Abstract
Background:
Infantile hemangioma (IH) is the most frequent vascular tumor of early childhood. Recently, propranolol, a nonselective β1- and β2-adrenoceptor inhibitor, was introduced into the therapy of severe proliferating IH with excellent results. However, the underlying mechanism of action of propranolol is still unclear.
Methods:
We performed immunohistochemistry for cluster of differentiation 31 (CD31), D2-40, glucose transporter-1 (GLUT-1), and Ki67 in order to characterize 21 vascular anomalies (nine IH, seven venous malformations (VMs), and five lymphatic malformations (LMs)). Furthermore, we analyzed the expression of β1-, β2-, and β3-adrenoceptor mRNA in these specimens as well as in hemangioma-derived stem cells by quantitative real-time PCR (qPCR).
Results:
We show that the expression of β1-adrenoceptor mRNA is 10.7-fold higher in IH independent of the proliferative or regressive phase as well as 2.5-fold higher in hemangioma-derived stem cells as compared with β2-adrenoceptor mRNA. In LM, the expression of β2-adrenoceptor mRNA was ninefold higher than that of β1-adrenoceptor mRNA. VM showed low expression levels of all β-adrenoceptor mRNAs, and β3-adrenoceptor mRNA was hardly detectable in any specimens examined.
Conclusion:
These results provide the first evidence of distinctions between IH and vascular malformations with regard to β-adrenoceptor subtype mRNA levels.
Similar content being viewed by others
Main
Recently, propranolol, a nonselective β-adrenoceptor inhibitor, was introduced into the therapy of severe proliferating infantile hemangioma (IH) (1). Propranolol treatment in IH results in fast growth inhibition and rapid tumor regression within a few days of therapy. This novel medical treatment has not been developed in a classic way; it was accidentally observed during corticosteroid therapy, the so far standard therapy for proliferating IH (2) when cardiac hypertrophy as a side effect is treated by propranolol. The molecular mechanism of propranolol on IH proliferation is unknown at present. A possible method of action could be vasoconstriction or induction of endothelial cell apoptosis (3). Inhibition of β-adrenoceptors has been suggested to have a negative impact on tumor development as well as a direct effect on endothelial cells by downregulation of angiogenesis factors such as vascular endothelial growth factor, matrix metalloproteinase-2, and matrix metalloproteinase-9 (4,5). A possible involvement of the β2-adrenoceptor/cyclic adenosine monophosphate pathway has been described in tumor vascularization of glioblastoma (4).
IH is the most frequent benign tumor of early childhood that shows specific biological features: after an initial phase of proliferation during the first year of life, a long-lasting period of spontaneous regression over several years is observed (6). The underlying molecular mechanism could not be completely determined because of a lack of models for IH. Until recently, neither IH cell lines nor animal models were available. Endothelial cells from IH show specific expression of glucose transporter-1 (GLUT-1) (7) and can be discriminated by this marker from endothelial cells of the vascular tumors and vascular malformations composed of veins, arteries, or lymphatic vessels. Furthermore, the clinical behavior, with lack of independent growth and regression in vascular malformations, clearly demonstrates a different biological background.
In this study, we studied the expression of β-adrenoceptor subtype mRNA in IH and vascular malformations, including venous malformations (VMs) and lymphatic malformations (LMs), to describe the molecular background of the new therapeutic approach with propranolol, a nonselective β-adrenoceptor inhibitor.
Results
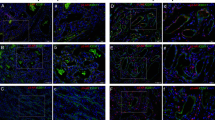
IHs of six children included in this study showed proliferation when surgery was performed. These children were younger than 1 y ( Table 1 ). IH from three children older than 1 y had passed a period of regression. All endothelial cells from IH showed GLUT-1 expression by immunohistochemistry, which confirms the diagnosis ( Figure 1 , Table 2 ). The six proliferating IHs additionally showed 10–15% Ki67-positive endothelial cells, confirming the growth potential. In contrast, Ki67 expression was not detectable in the IHs from three older children.
