Abstract
Background:
Sepsis continues to be a leading cause of death in infants and children. Natural killer (NK) cells serve as a bridge between innate and adaptive immunity, yet their role in pediatric sepsis has not been well characterized.
Methods:
We tested the hypothesis that decreased NK cell cytotoxicity is a common feature of pediatric systemic inflammatory response syndrome (SIRS)/sepsis patients by measuring, using flow cytometry, NK cell cytotoxicity and cell surface phenotype in the peripheral blood of 38 pediatric intensive care patients who demonstrated signs and symptoms of SIRS and/or sepsis.
Results:
NK cell cytotoxicity was significantly reduced in peripheral blood mononuclear cells (PBMCs) of pediatric SIRS/sepsis patients as compared with healthy controls, and the percentage of CD56dim CD16+ cytotoxic NK cells in PBMCs was lower in patients with SIRS/sepsis than in normal donors. However, on a per cell basis, CD56dim CD16+ NK cells in patients mediated cytotoxicity as well as those in normal donors.
Conclusion:
The NK cell dysfunction in pediatric SIRS/sepsis patients reflects a quantitative rather than a qualitative difference from healthy controls.
Similar content being viewed by others
Main
Sepsis continues to be a leading cause of mortality in children, with >40,000 cases of severe sepsis annually in the United States and millions worldwide; half of the children with severe sepsis in the United States are infants (1). Despite extensive research, our understanding of the mechanisms of the pathophysiology of sepsis remains poor (2), and we understand the role of the innate immune system even less. One cell type, the natural killer (NK) cell, may serve as a bridge between the innate and adaptive immune system, yet its role in pediatric sepsis has not been well described.
NK cells are small, bone marrow–derived granular lymphocytes that have the “natural” ability to kill abnormal targets such as virally-infected host cells or tumor cells, without prior sensitization (3). NK cells are believed to be important for the control of chronic viral infections and to play a role in tumor surveillance through direct cytolysis and the secretion of cytokines. Newer data suggest that NK cells may also have an immunoregulatory role, and they may exhibit a form of acquired immune memory (4). By flow cytometry, using forward scatter and side scatter, human NK cells are found in the lymphocyte gate, are negative for CD3 (as opposed to T cells and NKT cells), and are positive for either CD56 (neural cell adhesion marker) or CD16 (low-affinity Fc-γ receptor III). Among NK cells, CD56bright CD16neg (cytokine-producing) NK cells are less cytotoxic and produce more cytokines, whereas CD56dim CD16+ (cytotoxic) NK cells are more cytotoxic and produce fewer cytokines (5). A highly dysfunctional population of NK cells (CD56neg CD16+) has been described in chronic viral infections, although the role of these cells is not yet clear (6,7,8).
Deficiencies in NK cell functions have been described in many disease processes such as trauma and burns (9), and rheumatologic conditions such as juvenile rheumatoid arthritis (JRA) and systemic JRA (10,11). There are also data suggesting that NK cytotoxicity is reduced in adult (12) and neonatal (13) sepsis, although no mechanism for the deficiency has been described, and no such data exist for the pediatric population. We, therefore, investigated the frequency of NK cell subsets and NK activity in the peripheral blood of pediatric systemic inflammatory response syndrome (SIRS)/sepsis patients using flow cytometry–based assays.
Results
Patient and Control Populations
We performed a prospective observational study of patients admitted to our pediatric intensive care unit (PICU) with signs and symptoms of SIRS or sepsis (14). In an attempt to enroll patients early in their disease course, and because the distinction between SIRS and sepsis is made only by the eventual identification of an infectious agent, we chose to enroll patients with symptoms of SIRS. NK cell number, cell surface phenotype, and cytotoxicity against NK-susceptible K562 target cells were serially determined (whenever feasible) for each patient (as often as twice weekly) while the patient remained in the PICU and was equipped with a vascular access catheter. A total of 38 pediatric SIRS/sepsis patients were consented and enrolled between June 2010 and June 2011. Twenty-six patients had only a single sample draw, whereas seven patients donated three or more samples. Only values (lytic units, percentages) from the initial blood draw were used for comparison with controls. As is typically the case in pediatric studies, pediatric control patients were difficult to recruit. Furthermore, finding relatively “healthy” pediatric control patients in the PICU setting was especially difficult. However, blood samples from two pediatric control patients who were admitted to the PICU without sepsis or multiple organ failure, but who had vascular access catheters, were included. In lieu of additional pediatric control patients, blood samples from 15 healthy adult control patients were collected, prepared, and analyzed concurrently with the patient samples.
