Abstract
Background:
Phototherapy using light in the spectral range of 410–500 nm, which overlaps the absorption of bilirubin, is the common treatment for neonatal hyperbilirubinemia. Hemoglobin (Hb) absorbs light strongly throughout this same range and thus can compete with bilirubin for this light and consequently reduce the efficacy of phototherapy. Here, we determined the effect of hematocrit (Hct) on in vitro bilirubin photoalteration using narrow-band blue (450 nm) light-emitting diodes (LEDs).
Methods:
Suspensions with Hcts from 0 to 80% and 16 ± 1 mg/dl bilirubin were prepared by mixing red blood cells (RBCs), bilirubin (30 mg/dl) in 4% human serum albumin, and normal saline. Aliquots of each suspension were exposed to blue light at equal irradiances. Before and after 60 min of exposure, bilirubin levels in supernatants (n = 46) were measured using a diazo-dye method.
Results:
Bilirubin photoalteration steeply decreased by ~60% as Hct increased from 0 to 10%. Over the clinically relevant range of 30–70% Hct, the decrease was significant, but less drastic, exhibiting a quasi-linear dependence on Hct.
Conclusion:
Bilirubin photoalteration under blue light in vitro is significantly reduced as Hct increases. Clinical studies are warranted to confirm these in vitro observations that Hct can affect the efficacy of phototherapy.
Similar content being viewed by others
Main
Phototherapy is a standard treatment for excessive neonatal hyperbilirubinemia. Light exposure alters bilirubin into the structural-isomer lumirubin and configurational E-isomers, all of which have greater water solubility than natural bilirubin. Smaller amounts of colorless photo-oxidation products are also produced. All of these photoproducts can be excreted in bile and urine without hepatic processing, resulting in a reduction of circulating bilirubin levels (1). A number of phototherapy devices are currently available, incorporating different light sources, such as broad-spectrum fluorescent tubes, halogen lamps, and narrow-spectrum light-emitting diodes (LEDs) (1). Because bilirubin has its peak absorption at 450 nm, blue light sources are currently incorporated in most clinically used phototherapy devices (2).
In 1974, Lucey et al. (3) reported their qualitative observation that the loss of bilirubin in blood samples under blue light decreased with increasing hematocrit (Hct). They concluded that this phenomenon was due to a competition between hemoglobin (Hb) and bilirubin for the absorption of light. In a recent report, Lamola et al. (4) described a semi-empirical model that enables quantitative calculation of the effect of Hb in neonatal skin on the efficacy of phototherapy. For blue light, this model predicts a significant reduction—by as much as one-third—in efficacy as Hct increases from 40 to 60%. To test this semi-empirical model, we determined the in vitro effect of Hb (by varying Hct levels) on the loss of diazo-reactive bilirubin using blue LEDs with peak emission at 450 nm.
Results
Bilirubin Photoalteration in the Absence of Red Blood Cells
In order to ensure that the irradiance of the blue (λmax at 450 nm) LED panel was uniform over all wells of the 24-well tissue culture plates, measurements were taken at each well. A mean irradiance of 4.2 ± 0.5 × 1015 photons/cm2/s was selected, which corresponds to ~26 µW/cm2/nm as measured by a BiliBlanket II meter (GE HealthCare Technologies, Waukesha, WI). The optical density of the suspensions in the absence of Hb was calculated using the known extinction coefficient of bilirubin bound to albumin (>40,000 mol/cm/l), the bilirubin concentration (16 ± 1 mg/dl), and the sample depth (0.3 cm) and was >2 over the wavelength range of the LED bandwidth, meaning >99% of the emitted light was absorbed by the suspensions.
Next, to determine the optimal degree of bilirubin photoalteration, 450-µl aliquots of 30 mg/dl of bilirubin in 4% human serum albumin that contained no red blood cells (RBCs), but saline only ( Table 1 ), were placed into wells of a 24-well tissue culture plate and exposed to light at 450 nm for 60 min. The loss of diazo-reactive bilirubin was then measured in each suspension. We found that in the absence of RBCs, bilirubin photoalteration was 56.3 ± 6.4% (n = 13).