Characterization of IH. (a) H&E staining (a) and immunohistochemistry for (b) GLUT-1 and (c) Ki67 in a proliferative IH as well as (d) H&E staining and immunohistochemistry for (e) GLUT-1 and (f) Ki67 in IH after spontaneous regression (original magnification ×10). Proliferative IH of patient ID 10 showing (a) multiple capillaries with (b) strong expression of GLUT-1 and (c) 10–15% Ki67-positive endothelial cells. The IH of patient ID 6 after spontaneous regression shows vessels with (d) bigger lumen, (e) GLUT-1 positive immunostaining, and (f) 1% Ki67-positive endothelial cells. GLUT-1, glucose transporter-1; H&E, hematoxylin and eosin; IH, infantile hemangioma.
The age of patients with surgically removed vascular malformations varied from 4 wk to 18 y. Localization was in the head and neck region as well as at the extremities, which is in accordance with the literature (8). All vascular malformations showed cluster of differentiation 31 (CD31)–positive immunostaining of endothelial cells and all were GLUT-1 negative ( Figure 2 , Table 2 ). To discriminate between vascular malformations of blood and/or lymphatic vessels, D2-40 expression was determined and the result confirmed the clinical and radiological diagnosis identifying five LMs, which were D2-40 positive, and seven VMs.
Characterization of vascular malformations. (a) H&E staining and immunohistochemistry for (b) CD31 and (c) GLUT-1 in a venous malformation and (d) H&E staining and immunohistochemistry for (e) CD31 and (f) D2-40 in a lymphatic malformation (original magnification ×10). VM of patient ID 16 presenting (a) large and dilated caverna with (b) CD31-positive, but (c) GLUT-1 negative endothelium. Erythrocytes in the vessel lumen are GLUT-1 positive. (d) The LM shows collapsed vessels that are (e) CD31 and (f) D2-40 positive. CD31, cluster of differentiation 31; GLUT-1, glucose transporter-1; H&E, hematoxylin and eosin; LM, lymphatic malformation; VM, venous malformations.
Expression of β-adrenoceptor subtype mRNA was analyzed with quantitative real-time PCR (qPCR) and normalized to expression of ribosomal protein RPS29 mRNA ( Figure 3 ). Overall, β-adrenoceptor mRNA density was one order of magnitude higher in IH and LM samples as compared with VM samples. IH showed 10.7-fold higher expression of β1 as compared with β2-adrenoceptor mRNA ( Figure 3a ). β3-Adrenoceptor mRNA could be detected in all nine IH specimens, although at levels that were three orders of magnitude lower than β1-levels. β1-Adrenoceptor mRNA concentrations did not differ significantly between proliferative and nonproliferative IHs (proliferative 45 ± 10 vs. nonproliferative 90 ± 41 ADRB1 copies/1,000 RPS29 copies, P = 0.18). In VM specimens, mRNA levels for β1- and β2-adrenoceptors did not differ significantly ( Figure 3b ). In LM specimens, β2-adrenoceptor mRNA expression was ninefold higher than β1-adrenoceptor mRNA expression ( Figure 3c ). In all specimens examined, expression of β3-adrenoceptor mRNA was very low as compared with β1- and β2-subtypes.
Expression of β1-, β2-, and β3-adrenoceptors in (a) human IH, (b) venous malformations, and (c) lymphatic malformation. (a) In IH, β1-adrenoceptor (ADRB1) mRNA was more abundantly expressed than β2- or β3-adrenoceptor mRNA. (b) In VM, β1- and β2-adrenoceptor mRNA were detected at similar levels. (c) In LM specimens, β2-adrenoceptor expression (ADRB2) was higher than expression of β1- and β3-adrenoceptors. *P < 0.05, **P < 0.01, †P < 0.001. ADRB1, ADRB2, ADRB3, β1-, β2-, β3-adrenoceptor mRNA, respectively. IH, infantile hemangioma; LM, lymphatic malformation; VM, venous malformation.
Finally, we had the opportunity to study β-adrenoceptor mRNA expression in hemangioma-derived stem cells (9). Again, β1-adrenoceptor mRNA was the predominant subtype expressed in the hemangioma-derived stem cells ( Figure 4 ).
