Abstract
Introduction:
Iron-deficiency anemia (IDA) is recognized to have long-lasting effects on neurodevelopment, but there is little research on neuroendocrine systems.
Methods:
This study examined the effects of IDA in early or later infancy on plasma cortisol and prolactin stress–response patterns for 1 h after a venipuncture and catheter placement in 10-y-old healthy Chilean children. Children identified with IDA at 6 mo (IDA-6; n = 13) or 12 mo (IDA-12; n = 24) and who were iron sufficient (IS) at other infancy time points were compared to children who were IS at all time points during infancy (n = 23). All children received at least 6 mo of oral iron treatment in infancy.
Results:
At 10 y of age, IDA-6 and IDA-12 children demonstrated altered cortisol response patterns; both showed a more immediate decline and IDA-12 children showed a blunted curvature as compared to IS children. IDA-12 children showed significantly lower cortisol levels at 30 and 45 min after venipuncture and catheter placement than did IS children. There were no significant differences for stress–responsive plasma prolactin patterns between groups.
Discusion:
The results indicate that having IDA during infancy is associated with long-term neuroendocrine effects on stress–responsive cortisol patterns.
Similar content being viewed by others
Main
Iron is required for many central nervous system (CNS) processes (1). In recent decades, there has been an upsurge in research on the CNS effects of iron deficiency (ID), a common nutritional disorder that affects billions of people worldwide. Several CNS processes that are altered in ID are involved in neuroendocrine functioning (2), but few ID studies have considered neuroendocrine effects. Studies in the 1980s assessed prolactin because of the link of iron-deficiency anemia (IDA) to altered brain dopamine systems and because prolactin is under inhibitory dopaminergic control (3,4,5). Serum prolactin concentrations and hepatic prolactin receptors were elevated in iron-deficient rats (6,7). We previously explored serum prolactin in a relatively small sample of 12- to 23-mo-old Costa Rican infants who participated in a study of ID and infant development. Serum prolactin levels did not differ by iron status, but the behavioral profile typical of infants with IDA (wary and hesitant) was associated with elevated serum prolactin (8). Serum prolactin was measured again in the same children in early adolescence and those who had chronic ID in infancy showed an altered serum prolactin response pattern, suggesting long-term effects on this neuroendocrine system (9).
More than two decades ago, researchers also investigated the hypothalamic–pituitary–adrenal (HPA) axis and IDA in rats. Rat pups raised on an iron-deficient diet from birth had elevated basal corticosterone levels at 29–30 d of life and a lower incremental increase in corticosterone in response to ether-exposure stress, as compared with iron-sufficient pups (10). After a month of dietary iron treatment, basal corticosterone did not differ between previously IDA and control rats; however, behavioral differences persisted on mildly stressful tasks between these groups (11,12). In another study, prepubertal rats made iron deficient after weaning showed increased urinary norepinephrine at baseline and after surgical stress (13). Norepinephrine stimulates adrenocorticotropin production from the anterior pituitary, which in turn stimulates cortisol production, the end product of HPA axis activation. However, in the same postweaning IDA model, young adult rats with IDA did not show basal corticosterone differences. In addition, mild or severe stress was associated with only modest corticosterone changes in rats with IDA (14). Ultrastructural and cytochemical changes in adrenal cortex have also been observed in rats with IDA (15). Overall, in studies of IDA and the HPA system in rodents, the findings have been mixed.
There is little available research on IDA and cortisol responses in humans or nonhuman primates. In a preliminary report, children who were treated for IDA during infancy showed lower morning cortisol levels (16). Another study showed adults with IDA had blunted cortisol responses to adrenocorticotropin stimulation (17). In a monkey study, infants born to mothers that were randomly assigned to prenatal iron deprivation showed elevated cortisol levels in response to novel contexts at 4 mo of age. The 4-mo age in monkeys is roughly equivalent to older infancy/toddlerhood in humans (18). At no time were the monkey mothers or infants anemic in this study, indicating that the effects of iron deprivation occurred in the absence of anemia. Thus, there is evidence of effects of ID and IDA on the HPA system in human and nonhuman primates, but the few available studies involved different age periods and conditions.