Effect of Hct on Bilirubin Photoalteration
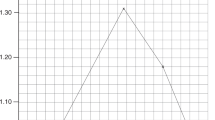
To determine the effect of Hct, we measured the percent of bilirubin photoalteration before and after 60 min of exposure to 450-nm light at Hcts ranging from 0 to 80%. After centrifuging the suspensions, aliquots of the supernatant were added to the standard sulfanilic acid reagent (diazo reagent) and assayed colorimetrically at 560 nm (n = 46). Data were expressed as the percent decrease in diazo-reactive bilirubin absorbance ( Figure 1 ). In the presence of RBCs, there was a steep 60% decrease in bilirubin photoalteration as Hcts increased from 0 to 10%. At a 10% Hct, ~20% of bilirubin was photoaltered. Over the clinically relevant range of Hcts—30 to 70% (5)—a significant and quasi-linear decrease in bilirubin photoalteration was observed ranging from 15% at 30% Hcts to 4% at 70% Hcts.
Effect of Hct on bilirubin photoalteration. A steep 60% decrease in bilirubin photoalteration observed from 0 to 10% Hcts, became more gradual at Hcts of 10–80%. The black squares represent the relative values for the rate of bilirubin photoalteration as predicted by the semi-empirical model with the black dotted line showing the best fit.
Assuming that the loss of diazo-reactive bilirubin after 60 min of exposure accurately reflected the initial rates of loss, we compared the degree of in vitro bilirubin photoalteration for different Hcts with that predicted by our semi-empirical model (4). To this end, we first calculated the relative rates of bilirubin photoalteration in the supernatant fraction of the bilirubin/RBC suspensions after exposure to light at 450 nm over the range of Hcts from 0 to 80% ( Figure 1 ). The relationship between Hct and bilirubin photoalteration from our semi-empirical model (4) reflects the relative initial rate of photoalteration. When we set the 0% Hct value to 56%, the model values for increasing Hcts fit very well with our in vitro data (points calculated using the model are shown as black squares). Noteworthy is that for both datasets, the effect of Hct in the clinically relevant range of 30 to 70% is nearly linear.
Discussion
This study confirmed that Hcts affect the effectiveness of in vitro bilirubin photoalteration. Under our experimental conditions, a steep 60% reduction in the loss of diazo-reactive bilirubin was observed as Hct increased from 0 to 10% with a significant, but less drastic reduction as Hcts were further increased. This relationship fits well with that predicted by our semi-empirical model, with both exhibiting a linear dependence on Hct in the clinically relevant range of 30 to 70%, over which the degree of bilirubin photoalteration is halved.
In both earlier work by Lucey and Hewitt in 1974 (3) and Granati et al. in 1983 (6), the irradiances of light used were not only very high, but the observed bilirubin loss may have been primarily due to oxidation processes and not only to the formation of photoisomers. In addition, Granati et al. (6) used the loss of bilirubin absorbance, which reflects the destruction of the bilirubin chromophore, as their measure of bilirubin photoalteration. Furthermore, they found photoisomers in only two of their samples, and then only in small quantities. Nonetheless, both early studies (3,6) still demonstrated that the presence of Hb does indeed impact bilirubin photochemistry. In our study, we exposed our samples to a much lower dose (~10 times lower than used in the aforementioned studies) and used the loss of diazo-reactive bilirubin as a measure of bilirubin photoalteration, which is considered indicative of lumirubin formation (1,7). Furthermore, because we applied an irradiance that would not result in a loss of diazo-reactive bilirubin greater than 50%, the limit for linearity for first-order process kinetics, in the absence of RBCs, all our loss measures closely represented loss rates.