Our cohort of patients was very heterogeneous and was a representative cross-section of the pediatric intensive care SIRS/sepsis population ( Table 1 ). An infectious agent was found in the majority of our patients (81.6%), and of these patients, bacterial infections were the most common type of pathogen, followed by viruses and fungi. The most common pathogen causing disease in our patients was respiratory syncytial virus, a common finding in PICUs. Two respiratory virus pathogens, respiratory syncytial virus and influenza B, were found in one patient (patient 83), hence the number discrepancy in Table 1 . Even though the Children’s Hospital of Pittsburgh PICU does admit and care for a large number of solid organ transplant patients, only two of these patients were enrolled in our study, and therefore they did not contribute significantly to our outcomes. Similar to other studies, children with chronic illness accounted for approximately half of our sample cohort (1). The majority of our patients had some degree of organ failure associated with their SIRS/sepsis event: 84.2% of our patients had severe sepsis, and 52.6% of our patients progressed to multiple organ dysfunction syndrome. Two of the patients (patients 41 and 90) did not survive their PICU admission: patient 41 died of fungus-associated pulmonary hemorrhage; and patient 90 had severe acute respiratory distress syndrome associated with respiratory syncytial virus and secondary infection with Staphylococcus aureus, and had an intracerebral hemorrhage on extracorporeal membrane oxygenation.
NK Cell Cytotoxicity
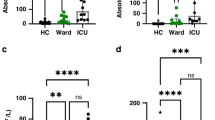
The spontaneous cytotoxicity of NK cells in peripheral blood mononuclear cells (PBMCs) was assayed using a 3-h flow cytometry-based assay ( Figure 1a , b ). NK cytotoxicity was reduced in PBMCs of virtually all of the pediatric SIRS/sepsis patients relative to controls. In several patients, the measured cytotoxicity was below the limit of detection (%cytotoxicity – %spontaneous death = negative number), and the lytic activities were assigned values of zero (0 lytic units). Initial NK cell cytotoxicity was significantly reduced in pediatric SIRS/sepsis patients as compared with healthy adult controls (20.7 ± 5.89 vs. 108 ± 25.1 LU20/107 PBMCs, P < 0.0001, Figure 1b ). Two healthy pediatric control patients had NK cell cytotoxicity that compared well with healthy adult controls (180 ± 68.1, Figure 1b ). Only two pediatric SIRS/sepsis patients (patients 65 and 79) had initial NK lytic activity (198 and 80.0 LU20/107 PBMCs, respectively) that compared to that of the healthy control patients. Patient 65 had SIRS from toxic shock syndrome and was only briefly admitted to the PICU, and patient 79 had severe sepsis from a gastric perforation. The rest of the pediatric SIRS/sepsis patients had markedly reduced lytic activity (<50 LU20/107 PBMCs).
Absolute natural killer (NK) cytotoxicity is reduced in pediatric systemic inflammatory response syndrome (SIRS)/sepsis patients. Carboxy-fluorescein succinimidyl ester (CFSE)-labeled K562 targets cells were incubated for 3 h at 37 °C with ficoll-separated PBMCs then stained with 7-AAD to quantify dead/dying CFSE-labeled K562 targets. Representative FACS plots of the flow cytometric cytotoxicity assay: (a) spontaneous cell death, (b) pediatric SIRS/sepsis patient, (c) healthy adult control. The numbers represent the frequency (%) of 7-AAD+ (dying) cells of CFSE-labeled K562 cells. The cytolytic activity of peripheral blood NK cells is expressed as lytic units per 107 PBMCs of pediatric SIRS/sepsis patients (solid circles), healthy pediatric controls (open circles), and healthy adult controls (open squares). (d) Statistical significance was determined by Wilcoxon’s rank-sum tests (*P < 0.0001). 7-AAD, 7-aminoactinomycin D; FACS, fluorescence-activated cell sorting; PBMC, peripheral blood mononuclear cell.