To clarify the relationship between IDA in infancy and neuroendocrine responses to stress later in life, we assessed stress–responsive plasma cortisol and prolactin concentrations in 10-y-old Chilean children who had been recruited in infancy and then followed for iron status and behavior. Using venipuncture and catheter placement, the same procedure as in our Costa Rica study, we collected sequential blood samples over 1 h in order to evaluate neuroendocrine responses to the stress of venipuncture. Based on our previous results, we predicted that children who had IDA as infants would show a faster decline in plasma prolactin concentration. We did not have specific predictions about cortisol changes because of mixed findings in the studies available on the HPA axis and IDA. The Chile study was also designed to consider whether the age at which IDA occurred in infancy affected outcomes.
Results
The characteristics of the 60 children (IDA-6 n = 13; IDA-12 n = 24; iron sufficient (IS) n = 23) included in this study are shown in Table 1 . At the time of this neuroendocrine assessment, children in the IDA-6 group were significantly younger than the comparison group of IS children, but the 0.1-y difference is equivalent to about 5 wk and not clinically meaningful at this age. There was also a lower percentage of boys in the IS group, but the percentage gender by group did not differ significantly. Socioeconomic status of the children in this study did not differ at enrollment in infancy or at the time of this follow-up by iron status group ( Table 1 ). Tables 2 3 4 show the hematology for the study children in infancy, and at 5 and 10 y of age, respectively, by iron status group. IDA in infancy responded well to iron therapy ( Table 2 ), and the majority of children maintained good iron status subsequently. Of the samples available at 5 y, only three children had ID ( Table 3 ). At 10 y, three children had ID ( Table 4 ). Removing the ID children from analysis did not change the results as outlined in the following.
Plasma Prolactin
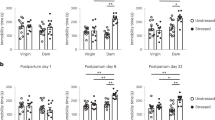
Over 1 h after venipuncture and catheter placement, there were no significant differences for plasma prolactin concentration or response pattern by iron status in infancy, whether unadjusted or adjusted for age, puberty, and gender. The plasma prolactin response patterns are shown in Figure 1a for each iron status group.
Neuroendocrine response to venipuncture by iron status in infancy. The hierarchical modeling curves for plasma prolactin and cortisol response were determined using samples obtained at 0, 15, 30, 45, and 60 min after venipuncture and catheter placement. (a) Plasma prolactin. There were no significant differences for intercept, initial slope, or curvature by iron status group. (b) Plasma cortisol. The curves for plasma cortisol response show significantly reduced initial slope for IDA-6 (−27.2) and IDA-12 (−79.1) as compared with IS (80.3), P = 0.05 and P < 0.001, respectively. The curvature of the response pattern was significantly blunted for IDA-12 as compared with IS (41.1 vs. −88.3, P < 0.001). The IDA-6 response pattern did not differ significantly from that of IDA-12 or IS. IDA-6 (long dashed line): infants with iron-deficiency anemia identified at 6 mo and iron sufficient at other times of measurement in infancy (n = 13). IDA-12 (solid line): infants with iron-deficiency anemia status at 12 mo and iron sufficient at other times of measurement in infancy (n = 24). IS (short dashed line): randomly selected iron sufficient infants (n = 23). IDA, iron-deficiency anemia.
Plasma Cortisol
There were no significant differences for plasma cortisol level at venipuncture and catheter placement (time 0). However, the IDA-12 group had significantly lower cortisol levels at 30 and 45 min after venipuncture than did the IS group (mean µg/dl ± SE); 30 min: IDA-12 43.4 ± 13.5, IS 97.8 ± 13.3; 45 min: IDA-12 36.4 ± 12.7, IS 90.1 ± 12.5. Children in the IDA-6 group did not differ significantly from the IS or IDA-12 groups at any time point. The plasma cortisol response patterns are shown in Figure 1b . The initial slope or rise was significantly greater for the IS (β = 80.3) group than for either the IDA-6 (β = −27.2) or IDA-12 (β = −79.1) groups, P = 0.05 and P < 0.001, respectively. The curvature of the response pattern was blunted for the IDA-12 (β = 41.1) group as compared with the IS group (β = −88.6), P < 0.001. The curvature of the IDA-6 group response pattern did not differ significantly from those of the IS or IDA-12 groups.