While we did not directly measure the production of bilirubin photoproducts (e.g., by using high-pressure liquid chromatography), it is likely that the loss in diazo-reactive bilirubin we measured reflects the transformation of bilirubin to lumirubin, a non–diazo-reacting photoproduct (1,7). The configurational photoisomers (Z,E isomers) of bilirubin are diazo-reactive (1,7) and thus do not contribute to the measured loss. As further evidence, the quantum yield for the loss of diazo-reactive bilirubin at 0% Hct was calculated from the quantity lost after 60 min of irradiation (6.6 ± 0.6 × 10−7 moles) divided by the photon dose (3.8 × 10−5 moles) to be 0.0018 ± 0.0002. This value is in good agreement with a mean value of 0.0015 for the quantum yield of formation of lumirubin in the wavelength range 410–450 nm reported by Greenberg at al. (7). In addition, we did observe Z to E conversion early after light exposure (<25 min) in the absence of RBCs. When we measured the absorbance of bilirubin at the maximum (450 nm) for 0% Hct samples after various lengths of light exposure, we found that absorbance dropped by 16% in the first 25 min, reaching a temporary plateau, which was followed by a much slower decrease (data not shown). The rapid decrease and plateau are most likely associated with the formation of the Z,E-configurational photoisomer reaching a photostationary concentration (8,9). We ascribe our observed later, slower decrease to the accumulation of lumirubin (8,9).
There are two in vivo studies investigating the effect of Hct on the efficacy of phototherapy. Granati et al. (6) compared the reduction in serum bilirubin levels in nonhemolytic infants with similar prephototherapy total bilirubin levels (13.5 mg/dl) with mean Hcts of 67 ± 5% (n = 30) and 50 ± 5% (n = 27) in the in vivo phase of their study. After 24 h of phototherapy (425–475 nm, 22 μW/cm2/nm) using blue broad-band fluorescent tubes (which includes UV and IR wavelengths), they observed that infants in the lower Hct group had a higher mean reduction (8%) of total bilirubin than those in the higher Hct group but was deemed not statistically significant.
Recently, Mreihil et al. (10) characterized the time course of the early formation of the Z,E-photoisomer in infants under phototherapy (using both fluorescent and LED light sources) by performing a post hoc analysis on the effect of the Hb on 36 infants. Because the conversion of Z,Z to the Z,E isomer is photoreversible, only the initial rate of formation they observed of the Z,E-isomer is relevant to comparisons to our findings. They found a 35% higher rate of conversion in the lower Hct group (Hb < 14.5 g/dl (calculated Hct <44%)) compared to that in the higher Hct group (Hb ≥ 14.5 g/dl (calculated Hct ≥44%)).
In summary, we found that the presence of RBCs can significantly reduce the rate of loss of diazo-reactive bilirubin (associated with the formation of lumirubin) under exposure to narrow-band blue (450 nm) light in vitro. These findings confirm our semi-empirical model, which predicted an effect of Hct on the efficacy of phototherapy based on the conclusion that Hb competes with bilirubin for light (4). As reported by others, there also appears to be significant effects of Hct (6) and Hb (10) on in vivo bilirubin photoalteration as well. In the current American Academy of Pediatrics practice guideline (11), the effect of an infant’s Hct in determining the duration or intensity of phototherapy used has not been considered. However, based on this study, infants with higher Hcts may require phototherapy either at higher irradiances or for longer durations in comparison to infants with lower Hcts. More rigorously controlled clinical studies are needed to fully delineate the effect of Hct on the efficacy of phototherapy and its implications on clinical practice.
Methods
Reagents
Bilirubin/human serum albumin. A 30 mg/dl stock solution of bilirubin containing 4% human serum albumin (Sigma-Aldrich, St Louis, MO), an albumin concentration sufficient to bind all the bilirubin, was prepared as follows: under low light conditions, 8-mg bilirubin (Sigma-Aldrich) was first dissolved in 12.5 µl 10 N NaOH and 800 µl distilled water. This solution was then added to a mixture containing 23.0 g of 0.1 mol/l potassium phosphate buffer (pH 7.4) and 1.0 g human serum albumin. The pH was titrated to 7.4 with 1.0 N HCl (12,13). All bilirubin solutions were stored as 1.5-ml aliquots in 2-ml polypropylene microfuge tubes at −20 °C in the dark.
Red blood cells. Adult RBCs were isolated from discarded human blood (Type O, Rh positive) obtained from the Stanford Hospital’s Transfusion Services (Stanford, CA). Because we used discarded blood, institutional review board approval was not required. The blood was centrifuged at 13,000g × 1 min to separate RBCs from plasma and washed twice with phosphate-buffered saline.