Frequency and Numbers of NK Cells in PBMCs
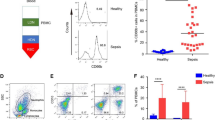
The low levels of NK cytotoxicity seen in the patients’ PBMCs could be due to poor cell function or to decreased numbers of NK cells present. NK cells are typically classified by surface expression of CD56 and/or CD16, with CD56dim CD16+ cells considered as being strongly cytotoxic and weak cytokine producers whereas CD56bright CD16neg NK cells are weakly cytotoxic but strong cytokine producers (5). Another subpopulation of NK cells, CD56neg CD16+, has also been described in viral disease processes such as hepatitis C, HIV, and hantavirus, and may be dysfunctional (15,16,17). Pediatric SIRS/sepsis patients did not have any significant change in the proportion of CD56bright CD16neg NK cells within total NK cells ( Figure 2a , b ). However, patients did seem to have a skewing of their CD16+ NK population in that they demonstrated a significant decrease in the proportion of CD56dim CD16+ cytotoxic NK cells (55.0 ± 3.52 vs. 74.8 ± 2.88%, pediatric SIRS/sepsis patients vs. healthy adult controls, P < 0.005) and an increase, although not significant (P < 0.06), in the proportion of CD56neg CD16+ NK population (33.7 ± 3.83 vs. 17.9 ± 3.05%, P < 0.06). The absolute number of CD56dim CD16+ NK cells was also significantly reduced (46.8 ± 10.8 vs. 71.0 ± 11.3 × 103 CD56dim CD16+ cells/ml, P < 0.05), whereas there was no increase in the absolute number of CD56neg CD16+ cells (16.1 ± 3.90 vs. 22.3 ± 7.76 × 103 CD56neg CD16+ cells/ml, Figure 2c ). Therefore, there was not so much a skewing of the CD16+ population as there was a loss of CD56dim CD16+ cells in the peripheral blood. Our healthy pediatric control patients had NK subpopulation frequencies and numbers that were similar to those of healthy adult controls ( Figure 2c ).
Natural killer (NK) cells from pediatric systemic inflammatory response syndrome (SIRS)/sepsis patients have a skewed cell surface phenotype. Representative FACS plots showing cell surface staining of NK cells for CD56 and CD16 on NK cells from (a) a pediatric SIRS/sepsis patient and (b) a healthy adult control patient where the numbers adjacent to the gates represent the frequency (%) of the different NK cell populations. NK cells were initially defined by gating on lymphocytes by forward scatter (FSC) and side scatter (SSC), then gating on CD3 negative lymphocytes. The frequency of the different NK cell populations: (c) CD56bright CD16neg, (d) CD56dim CD16+, and (e) CD56neg CD16+ of all NK cells in pediatric SIRS/sepsis patients (solid circles), healthy pediatric controls (open circles), and healthy adult controls (open squares). (d) Pediatric SIRS/sepsis patients (solid circles) have a decreased proportion of cytotoxic CD56dim CD16+ NK cells (55.0 ± 3.52 vs. 74.8 ± 2.88%, pediatric SIRS/sepsis patients vs. healthy adult controls, *P < 0.005) as compared with adult controls (open squares) and an (e) increased, but not significant, proportion of CD56neg CD16+ NK cells as compared to pediatric controls. (f) When analyzing the absolute numbers of NK cells in peripheral blood, only the number of cytotoxic CD56dim CD16+ NK cells (46.8 ± 10.8 vs. 71.0 ± 11.3 × 103 CD56dim CD16+ cells/ml, **P < 0.05) is reduced, (g) while the number of CD56neg CD16+ NK cells was not significantly different between groups. FACS, fluorescence-activated cell sorting.