Discussion
This study demonstrates that IDA in infancy is associated with long-lasting differences in the HPA neuroendocrine system. As compared to children who were IS during infancy, children who had IDA at either 6 or 12 mo lacked the typical rise of cortisol after an acute stress of venipuncture and catheter placement at 10 y of age. In addition, children who had IDA identified at 12 mo had a blunted cortisol response pattern, and plasma cortisol concentrations, at 30 and 45 min after venipuncture and catheter placement compared with those who were IS in infancy. Thus, years later, IDA during infancy appears to alter the initial cortisol response to stress, and for those who had IDA at 12 mo, IDA resulted in a blunting of the typical stress–responsive pattern.
The HPA axis is the major stress–response system in humans, and cortisol responses are affected by several factors including the type of stress, the context the individual is experiencing around the time of measurement, and the individual’s previous experiences, e.g., during the development of the HPA axis (19,20). In this study, the type of stress was standardized to venipuncture and catheter placement. The context was standardized to a process of a brief physical exam and being prepared for a polysomnographic sleep study, a protocol that was familiar to the children without affecting the individual bedtime routines. The last aspect is relevant because sleep restriction/deprivation is a modifier of plasma cortisol concentration values (21). Cortisol also has diurnal or circadian modulation of the HPA axis; time of day influences plasma cortisol concentration, which, in turn, affects cortisol responses to stress. Cortisol levels are typically highest during the last part of nighttime sleep and 30 min of awakening, and after an initial rapid decline, levels decrease more gradually during the afternoon, reaching a nadir by the late evening, typically at waking–sleep transition and sleep onset (20,22,23). The extent of rise of cortisol after a stress is constrained when the cortisol levels are already elevated, as in the early morning (24). In this study, the neuroendocrine measurements were taken at least 1 h before the expected individual sleep onset.
The first blood sample in this study was obtained at the nadir of the cortisol circadian pattern and showed no differences by infancy iron status. This suggests the evening nadir of the diurnal HPA system was not significantly altered by infant IDA; however, another study assessed IDA and diurnal HPA cortisol levels and is important to consider. The study by Yehuda et al. demonstrated lower morning salivary cortisol levels in 10-y-old children who had IDA during infancy (16). This suggested a blunting of the normal morning rise of the diurnal cortisol pattern. Although there are important design differences between the Yehuda study and our study (time of day of assessment, sample type, and collection method (venipuncture vs. salivary collection), among others), the possibility that IDA during early life has a long-lasting effect on the diurnal circadian cortisol system deserves further investigation.
Differences in the stress–responsive cortisol system were demonstrated for study children who had IDA at 12 mo: lower plasma cortisol concentrations and a blunted response pattern after a stress as compared with children who were IS in infancy. Flattening of the cortisol stress–responsive and circadian patterns has been described in animal models and in humans. Termed hypocortisolism, such alterations of HPA functioning have been linked to adverse or challenging experiences early in life (20,25). Investigations in animal models suggest there are sensitive periods during the development of the HPA axis whereby stress and high cortisol (corticosterone) exposure are associated with reduced amplitude of the circadian modulation (e.g., lower morning levels) and blunted cortisol responses to stress later in life. This blunted cortisol response pattern for infants who had IDA at 12 mo is consistent with some of the previous findings in rodent studies (10,11). The mechanisms that underlie the blunted stress–responsive cortisol pattern long-term are unknown. However, like other challenges early in life, this nutritional deficiency may result in long-lasting, key alterations in HPA functioning.
In this study, we assessed the timing of IDA and used strict criteria that limited the possibility of ID without anemia at other time points. This was because of the finding from a previous study involving monkeys that iron deficiency without anemia was associated with altered cortisol responses (18). We found that children who had IDA between 6 and 12 mo appeared to have an intermediate cortisol response, lower than the IS group but not significantly different from either the IS or IDA-12 group. Unfortunately, a limitation of assessing discrete IDA time periods is that it likely limited our power to determine other smaller but true differences in cortisol level and response patterns.