Discussion
In this study, we could demonstrate for the first time that β1-adrenoceptors represent the most abundant β-adrenoceptor subtype expressed in IH and in hemangioma-derived stem cells. By contrast, in LM, β2-adrenoceptors were the predominant subtype. Unfortunately, we could not study β-adrenoceptor expression on the cellular level in these specimens due to the lack of sufficiently specific antibodies and the condition of the material, which did not allow performance of in situ hybridization. Therefore, it is not clear whether the endothelium or other cellular components such as pericytes, lymphocytes, or fibroblasts in IH, VM, and LM, express the β-adrenoceptors. A recent study using a tissue microarray showed high expression of β2-adrenoceptors on the protein level in proliferating and involuting IH (10). Low receptor expression was noted in VMs. Unfortunately, β1-adrenoceptor expression was not investigated in that study, and therefore expression levels of receptor mRNA and protein cannot be directly compared with those of the current investigation. Furthermore, β1-adrenoceptors are abundant in the walls of arteries, and it is highly probable that in IH arteries could be present due to the abundant vascularization of the tumor. On the other hand, arteries are less abundant in VMs and LMs. In addition, it is not clear whether the β1-adrenoceptors found in IH are functional. These are all limitations of our study. However, the difference of mRNA levels is statistically significant and could therefore be relevant for the clinical behavior of the vascular anomalies. Of note, cells derived from hemangiomas (9) also expressed β1-adrenoceptors at 2.5-fold higher levels than β2-adrenoceptors, suggesting that the β-adrenoceptor subtype profile identified in IH tumor samples is also representative of the subtype profile in the hemangioma cells.
β-Adrenoceptors are members of the G protein–coupled receptor superfamily. Downstream activation of β-adrenoceptors leads to activation of guanine nucleotide–binding proteins causing dissociation of Gα-guanosine-5′-triphosphate and Gβγ subunits, thereby activating intracellular signaling pathways via adenylyl cyclase activation (11,12). Inhibition of this pathway by β-blockers has not been studied in detail with respect to inhibition of cell proliferation. Furthermore, data on inhibition of downstream signaling of β-adrenoceptors in endothelial cells from IH are not available and future studies using hemangioma-derived stem cells are warranted (9). However, models of the antiangiogenic effect of β-blockers by downregulation of angiogenesis factor expression or induction of endothelial cell apoptosis in IH have been postulated (3).
Our results demonstrate that IH, VM, and LM can be discriminated based on their difference in β-adrenoceptor subtype mRNA expression. Of note, until today only proliferating IHs have been reported to show growth inhibition by propranolol, a nonselective β-adrenoceptor antagonist. Interaction on the β1-adrenoceptor level could be a possible mechanism of action.
Methods
Hemangioma Cells
Hemangioma-derived stem cells were obtained from Joyce Bischoff (Harvard Medical School, Boston, MA). The derivation, sources, and culture conditions for the hemangioma-derived stem cells have been detailed previously (9).
Tissue
Tissue specimens from IHs (n = 9), VMs (n = 7), and LMs (n = 5) of patients who underwent surgery from 2004 to 2008 at the University Medical Hospital Freiburg were available in our tumor tissue bank. Informed consent of the families was provided, and the study was approved by the ethics committee of the University Medical Center of Freiburg. Table 1 summarizes the localization of the vascular anomaly, age at surgery, previous therapy, and growth stage.
Immunohistochemistry for Ki67, CD31, GLUT-1, and D2-40
Sections of 6 µm from formalin-fixed tissue material were provided to perform hematoxylin and eosin staining and immunohistochemistry. Antibodies against Ki67 (NeoMarkers, Fremont, CA, dilution 1:300), CD31 (NeoMarkers, dilution 1:200), GLUT-1 (Diagnostic BioSystems, Pleasanton, CA, dilution 1:50), and D2-40 (Covance, Emeryville, CA, dilution 1:100) were used following a standard protocol of the manufacturer of the automated immunohistochemistry system (A. Menarini Diagnostics, Berlin, Germany). Ki67 staining was defined as the percentage of positive nuclei from a total of 2,000 cells counted using an eyepiece grid. The positive nuclei were counted by one investigator (E.J.).