The presumably dysfunctional population of CD56neg CD16+ cells has been infrequently described; therefore, we performed additional cell surface and intracellular staining to show they had properties of NK cells. The CD56neg CD16+ cells were found in the lymphocyte gate by forward scatter and side scatter, were negative for CD3 (T cells), CD14 (monocytes), and CD19 (B cells), and had comparable expression of NK cell markers, including CD2, CD7, CD11b, CD62L, NKG2A, NKp30, NKp46, and intracellular perforin, as the CD56bright CD16neg and CD56dim CD16+ NK populations. We could find no pattern of difference of NK activation receptor or killer inhibitory receptor expression between patients’ and control NK cells.
Quality of Cytotoxic NK Cells
We attempted to determine whether the reduced cytolytic activity in PBMCs of pediatric SIRS/sepsis patients was due to the quality of the NK cells or just the reduced frequency. Using flow cytometry without purifying NK cells, we were able to determine the frequency of cytotoxic CD56dim CD16+ NK cells in patients’ PBMCs. The percentage of these cytolytic NK cells was significantly reduced in pediatric SIRS/sepsis patients relative to healthy controls (4.25 ± 0.564 vs. 1.66 ± 0.342%, P < 0.001, Figure 3a ). When the NK cell cytotoxicity was adjusted to account only for the percentage of CD56dim CD16+ cytotoxic NK cells in PBMCs, no difference in cytotoxicity between the patients and the controls was evident: 32.6 ± 16.5 vs. 27.9 ± 8.29 LU20/105 CD56dim CD16+ NK cells ( Figure 3b ). These data are consistent with the conclusion that it is the low frequency of CD56dim CD16+ NK cells in the peripheral blood of pediatric SIRS/sepsis patients and not cellular dysfunction that is responsible for the observed low levels of cytolytic activity.
Cytotoxic NK cells from pediatric SIRS/sepsis patients are decreased in frequency but have normal cytotoxic function. (a) The frequency of cytotoxic CD56dim CD16+ NK cells of PBMCs is greatly reduced in pediatric SIRS/sepsis patients (solid circles) as compared with the adult controls (open squares, *P < 0.001). However, correcting the LU20/107 PBMCs with the frequency of cytotoxic CD56dim CD16+ NK cells in PBMCs gives cytotoxicity on a per cell basis with the units of lytic units per 105 cytotoxic CD56dim CD16+ NK cells (LU20/105 CD56dim CD16+ NK cells). (b) On a per cell basis, the cytotoxic NK cells from pediatric SIRS/sepsis patients (solid circles) had similar cytotoxicity as compared with the pediatric controls (open circles) or adult controls (open squares). PBMC, peripheral blood mononuclear cell; SIRS, systemic inflammatory response syndrome.
Discussion
Whereas NK cytotoxicity deficiencies have been reported in adult and neonatal sepsis, this is the first report to show that the same deficiency occurs in pediatric SIRS/sepsis patients, and furthermore, we found that the mechanism of the deficiency is not a qualitative one but a quantitative deficit of peripheral blood cytotoxic CD56dim CD16+ NK cells. Pediatric SIRS/sepsis can now be added to the list of disease processes that are characterized by low/absent NK activity as measured by the basic NK cytotoxicity assay. By measuring both the percentage of cytotoxic NK cells in each sample and its cytotoxicity, we were able to show that the cytotoxic deficiency in pediatric SIRS/sepsis patients is due to the low frequency of cytotoxic NK cells and presumably not to any cellular defects in cytotoxicity. Ideally, qualitative differences in cytotoxicity could have been measured by isolating NK cells prior to the cytotoxicity assay; however, this was not feasible in our study due to the low numbers of NK cells in pediatric SIRS/sepsis patients.
The quantitative cytotoxic deficiency seems to be in large part due to the reduced frequency of NK cells in the peripheral blood of pediatric SIRS/sepsis patients. Peripheral white blood cell counts in pediatric sepsis can either be increased or decreased, but there is generally an increase in absolute neutrophil counts with or without a left shift or bandemia. This neutrophilia is accompanied by stable or reduced absolute lymphocyte counts, which lead to a relative decreased percentage of peripherally circulating lymphocytes and therefore a decreased frequency of NK cells of PBMCs. Our results also raise the possibility that quantitative rather than qualitative deficiencies in NK cells may be at the heart of other disease processes, such as rheumatologic conditions, burns, and trauma, in which NK activity has been shown to be low/absent. Further studies are warranted to address this possibility.