Plasma prolactin concentration and response patterns after venipuncture and catheter placement at age 10 y did not differ by iron status in infancy. These results differed from our previous finding in Costa Rica and our predictions for this study. In the Costa Rica study, chronic ID in infancy was associated with a blunted serum prolactin response pattern after venipuncture and catheter placement in early adolescence (9). There are several important differences that may explain the discrepant findings between studies. The children in the Costa Rica study likely experienced a more chronic period of ID/IDA because they were identified later in infancy (at 17 mo, on average), and iron status before identification was unknown. In contrast, the children described in this study could not have had IDA in infancy for more than a few months because their iron status was measured at 6, 12, and 18 mo, and they received prompt iron treatment upon detection of IDA. In addition, the adolescents in the Costa Rica study were, on average, 2 y older than the children in this study and many were pubertal; the majority in this study were not. Furthermore, the time of day of assessment in the Costa Rica study was morning contrary to the evening measurements in this study. Plasma prolactin also has a circadian pattern, with higher levels during sleep. Thus, the differences in study findings could reflect the timing, duration, and/or severity of IDA in infancy, developmental stage at follow-up, and/or time of day of assessment. Finally, as plasma prolactin concentration and rapid-eye-movement sleep regulation are closely related (26), and former subjects with IDA showed altered rapid-eye-movement sleep patterns (27), differences in prolactin concentration between groups could become apparent during sleep. Given the strong theoretical basis for expecting an effect of early IDA on the dopaminergic regulation of the prolactin system, further work is needed (4,28).
This study is limited by the relatively small number of subjects and a lower percentage of males in one group; however, our aim was to examine the effect of time of IDA during infancy on neuroendocrine measures. By design, we included only those children who had IDA at one time point in infancy and were otherwise IS, or for the IS group, only those children who were IS throughout infancy. A second limitation is that we measured only the end products, cortisol and prolactin, of two neuroendocrine systems. Future studies should consider measuring adrenocorticotropin at the time of blood collection to help understand potential mechanisms underlying the differences in response pattern observed (e.g., hypothalamic or adrenal). Measuring salivary cortisol in the time period before, at, and after catheter placement might also help determine whether those children who had IDA in infancy had anticipatory cortisol responses to the procedures, which might have obscured initial differences. Other limitations of this study include a lack of data about specific genetic differences for the study children, dietary factors over the course of childhood, and that we could not control changes in iron status during the periods between 12 mo and 5 and 10 y. Taken together, these limitations reduce the stringency of our conclusions. There is no clear explanation of why neuroendocrine alterations associated with early IDA should be that long-lasting, and we cannot rule out that these uncontrolled confounders or some other unidentified factor(s) may account for the association. Still, we suggest that these neuroendocrine alterations may relate to persisting modifications of the brain processes in which iron is involved.
In summary, although long-term effects of IDA in infancy have been shown for several aspects of neurodevelopment across species, this study demonstrated that IDA in infancy also affects the HPA neuroendocrine system later in life. IDA around a year of age blunted serum cortisol response patterns to a stress at age 10 y. IDA in infancy was not associated with differences in the prolactin stress–responsive system. However, findings from previous studies and the theoretical basis for IDA effects suggest that further evaluation of this system is warranted.
Methods
Subjects
Children who had IDA at 6, 12, or 18 mo of age and children who had been nonanemic in infancy were invited to participate in a neuromaturation follow-up at 10 y of age. The subjects were participants in an ongoing research project on sensory, motor, social-emotional, cognitive, and neuromaturation effects of IDA in infancy. Detailed descriptions of the subjects and findings during infancy and preschool age have been published (27,29,30,31). Briefly, study participants were healthy, full-term Chilean infants (birth weight ≥ 3 kg, no perinatal complications, and no acute or chronic illnesses). Anemia was defined as venous hemoglobin (Hb) ≤ 100 g/I at 6 mo and < 110 g/I at 12 or 18 mo (32). ID was defined as two or more of three iron measures in the deficient range (mean cell volume (MCV) < 70 fl, erythrocyte protoporphyrin (FEP) ≥ 100 µg/dl red blood cells (1.77 μmol/l), serum ferritin <12 µg/I). For each infant with IDA identified at 6, 12, or 18 mo, an infant of the same age who was nonanemic (venous Hb >115 g/I) was randomly selected. Six-mo-old infants were treated for 1 y with 15 mg/d of elemental iron as oral ferrous sulfate; infants identified at 12 or 18 mo were treated with oral iron (30 mg/d) for a minimum of 6 mo. To reduce the chances that infants from the nonanemic group became iron deficient, they underwent the same iron treatment.