Bilirubin/RBC suspensions. To create the bilirubin/RBC suspensions, the bilirubin solution, packed RBCs, and normal saline were combined in varying volumes to form suspensions with Hcts ranging from 0 to 80%, as shown in Table 1 .
Diazo reagents. The diazo reagents were prepared as follows: Solution A consisted of 1 g of sulfanilic acid (0.1% final concentration) dissolved in 500-ml 50% dimethylsulfoxide. Solution B consisted of 17-ml 11.8 N HCl mixed with 483 ml of distilled water. Solutions A and B were combined to form Solution C. Fifty milliliters (out of the total 1,000 ml) of Solution C was added to 1.66 ml of sodium nitrite (0.07 mol/l) to create the final working reagent (12,13).
Light Source
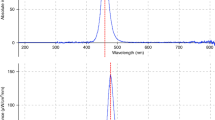
A panel containing 32 blue narrow-spectrum LEDs (Bright Light LED, Canoga Park, CA), with a peak wavelength of 450 nm and 19-nm bandwidth, was used ( Figure 2a ).
Exposure setup. (a) The emission spectrum of the blue LED panel, which has a peak wavelength at 450 nm. (b) The 24-well (4 ×
6) tissue culture plate containing the RBC/bilirubin suspensions were placed on a shaker placed 11-cm below a blue (450 nm) LED panel emitting a constant irradiance of 4.2 × 1015 photons/cm2/s (or 1,830 μW/cm2) for 60 min. (c) Custom-made blue LED panel. The red square marks the LEDs directly illuminating the bilirubin/RBC suspensions.
Exposure Setup
450-μl aliquots of each bilirubin/RBC suspension were first pipetted into the 16-mm-diameter wells of a 24-well (4 × 6) tissue culture plate (Becton Dickinson Labware, Franklin, NJ). The depth of the suspension in the well was 0.3 cm. The plates were then placed on an Orbis Plus shaker (Fischer Scientific, Waltham, MA) 11 cm below the blue LED panel and were left uncovered to ensure unimpeded light access and oxygen diffusion ( Figure 2b ). The well plates were always placed in the same position, centered under the nine LEDs ( Figure 2c ), where the irradiance over this area was found to be 4.2 × 1015 photons/cm2/s (1,830 μW/cm2) as measured using a spectroradiometer (Model S2000; Ocean Optics, Dunedin, FL), which is equivalent to 26.2 µW/cm2/nm as measured by a BiliBlanket II meter (GE HealthCare Technologies). The temperature was kept at 21 °C.
Protocol
Bilirubin/RBC suspensions were diluted with differing volumes of normal saline (EMD Chemicals, Gibbstown, NJ) to yield suspensions all containing a “serum/plasma” bilirubin concentration of 16 ± 1 mg/dl, which was confirmed using a UB Analyzer UA-2 (Arrows, Osaka, Japan). To confirm Hct levels in each bilirubin/RBC suspension, 35 µl of each suspension was pipetted into a hematocrit capillary tube and centrifuged for 1 min using a microhematocrit centrifuge, and the Hct determined using a microcapillary reader (Damon, Needham Heights, MA).
Both before and after 60 min of light exposure, bilirubin concentrations in supernatants were measured in triplicate using the diazo method (12,13). Both before and after light exposures, aliquots of the bilirubin/RBC suspensions were centrifuged at 13,000g × 1 min in CapiJect tubes (Terumo, Tokyo, Japan). Twenty-five microliters of the supernatant was added to 1.3 ml of diazo reagent with 650 µl of water and left for 10 min in the dark. Absorbance at 560 nm was then measured using a UV-1800 Spectrophotometer (Shimadzu, Kyoto, Japan).
Efficacy, defined as the % bilirubin photoalteration or the loss of diazo-reactive bilirubin, was calculated as the % decrease in absorbance of the diazo-reacted supernatants before and after light exposures, as follows:

Statement of Financial Support
This work was supported by the Mary L Johnson Research Fund (USA), the Christopher Hess Research Fund (USA), and the Undergraduate Advising and Research Major Grant (Stanford University, USA)
Disclosure
None of the authors has any financial ties to products in the study or potential conflicts of interests.