Within the NK cell population, we showed a significant reduction in the proportion and absolute number of cytotoxic CD56dim CD16+ NK cells. There could be several explanations for this observation: (i) the cytotoxic NK cells may have migrated out of peripheral blood to the site of infection or to lymph nodes, (ii) the cytotoxic NK cells may be undergoing apoptosis at an increased rate, and (iii) the cytotoxic NK cells may be being skewed toward a dysfunctional, highly activated CD56neg CD16+ phenotype as this temporal phenomenon has been described in patients with HIV (7). The significance of the increased proportion of the dysfunctional CD56neg CD16+ NK cell population is unclear and may simply reflect a stable population of CD56neg CD16+ NK cells (numerator) being divided by a smaller denominator because of the loss of cytotoxic CD56dim CD16+ NK cells. In our patients, generally, the frequency of this dysfunctional NK subset was highest early in the PICU admission/study and gradually declined over time (data not shown). However, a few patients had steady or increasing frequency of the dysfunctional NK subset over time, and several patients had extremely high percentages (>50% of NK cells) of the dysfunctional CD56neg CD16+ subset, although the patients did not share any other laboratory or clinical findings. Further research is needed to elucidate the nature of this NK subset population.
We chose to examine the final product of NK cytotoxicity, target cell death, as the marker of NK cell function. NK cellular cytotoxicity is a granule-dependent exocytic process. Exocytosis is a fundamental cellular process that is composed of many steps, including polarization, docking, priming, and fusion. Through granule-dependent exocytosis, NK cells are able to deliver its various cytotoxic proteins, including perforin, granzymes, granulysin and other lysosomal enzymes, to the target cell. Ultimately, defects in any of the steps, or absence of key proteins, can lead to dysfunctional cellular cytotoxicity (18) and can result in familial hemophagocytic lymphohistiocytosis (FHL) or other syndromes in which hemophagocytic lymphohistiocytosis can be seen. To date, the underlying genetic defect of FHL has been described for four loci, whereas other syndromes have also been shown to have defects of the exocytosis process. Lysosomal trafficking regulator, whose defect causes Chediak-Higashi syndrome, and adaptor protein 3 are involved in polarization: the transport of proteins from the Golgi to cytotoxic granules. Other proteins such as Rab27a (Griscelli syndrome type 2), syntaxin 11 (FHL4), Munc13-4 (FHL3), and Munc18-2 (FHL5) are involved in the regulation of granule docking and membrane fusion and can be identified by varying degrees of defective CD107a (LAMP-1) staining, also known as the degranulation assay, which estimates cellular cytotoxicity. Whereas the exact mechanism of perforin is controversial, the absence of intracytoplasmic staining of perforin (PRF1 mutations) can identify patients with FHL2 (19). Functional assays, including CD107a mobilization, intracellular cytokine production, and intracytoplasmic perforin staining, can be combined to help differentiate the subtypes of FHL (20,21).
There were several limitations of our study. Many of our patients had NK activity that was below the limit of detection of our assay (the measure cytotoxicity was lower than the spontaneous death of target cells). This is not a limitation of the flow cytometric assay as compared with the 51Cr-release assay. In fact, it is our experience that the flow cytometric assay has a better dynamic range than the 51Cr-release assay. Another limitation of our study is the low number of pediatric control patients. Whereas we had hoped to enroll more pediatric control patients, we did not wish to contaminate our control population with patients who had recently been stressed, such as postoperative patients and patients in the recovery phase after illness. Given that our healthy pediatric control NK activities were comparable with those of healthy adult controls, we are confident in our data. Other studies have used historic controls for their comparison groups. We feel that this is not appropriate because the NK activity assay, being a functional cellular assay, is highly dependent on the health of the target cells, which can change over time, and therefore concurrent measurement of controls and samples is necessary.