For the 10-y follow-up, we attempted to contact the 269 children from the larger study who had neurophysiology studies in infancy or the preschool period. Thirty-one children did not participate in the follow-up: 19 had moved, 8 declined participation or had a scheduling conflict, 3 could not be located, and 1 was disqualified because of health problems. Participating children and their families were free to agree to some but not all parts of the follow-up. Therefore, of the families contacted, 114 agreed to sequential blood samples following venipuncture and catheter placement. There were no statistically significant differences in background characteristics among former IDA children who had catheter placement for neuroendocrine assessments and those who participated only in other aspects of the follow-up study. Comparing the children who had been nonanemic in infancy and had the neuroendocrine assessments at 10 y of age with those who did not, those with neuroendocrine studies had been born to older mothers (28.5 vs. 25.0 y, P = 0.01), and a higher proportion lived in father-absent households at follow-up (31% vs. 13%, P = .04). Socioeconomic status evaluation was available as previously described for infancy and the 10-y follow-up (32,33).
The original infant study and follow-up protocols were approved and reviewed annually by the institutional review boards of the University of Michigan Medical Center, Ann Arbor, MI, and Institute of Nutrition and Food Technology, University of Chile, Santiago. Parents provided signed informed consent, and children provided assent beginning at 10 y of age.
Procedures
Children and their mothers were transported to the laboratory for an overnight sleep study. On arrival in the early evening, they became familiar with personnel and setting; individuals’ bedtime routines were maintained. Blood was sampled at venipuncture and catheter placement and then every 15 min thereafter for 1 h. During this hour, the children were prepared for polysomnography (e.g., reminding them of the procedure, placing electrodes, etc.) by experienced and familiar project personnel. Tanner stage was determined by one of the physician investigators (34).
Iron Status and Neuroendocrine Analyses
Iron status was based on venous Hb, MCV, FEP, transferrin saturation, and ferritin in infancy and at this 10-y follow-up. Children in the parent study were also assessed at 5 y, and available hematology was also reviewed for the subjects in this study. As in the past, we defined anemia as Hb below normal for age and sex and ID as two or more abnormal iron measures. Cutoffs for 10-y-olds in NHANES III were used to determine ID (≥2 abnormal measures and IDA (low Hb as well): Hb <118 g/l, MCV <76 fl, transferrin saturation <14%, >1.24 μmol/l red blood cells (70 μg/dl), and plasma ferritin <12 mcg/l). For neuroendocrine assays, blood samples were promptly centrifuged and plasma samples were frozen at −80 °C. Plasma was assayed in duplicate for prolactin and cortisol concentrations using electrochemiluminescence assays (Roche, Basel, Switzerland). Samples were assayed once for each neuroendocrine measure. The coefficient of variation was 1.94% for cortisol and 0.42% for prolactin.
Data Analysis
Due to the nature of the original Chile study, infants could have been iron deficient at time points other than the age at which IDA was identified. For example, an infant identified as having IDA at 12 mo could have been iron deficient at 6 or 18 mo. Including such infants would complicate our ability to see a primary effect of timing of IDA. We therefore used all available hematology data at 6, 12, and 18 mo to identify clear-cut groups with regard to iron status and timing, specifically children who had been IDA only at the time point of identification. Similarly, nonanemic infants could have been iron deficient at one or more time points, as the nonanemic group was selected based on hemoglobin only. To have a clear-cut IS control group for comparison, we excluded children who were iron deficient as infants. Only six infants identified with IDA at 18 mo were IS at other time points; this n was too small for separate comparisons. Consequently, we compared the following three groups: IDA at 6 mo (IDA-6), IDA at 12 mo (IDA-12)) and IS, always IS. The final subject sample was 60.