References
Vreman HJ, Wong RJ, Stevenson DK. Phototherapy: current methods and future directions. Semin Perinatol 2004;28:326–33.
Dennery PA, Seidman DS, Stevenson DK. Neonatal hyperbilirubinemia. N Engl J Med 2001;344:581–90.
Lucey J, Hewitt J. Recent observations on light and neonatal jaundice. In: Brown AK, Showacre J, eds. Phototherapy for Neonatal Hyperbilirubinemia. DHEW Publication No (NIH) 76–1075. Washington, DC: Department of Health, Education, and Welfare, 1977:123–34.
Lamola AA, Bhutani VK, Wong RJ, Stevenson DK, McDonagh AF. The effect of hematocrit on the efficacy of phototherapy for neonatal jaundice. Pediatr Res 2013;74:54–60.
Jopling J, Henry E, Wiedmeier SE, Christensen RD. Reference ranges for hematocrit and blood hemoglobin concentration during the neonatal period: data from a multihospital health care system. Pediatrics 2009;123:e333–7.
Granati B, Felice M, Fortunato A, Giancola G, Rubaltelli FF. Sites of action of light during phototherapy. Biol Neonate 1983;43:1–8.
Greenberg JW, Malhotra V, Ennever JF. Wavelength dependence of the quantum yield for the structural isomerization of bilirubin. Photochem Photobiol 1987;46:453–6.
Lightner DA, Wooldridge TA, McDonagh AF. Configurational isomerization of bilirubin and the mechanism of jaundice phototherapy. Biochem Biophys Res Commun 1979;86:235–43.
McDonagh AF, Lightner DA. Phototherapy and the photobiology of bilirubin. Semin Liver Dis 1988;8:272–83.
Mreihil K, Madsen P, Nakstad B, Benth JŠ, Ebbesen F, Hansen TW. Early formation of bilirubin isomers during phototherapy for neonatal jaundice: effects of single vs. double fluorescent lamps vs. photodiodes. Pediatr Res 2015;78:56–62.
American Academy of Pediatrics. Management of hyperbilirubinemia in the newborn infant 35 or more weeks of gestation. Pediatrics 2004;114:297–316.
Vreman HJ, Wong RJ, Murdock JR, Stevenson DK. Standardized bench method for evaluating the efficacy of phototherapy devices. Acta Paediatr 2008;97:308–16.
Schulz S, Vreman HJ, Wong RJ, Cline BK, Stevenson DK. Bilirubin photodestruction action spectrum revisited. J Invest Med 2012;60:210 (Abstract #302).
Acknowledgements
We thank Adam Blankespoor for constructing the LED panel with Hendrik J. Vreman. We would also like to thank Stephanie Kourula for her helpful discussions concerning the Ocean Optics Spectroradiometer. Rami Vardi of Bright Light, Inc., donated the LEDs and power supply.
Author information
Authors and Affiliations
Corresponding author
PowerPoint slides
Rights and permissions
About this article
Cite this article
Linfield, D., Lamola, A., Mei, E. et al. The effect of hematocrit on in vitro bilirubin photoalteration. Pediatr Res 79, 387–390 (2016). https://doi.org/10.1038/pr.2015.240
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/pr.2015.240
This article is cited by
-
Sixty years of phototherapy for neonatal jaundice – from serendipitous observation to standardized treatment and rescue for millions
Journal of Perinatology (2020)
-
The effect of light wavelength on in vitro bilirubin photodegradation and photoisomer production
Pediatric Research (2019)
-
Double versus single intensive phototherapy with LEDs in treatment of neonatal hyperbilirubinemia
Journal of Perinatology (2018)
-
Efficient Photocatalytic Bilirubin Removal over the Biocompatible Core/Shell P25/g-C3N4 Heterojunctions with Metal-free Exposed Surfaces under Moderate Green Light Irradiation
Scientific Reports (2017)
-
The impact of hemoglobin on the efficacy of phototherapy in hyperbilirubinemic infants
Pediatric Research (2017)