RNA Extraction, cDNA Synthesis, and β-Adrenoceptor Expression Analysis by qPCR
Gene expression analysis from the available tissues was performed by qPCR as described previously (13). In brief, RNA was isolated from snap-frozen tissue using the RNeasy Mini Kit (Qiagen, Hilden, Germany). Total RNA (1 µg per probe) was reverse-transcribed according to the manufacturer’s instructions (QuantiTect Rev. Transcription Kit; Qiagen). For qPCR, 30 μl of the amplification mixture (Quantitect SYBR Green kit; Qiagen) was used containing 20 ng of reverse-transcribed RNA and 300 nmol/l primers (MWG, Ebersberg, Germany) specific for the human β-adrenoceptor subtypes or the ribosomal protein RPS29. The cycling conditions were as follows: 15 s polymerase activation at 95 °C and 40 cycles at 95 °C for 15 s, at 58 °C for 30 s, and at 72 °C for 30 s. Results were normalized to RPS29 values. Primer sequences used for qPCR experiments are listed in Table 3 .
Statistics
Data are presented as means ± SEM of individual data points. Data were analyzed using one-way ANOVA followed by Bonferroni post hoc tests. A P value of <0.05 was considered statistically significant.
Statement of Financial Support
This study was supported by the Deutsche Forschungsgemeinschaft.
References
Léauté-Labrèze C, Dumas de la Roque E, Hubiche T, Boralevi F, Thambo JB, Taïeb A . Propranolol for severe hemangiomas of infancy. N Engl J Med 2008;358:2649–51.
Rössler J, Wehl G, Niemeyer CM . Evaluating systemic prednisone therapy for proliferating haemangioma in infancy. Eur J Pediatr 2008;167:813–5.
Storch CH, Hoeger PH . Propranolol for infantile haemangiomas: insights into the molecular mechanisms of action. Br J Dermatol 2010;163:269–74.
Annabi B, Lachambre MP, Plouffe K, Moumdjian R, Béliveau R . Propranolol adrenergic blockade inhibits human brain endothelial cells tubulogenesis and matrix metalloproteinase-9 secretion. Pharmacol Res 2009;60:438–45.
Hajighasemi F, Hajighasemi S . Effect of propranolol on angiogenic factors in human hematopoietic cell lines in vitro. Iran Biomed J 2009;13:223–8.
Frieden IJ, Haggstrom AN, Drolet BA, et al. Infantile hemangiomas: current knowledge, future directions. Proceedings of a research workshop on infantile hemangiomas, April 7–9, 2005, Bethesda, Maryland, USA. Pediatr Dermatol 2005;22:383–406.
North PE, Waner M, Mizeracki A, Mihm MC Jr . GLUT1: a newly discovered immunohistochemical marker for juvenile hemangiomas. Hum Pathol 2000;31:11–22.
Steven M, Kumaran N, Carachi R, Desai A, Bennet G . Haemangiomas and vascular malformations of the limb in children. Pediatr Surg Int 2007;23:565–9.
Greenberger S, Boscolo E, Adini I, Mulliken JB, Bischoff J . Corticosteroid suppression of VEGF-A in infantile hemangioma-derived stem cells. N Engl J Med 2010;362:1005–13.
Chisholm KM, Chang KW, Truong MT, Kwok S, West RB, Heerema-McKenney AE . β-Adrenergic receptor expression in vascular tumors. Mod Pathol 2012;25:1446–51.
DeGeorge BR Jr, Koch WJ . Beta blocker specificity: a building block toward personalized medicine. J Clin Invest 2007;117:86–9.
Takahata Y, Takarada T, Iemata M, et al. Functional expression of beta2 adrenergic receptors responsible for protection against oxidative stress through promotion of glutathione synthesis after Nrf2 upregulation in undifferentiated mesenchymal C3H10T1/2 stem cells. J Cell Physiol 2009;218:268–75.
Gilsbach R, Brede M, Beetz N, et al. Heterozygous alpha 2C-adrenoceptor-deficient mice develop heart failure after transverse aortic constriction. Cardiovasc Res 2007;75:728–37.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Rössler, J., Haubold, M., Gilsbach, R. et al. β1-Adrenoceptor mRNA level reveals distinctions between infantile hemangioma and vascular malformations. Pediatr Res 73, 409–413 (2013). https://doi.org/10.1038/pr.2013.16
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/pr.2013.16
This article is cited by
-
Vascular endothelial cell specification in health and disease
Angiogenesis (2021)
-
Signaling pathways in the development of infantile hemangioma
Journal of Hematology & Oncology (2014)
-
Medikamentöse Behandlungsansätze für infantile Hämangiome und lymphatische Malformationen
HNO (2014)