Prolactin and cortisol concentrations were compared by iron status in infancy by using mixed models with random effects fitted with proc mixed in the Statistical Analysis System (35). To account for the within-subject correlations due to sequential measurements on the same subject, we used a hierarchical modeling approach in which each subject had his or her own random intercept and slope. Gender, age at 10-y assessment, and Tanner stage were considered as controlling covariates in the mixed models. A three-way interaction term of time (both linear and quadratic), infancy iron status, and gender was also included to assess whether any group differences in neuroendocrine response trajectories varied by gender.
Statement of Financial Support
This study was supported by National Institutes of Health grant HD33487.
References
Beard JL, Connor JR . Iron status and neural functioning. Annu Rev Nutr 2003;23:41–58.
Lozoff B, Beard J, Connor J, Felt B, Georgieff M, Schallert T . Long-lasting neural and behavioral effects of iron deficiency in infancy. Nutr Rev 2006;64(5 Pt 2):S34–43; discussion S72–91.
Youdim MBH . Brain iron metabolism biochemical and behavioral aspects in relation to dopaminergic neurotransmission. In: Lagoth A, ed. Handbook of Neurochemistry. New York: Plenum Press, 1985:731–55.
Ben-Jonathan N, Hnasko R . Dopamine as a prolactin (PRL) inhibitor. Endocr Rev 2001;22:724–63.
Youdim MB, Ben-Shachar D, Yehuda S . Putative biological mechanisms of the effect of iron deficiency on brain biochemistry and behavior. Am J Clin Nutr 1989;50:607–17.
Barkey RJ, Ben-Shachar D, Amit T, Youdim MB . Increased hepatic and reduced prostatic prolactin (PRL) binding in iron deficiency and during neuroleptic treatment: correlation with changes in serum PRL and testosterone. Eur J Pharmacol 1985;109:193–200.
Barkey RJ, Amit T, Ben-Shachar D, Youdim MB . Characterization of the hepatic prolactin receptors induced by chronic iron deficiency and neuroleptics. Eur J Pharmacol 1986;122:259–67.
Lozoff B, Felt BT, Nelson EC, Wolf AW, Meltzer HW, Jimenez E . Serum prolactin levels and behavior in infants. Biol Psychiatry 1995;37:4–12.
Felt B, Jimenez E, Smith J, et al. Iron deficiency in infancy predicts altered serum prolactin response 10 years later. Pediatr Res 2006;60:513–7.
Weinberg J, Dallman PR, Levine S . Iron deficiency during early development in the rat: behavioral and physiological consequences. Pharmacol Biochem Behav 1980;12:493–502.
Weinberg J, Levine S, Dallman PR . Long-term consequences of early iron deficiency in the rat. Pharmacol Biochem Behav 1979;11:631–8.
Weinberg J, Brett LP, Levine S, Dallman PR . Long-term effects of early iron deficiency on consummatory behavior in the rat. Pharmacol Biochem Behav 1981;14:447–53.
Groeneveld D, Smeets HG, Kabra PM, Dallman PR . Urinary catecholamines in iron-deficient rats at rest and following surgical stress. Am J Clin Nutr 1985;42:263–9.
Dallman PR, Refino CA, Dallman MF . The pituitary-adrenal response to stress in the iron-deficient rat. J Nutr 1984;114:1747–53.
Coleman R, Tanne Z, Nahir M, Shomrat D, Miller-Lotan R, Youdim MB . Ultrastructural changes in mitochondria of the adrenal cortex of iron-deficient rats. Acta Anat (Basel) 1995;152:33–40.
Yehuda S, Yehuda M . Long lasting effects of infancy iron deficiency–preliminary results. J Neural Transm Suppl 2006:197–200.
Saad MJ, Morais SL, Saad ST . Reduced cortisol secretion in patients with iron deficiency. Ann Nutr Metab 1991;35:111–5.