Methods
Patients
Pediatric patients were eligible for enrollment when the following criteria were fulfilled: (i) admission to the PICU, (ii) presence of an indwelling catheter for sample collection, and (iii) presence of clinical signs and symptoms of sepsis and/or SIRS (14). The study was approved by the University of Pittsburgh Institutional Review Board (IRB no. PRO09060070), and blood samples were collected during the acute phase of the syndrome after obtaining informed consent from parents or guardians.
Sample Preparation
Blood samples from pediatric SIRS/sepsis patients were collected in sodium heparin tubes. PBMCs were separated by density centrifugation (Ficoll-Paque Plus, density 1.077 g/ml; GE Healthcare Bio-Sciences AB, Uppsala, Sweden). Cells were resuspended in Aim-V medium (Gibco Products, Grand Island, NY). All cell counts were performed using a Z1 Coulter Particle Counter (Beckman Coulter, Fullerton, CA) after red blood cell lysis with Zap-O-Globin II Lytic Reagent (Beckman Coulter).
Control Subjects
Blood samples from healthy adult controls were similarly collected in sodium heparin tubes, PBMCs were separated by density centrifugation, and samples were analyzed in parallel with samples from pediatric SIRS/sepsis patients. To control for age variability, we were able to enroll two pediatric control patients (2-mo-old female, 7-y-old male) who fulfilled criteria i and ii above but did not exhibit any signs or symptoms of SIRS/sepsis.
Cell Surface Flow Cytometric Analysis
PBMCs were surface stained with the following antibody fluorochrome combinations: CD16-fluorescein isothiocyanate, CD3-phycoerythrin/Texas red (ECD), and CD56-phycoerythrin/Cy5 (PE-Cy5). The FL2 channel, using the phycoerythrin fluorochrome, was used for additional analysis of multiple cell surface markers, including CD2, CD7, CD14, CD19, NKG2A, killer inhibitory receptors, NKG2D, NKp30, NKp46, and CD94. Cells were stained and washed in phosphate-buffered saline with 3% heat-inactivated fetal bovine serum and 0.01% sodium azide. Cell staining was performed at 4 °C for 20 min with a volume of 20 μl per stain. Cells were washed once and fixed with 1% paraformaldehyde. All flow cytometry data were acquired on a 4-color Epics XL (Beckman Coulter) flow cytometer. For cell surface phenotype determination, a minimum of 100,000 events were acquired and analyzed using FlowJo 9.3.1 software (TreeStar, Mountain View, CA).
Intracellular Staining
For analysis of intracellular perforin, unstimulated cells were first surface stained as described above and then the cells were fixed and permeabilized using eBioscience Fixation & Permeabilization buffers (eBioscience, San Diego, CA). Cells were stained for intracellular perforin with anti–perforin-phycoerythrin (Beckman Coulter) for 15 min at 4 °C. Cells were washed using Permeabilization buffer and fixed with 1% (wt/vol) paraformaldehyde in phosphate-buffered saline. For intracellular phenotype determination, a minimum of 100,000 events were acquired.
NK Cytotoxicity Assay
NK cell cytotoxicity was performed as previously described (22), with subtle modifications, in the Clinical Laboratory Improvement Amendments–certified University of Pittsburgh Cancer Institute Immunologic Monitoring and Cellular Products Laboratory. Whereas the laboratory performs the 51Cr-release cytotoxicity assay, we chose to use the fluorochrome-based flow cytometry cytotoxicity assay instead because we have found it to be more sensitive and safer because no radiation is used. The modified flow cytometry cytotoxicity assay was validated against the 51Cr-release assay prior to the enrollment of pediatric patients. In brief, target cells (K562 cells) were washed with serum-free phosphate-buffered saline and then stained with carboxy-fluorescein succinimidyl ester (CFSE) at a concentration of 2 μmol/l for 5 min. The reaction was stopped by the addition of 1 ml of heat-inactivated fetal bovine serum. The cells were washed with phosphate-buffered saline and resuspended in AIM-V media (Invitrogen, Carlsbad, CA) to give a concentration of 0.5 × 106 cells/ml. Target cells (5 × 105 cells) were mixed with varying numbers of PBMCs to give varying effector to target (E:T) ratios in 12 × 75 mm polypropylene round-bottom tubes for 3 h at 37 °C. At the end of the incubation period, 7-aminoactinomycin D (7-AAD; Invitrogen) was added to the tubes at a final concentration of 1 μg/ml. Events (minimum 5,000 events) were acquired on a 4-color Epics XL flow cytometer and analyzed using FlowJo software (TreeStar). Percentage of target cell death equaled the percentage of 7AAD+/CFSE+ cells of total CFSE+ cells ( Figure 1a ). Spontaneous K562 cell death as measured by 7-AAD positivity was typically 3–6%.
