laurberg P. Low birth weight in children born to mothers with hyperthyroidism and high birth weight in hypothyroidism, whereas preterm birth is common in both conditions: a Danish National Hospital Register Study. Eur Thyroid J 2013;2:135–44.
Casey BM, Dashe JS, Wells CE, et al. Subclinical hypothyroidism and pregnancy outcomes. Obstet Gynecol 2005;105:239–45.
Benhadi N, Wiersinga WM, Reitsma JB, Vrijkotte TG, Bonsel GJ. Higher maternal TSH levels in pregnancy are associated with increased risk for miscarriage, fetal or neonatal death. Eur J Endocrinol 2009;160:985–91.
Ashoor G, Maiz N, Rotas M, Jawdat F, Nicolaides KH. Maternal thyroid function at 11 to 13 weeks of gestation and subsequent fetal death. Thyroid 2010;20:989–93.
Negro R, Schwartz A, Gismondi R, Tinelli A, Mangieri T, Stagnaro-Green A. Universal screening versus case finding for detection and treatment of thyroid hormonal dysfunction during pregnancy. J Clin Endocrinol Metab 2010;95:1699–707.
Negro R, Schwartz A, Gismondi R, Tinelli A, Mangieri T, Stagnaro-Green A. Increased pregnancy loss rate in thyroid antibody negative women with TSH levels between 2.5 and 5.0 in the first trimester of pregnancy. J Clin Endocrinol Metab 2010;95:E44–8.
Negro R, Mestman JH. Thyroid disease in pregnancy. Best Pract Res Clin Endocrinol Metab 2011;25:927–43.
Olsen J. [Fetal programming of chronic diseases]. Ugeskr Laeger 2003;165:4384–6.
Ahmed OM, El-Gareib AW, El-Bakry AM, Abd El-Tawab SM, Ahmed RG. Thyroid hormones states and brain development interactions. Int J Dev Neurosci 2008;26:147–209.
Bernal J, Nunez J. Thyroid hormones and brain development. Eur J Endocrinol 1995;133:390–8.
Haddow JE, Palomaki GE, Allan WC, et al. Maternal thyroid deficiency during pregnancy and subsequent neuropsychological development of the child. N Engl J Med 1999;341:549–55.
Man EB, Brown JF, Serunian SA. Maternal hypothyroxinemia: psychoneurological deficits of progeny. Ann Clin Lab Sci 1991;21:227–39.
Lazarus JH, Bestwick JP, Channon S, et al. Antenatal thyroid screening and childhood cognitive function. N Engl J Med 2012;366:493–501.
Henrichs J, Bongers-Schokking JJ, Schenk JJ, et al. Maternal thyroid function during early pregnancy and cognitive functioning in early childhood: the generation R study. J Clin Endocrinol Metab 2010;95:4227–34.
Ghassabian A, Henrichs J, Tiemeier H. Impact of mild thyroid hormone deficiency in pregnancy on cognitive function in children: lessons from the Generation R Study. Best Pract Res Clin Endocrinol Metab 2014;28:221–32.
Li Y, Shan Z, Teng W, et al. Abnormalities of maternal thyroid function during pregnancy affect neuropsychological development of their children at 25-30 months. Clin Endocrinol (Oxf) 2010;72:825–9.
Andersen SL, Laurberg P, Wu CS, Olsen J. Attention deficit hyperactivity disorder and autism spectrum disorder in children born to mothers with thyroid dysfunction: a Danish nationwide cohort study. BJOG 2014;121:1365–74.
Andersen SL, Laurberg P, Wu CS, Olsen J. Maternal thyroid dysfunction and risk of seizure in the child: a Danish nationwide cohort study. J Pregnancy 2013;2013:636705.
Andersen SL, Olsen J, Laurberg P. Foetal programming by maternal thyroid disease. Clin Endocrinol (Oxf) 2015;83:751–8.
Mittag J, Lyons DJ, Sällström J, et al. Thyroid hormone is required for hypothalamic neurons regulating cardiovascular functions. J Clin Invest 2013;123:509–16.
Vujovic M, Nordström K, Gauthier K, et al. Interference of a mutant thyroid hormone receptor alpha1 with hepatic glucose metabolism. Endocrinology 2009;150:2940–7.
Godoy GA, Korevaar TI, Peeters RP, et al. Maternal thyroid hormones during pregnancy, childhood adiposity and cardiovascular risk factors: the Generation R Study. Clin Endocrinol (Oxf) 2014;81:117–25.
Stagnaro-Green A, Abalovich M, Alexander E, et al.; American Thyroid Association Taskforce on Thyroid Disease During Pregnancy and Postpartum. Guidelines of the American Thyroid Association for the diagnosis and management of thyroid disease during pregnancy and postpartum. Thyroid 2011;21:1081–125.
Author information
Authors and Affiliations
Corresponding author
Supplementary information
Supplementary Tables
(DOC 56 kb)
PowerPoint slides
Rights and permissions
About this article
Cite this article
Rytter, D., Andersen, S., Bech, B. et al. Maternal thyroid function in pregnancy may program offspring blood pressure, but not adiposity at 20 y of age. Pediatr Res 80, 7–13 (2016). https://doi.org/10.1038/pr.2016.56
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/pr.2016.56
This article is cited by
-
Maternal thyroid hormone receptor β activation in mice sparks brown fat thermogenesis in the offspring
Nature Communications (2023)
-
Changes in nitric oxide synthase levels are associated with impaired cardiac function and tolerance to ischemia-reperfusion injury in male rats with transient congenital hypothyroidism
Naunyn-Schmiedeberg's Archives of Pharmacology (2020)
-
Antenatal/early postnatal hypothyroidism increases the contribution of Rho-kinase to contractile responses of mesenteric and skeletal muscle arteries in adult rats
Pediatric Research (2018)