Golub MS, Hogrefe CE, Tarantal AF, et al. Diet-induced iron deficiency anemia and pregnancy outcome in rhesus monkeys. Am J Clin Nutr 2006;83:647–56.
Lopez NL, Vazquez DM, Olson SL . An integrative approach to the neurophysiological substrates of social withdrawal and aggression. Dev Psychopathol 2004;16:69–93.
Gunnar MR, Vazquez DM . Low cortisol and a flattening of expected daytime rhythm: potential indices of risk in human development. Dev Psychopathol 2001;13:515–38.
Copinschi G . Metabolic and endocrine effects of sleep deprivation. Essent Psychopharmacol 2005;6:341–7.
Bailey SL, Heitkemper MM . Morningness-eveningness and early-morning salivary cortisol levels. Biol Psychol 1991;32:181–92.
Schmidt-Reinwald A, Pruessner JC, Hellhammer DH, et al. The cortisol response to awakening in relation to different challenge tests and a 12-hour cortisol rhythm. Life Sci 1999;64:1653–60.
Benjamin LS . Statistical treatment of the law of initial values (liv) in autonomic research: a review and recommendation. Psychosom Med 1963;25:556–66.
Heim C, Ehlert U, Hellhammer DH . The potential role of hypocortisolism in the pathophysiology of stress-related bodily disorders. Psychoneuroendocrinology 2000;25:1–35.
Obal F Jr, Krueger JM . Hormones, cytokines, and sleep. In: McEwen BS, ed. Coping With the Environment: Neural and Endocrine Mechanisms: Handbook of Physiology. New York: Oxford University Press, 2000:331–49.
Peirano PD, Algarín CR, Garrido MI, Lozoff B . Iron deficiency anemia in infancy is associated with altered temporal organization of sleep states in childhood. Pediatr Res 2007;62:715–9.
Freeman ME, Kanyicska B, Lerant A, Nagy G . Prolactin: structure, function, and regulation of secretion. Physiol Rev 2000;80:1523–631.
Roncagliolo M, Garrido M, Walter T, Peirano P, Lozoff B . Evidence of altered central nervous system development in infants with iron deficiency anemia at 6 mo: delayed maturation of auditory brainstem responses. Am J Clin Nutr 1998;68:683–90.
Algarín C, Peirano P, Garrido M, Pizarro F, Lozoff B . Iron deficiency anemia in infancy: long-lasting effects on auditory and visual system functioning. Pediatr Res 2003;53:217–23.
Peirano P, Algarín C, Garrido M, Algarín D, Lozoff B . Iron-deficiency anemia is associated with altered characteristics of sleep spindles in NREM sleep in infancy. Neurochem Res 2007;32:1665–72.
Lozoff B, De Andraca I, Castillo M, Smith JB, Walter T, Pino P . Behavioral and developmental effects of preventing iron-deficiency anemia in healthy full-term infants. Pediatrics 2003;112:846–54.
Alvarez ML, Muzzo S, Ivanovic D . Scale for measurement of socioeconomic level, in the health area. Rev Med Chil 1985;113:243–9.
Tanner JM . Growth at Adolescence. Oxford, UK: Blackwell Scientific, 1962.
Littell RC, Milliken GA, Stroup WW, Wolfinger RD . SAS System for Mixed Models. Cary, NC: SAS Institute, 1996.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Felt, B., Peirano, P., Algarín, C. et al. Long-term neuroendocrine effects of iron-deficiency anemia in infancy. Pediatr Res 71, 707–712 (2012). https://doi.org/10.1038/pr.2012.22
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/pr.2012.22
This article is cited by
-
The Impact of Early-Life Exposures on Women’s Reproductive Health in Adulthood
Current Epidemiology Reports (2021)
-
The impact of maternal and early life malnutrition on health: a diet-microbe perspective
BMC Medicine (2020)
-
Prevalence of and factors associated with anemia in school children from Maceió, northeastern Brazil
BMC Public Health (2016)
-
Inhibitory control in otherwise healthy overweight 10-year-old children
International Journal of Obesity (2015)