% Cytotoxicity (of a given E:T ratio) = % target cell death (of a given E:T ratio) – % spontaneous cell death.
Because often the percentage lysis and E:T ratio relationship is not linear (lysis often reaches a maximum at high E:T ratios) (23), we used a computer program (David Coggin, 1991) that fits cytotoxicity data to the exponential curve:

In this equation, A equals 1 (curve maximum, maximum cell death being 100%), k is the slope of the exponential equation, y is 0.2 (20% target cell lysis), and x is the number of PBMCs. The resulting lytic unit (LU20/107 PBMCs) is the reciprocal of the number of PBMCs required to cause 20% lysis of 50,000 K562 target cells. However, because the definition of a lytic unit is based on the number of effectors needed for death of 20% target cells in 51Cr-release assays, and because the flow cytometry-based assay uses 10-fold more targets than the 51Cr-release assay (50,000 vs. 5,000 K562 cells), a 10-fold correction factor has been added so that the results of the flow assay are comparable with those of the 51Cr-release assay. The lytic unit calculation was taken one step further to account for the percentage of cytotoxic (CD56dim CD16+) NK cells present in PBMCs: the lytic unit value (LU20/107 PBMCs) was divided by the frequency (percentage) of CD56dim CD16+ of PBMCs to calculate the LU20/105 CD56dim CD16+ NK cells.
Statistics
All cytotoxicity data in lytic units were expressed as mean values ± SEM. Results were compared using the Wilcoxon’s rank-sum test because the data were not normally distributed. All statistical analyses were performed using JMP 8.0 for Mac OS X software (SAS Institute, Cary, NC). Results were considered significant when P < 0.05.
Statement of Financial Support
This work was supported in part by a departmental seed grant from the Department of Critical Care Medicine, University of Pittsburgh School of Medicine, Pittsburgh, PA.
References
Watson RS, Carcillo JA . Scope and epidemiology of pediatric sepsis. Pediatr Crit Care Med 2005;6:Suppl 3:S3–5.
Rittirsch D, Flierl MA, Ward PA . Harmful molecular mechanisms in sepsis. Nat Rev Immunol 2008;8:776–87.
Kiessling R, Klein E, Wigzell H . “Natural” killer cells in the mouse. I. Cytotoxic cells with specificity for mouse Moloney leukemia cells. Specificity and distribution according to genotype. Eur J Immunol 1975;5:112–7.
Paust S, von Andrian UH . Natural killer cell memory. Nat Immunol 2011;12:500–8.
Cooper MA, Fehniger TA, Caligiuri MA . The biology of human natural killer-cell subsets. Trends Immunol 2001;22:633–40.
Mavilio D, Lombardo G, Benjamin J, et al. Characterization of CD56-/CD16+ natural killer (NK) cells: a highly dysfunctional NK subset expanded in HIV-infected viremic individuals. Proc Natl Acad Sci USA 2005;102:2886–91.
Alter G, Teigen N, Davis BT, et al. Sequential deregulation of NK cell subset distribution and function starting in acute HIV-1 infection. Blood 2005;106:3366–9.
Björkström NK, Ljunggren HG, Sandberg JK . CD56 negative NK cells: origin, function, and role in chronic viral disease. Trends Immunol 2010;31:401–6.
Blazar BA, Rodrick ML, O’Mahony JB, et al. Suppression of natural killer-cell function in humans following thermal and traumatic injury. J Clin Immunol 1986;6:26–36.
Villanueva J, Lee S, Giannini EH, et al. Natural killer cell dysfunction is a distinguishing feature of systemic onset juvenile rheumatoid arthritis and macrophage activation syndrome. Arthritis Res Ther 2005;7:R30–7.
Grom AA, Villanueva J, Lee S, Goldmuntz EA, Passo MH, Filipovich A . Natural killer cell dysfunction in patients with systemic-onset juvenile rheumatoid arthritis and macrophage activation syndrome. J Pediatr 2003;142:292–6.
von Muller L, Klemm A, Durmus N, et al. Cellular immunity and active human cytomegalovirus infection in patients with septic shock. J Infect Dis 2007;196:1288–95.
el-Sameea ER, Metwally SS, Mashhour E, et al. Evaluation of natural killer cells as diagnostic markers of early onset neonatal sepsis: comparison with C-reactive protein and interleukin-8. Egypt J Immunol 2004;11:91–102.
Goldstein B, Giroir B, Randolph A ; International Consensus Conference on Pediatric Sepsis. International pediatric sepsis consensus conference: definitions for sepsis and organ dysfunction in pediatrics. Pediatr Crit Care Med 2005;6:2–8.
Gonzalez VD, Falconer K, Björkström NK, et al. Expansion of functionally skewed CD56-negative NK cells in chronic hepatitis C virus infection: correlation with outcome of pegylated IFN-alpha and ribavirin treatment. J Immunol 2009;183:6612–8.
Reeves RK, Gillis J, Wong FE, Yu Y, Connole M, Johnson RP . CD16- natural killer cells: enrichment in mucosal and secondary lymphoid tissues and altered function during chronic SIV infection. Blood 2010;115:4439–46.
Björkström NK, Lindgren T, Stoltz M, et al. Rapid expansion and long-term persistence of elevated NK cell numbers in humans infected with hantavirus. J Exp Med 2011;208:13–21.
Sieni E, Cetica V, Mastrodicasa E, et al. Familial hemophagocytic lymphohistiocytosis: a model for understanding the human machinery of cellular cytotoxicity. Cell Mol Life Sci 2012;69:29–40.
Stepp SE, Dufourcq-Lagelouse R, Le Deist F, et al. Perforin gene defects in familial hemophagocytic lymphohistiocytosis. Science 1999;286:1957–9.
Aricò M, Allen M, Brusa S, et al. Haemophagocytic lymphohistiocytosis: proposal of a diagnostic algorithm based on perforin expression. Br J Haematol 2002;119:180–8.
Marcenaro S, Gallo F, Martini S, et al. Analysis of natural killer-cell function in familial hemophagocytic lymphohistiocytosis (FHL): defective CD107a surface expression heralds Munc13-4 defect and discriminates between genetic subtypes of the disease. Blood 2006;108:2316–23.
Kim GG, Donnenberg VS, Donnenberg AD, Gooding W, Whiteside TL . A novel multiparametric flow cytometry-based cytotoxicity assay simultaneously immunophenotypes effector cells: comparisons to a 4 h 51Cr-release assay. J Immunol Methods 2007;325:51–66.
Bryant J, Day R, Whiteside TL, Herberman RB . Calculation of lytic units for the expression of cell-mediated cytotoxicity. J Immunol Methods 1992;146:91–103.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Halstead, E., Carcillo, J., Schilling, B. et al. Reduced frequency of CD56dim CD16pos natural killer cells in pediatric systemic inflammatory response syndrome/sepsis patients. Pediatr Res 74, 427–432 (2013). https://doi.org/10.1038/pr.2013.121
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/pr.2013.121
This article is cited by
-
Sepsis with liver dysfunction and coagulopathy predicts an inflammatory pattern of macrophage activation
Intensive Care Medicine Experimental (2022)
-
Pathological alteration and therapeutic implications of sepsis-induced immune cell apoptosis
Cell Death & Disease (2019)
-
Occurrence of marked sepsis-induced immunosuppression in pediatric septic shock: a pilot study
Annals of Intensive Care (2018)