Abstract
Aim of this study was to evaluate the effect of preimplantation genetic screening (PGS) on neurodevelopmental outcome in children. We conducted a prospective follow-up study of children born to women randomly assigned to in vitro fertilization with or without PGS. Primary outcome was adverse neurologic outcome at 18 mo; secondary outcomes were types of minor neurologic dysfunction (MND), neurologic outcome before 18 mo, neonatal intensive care admission, and congenital malformations. Twenty women in the PGS group participated with 25 children and 26 women in the control group participated with 31 children. Five PGS pregnancies (25%) and four control pregnancies (15%) resulted in birth of at least one child with an adverse neurologic outcome (adjusted odds ratio: 2.3 [0.4–12.0]). Dysfunction in fine motor abilities and posture and muscle tone dysregulation tended to be present more frequently after PGS. Neurologic outcome before 18 mo, neonatal intensive care admission, and prevalence of congenital malformations were similar in study and control pregnancies. Nevertheless, at child level, rates of adverse outcome were higher after PGS. In conclusion, outcome in pregnancies after in vitro fertilization (IVF) with and without PGS was similar. The small sample size precludes the conclusion that PGS is not associated with less favorable neurologic outcome. Safety of new assisted reproductive techniques should be evaluated before large-scale implementation.
Similar content being viewed by others
Main
Children born after assisted reproduction represent a sizeable part of the population; therefore, their health is of general concern. Nowadays, in Europe and the United States, 1–4% of children are born after assisted reproduction, and the numbers are still increasing (1,2).
One of the major goals for new reproductive techniques is to enhance efficiency of assisted reproduction, and newer methods are regularly introduced to achieve this goal. One of these methods is in vitro fertilization (IVF) with preimplantation genetic screening (PGS) for aneuploidy. In this procedure, embryos obtained with IVF are biopsied, which implies that a hole is made in the zona pellucida with laser or by chemical means. One or two blastomeres are aspirated, so that copy numbers of several sets of chromosomes can be determined. The concept behind this procedure is to identify and discard embryos with an abnormal chromosomal constitution because these might have a lower implantation potential or eventually lead to miscarriage (3). Higher ongoing pregnancy rates were expected due to selection of the most viable embryos but have not been demonstrated in recent randomized controlled trials (RCTs) (4–7). In fact, two trials have shown a reduction in ongoing pregnancies (8,9).
Besides efficacy, another important issue in assisted reproduction is the safety of the procedure for the developing fetus. Despite the invasiveness of PGS, information on developmental outcome after this procedure is scarce (10–12), and information on neurologic outcome is completely lacking. Therefore, we conducted a prospective, assessor-blinded follow-up study of children born to women randomly assigned to IVF with or without PGS. The aim of our study was to investigate the effect of PGS on the child's neurodevelopmental outcome. The primary outcome measure of this study was adverse neurologic outcome at the age of 18 mo. Secondary outcome measures were specific types of minor neurologic dysfunction (MND) at 18 mo, neurologic outcome before 18 mo, admission to a neonatal intensive care unit, and congenital malformations.
METHODS
Eligible for this follow-up study were children of women participating in one center (University Medical Center Groningen) of a multicenter trial on the efficiency of PGS to establish ongoing pregnancies (8). In the trial, exclusion criteria for women were age of IVF candidate younger than 35 or older than 41 y, previously failed IVF cycles, and objections against a possible double-embryo transfer. Randomization of women was performed centrally with minimization for age (35–37 and 38–41 y) and reproductive technique (IVF and intracytoplasmic sperm injection), with stratification according to study center before the start of the IVF procedures. Information concerning IVF treatment procedures has been reported previously (8). For this study, women who conceived naturally during the trial were excluded from the follow-up study because the aim of our study was to investigate the effect of PGS on child outcome in IVF treatment. The protocol of the follow-up study was approved by the Medical Ethics Committee of the University Medical Center Groningen.
Couples were invited for the follow-up study during the third trimester of pregnancy. After written informed consent, inclusion took place during the first 2 wk after birth. At the first appointment, demographic information (including e.g. parity, gestational age, birth weight, neonatal intensive care unit admission, parental age, and educational level) was collected on standardized charts. Time to pregnancy was retrieved from fertility charts. Information on child health up to 18 mo was collected by history taking during assessments. Major congenital malformations were classified as malformations that generally cause functional impairment or require surgical correction (13). All other malformations were classified as minor.
Neurodevelopmental assessments.
Follow-up consisted of standardized, age-specific neurologic assessments at the ages of 2 wk and 3, 4, 10, and 18 mo postterm. Age-specific testing is necessary in children due to abundant structural and functional changes in the nervous system, which induce changes in expression and prevalence of neurologic dysfunction (14). At 2 wk and 3 mo, quality of general movements (GMs) was assessed. Four classes of GM quality can be distinguished, being normal-optimal, normal-suboptimal, mildly abnormal, and definitely abnormal GMs (15). At 4 and 10 mo, the Touwen Infant Neurologic Examination was used, which resulted in the classification of normal, normal-suboptimal, MND, and definitely abnormal (16).
The neurologic examination according to Hempel was used at 18 mo (14). In this assessment, children perform various motor tasks while playing in a standardized free-field situation. Functionality is tested in five different domains: fine motor function, gross motor function, posture and muscle tone, reflexes, and visuomotor function. Multiple signs in a domain result in a dysfunctional cluster. Children are classified as neurologically normal, simple MND, complex MND, or major neurologic dysfunction based on the number of dysfunctional clusters (17). Neurologically normal implies the presence of no dysfunctional clusters or only the presence of the cluster reflexes. Simple MND means the presence of one cluster of dysfunction, i.e. the isolated presence of fine motor, gross motor, visuomotor dysfunction, or mild dysregulation of posture and muscle tone. It is considered to reflect a normal, but nonoptimal form of brain function. Complex MND denotes the presence of two or more dysfunctional clusters; it is the form of MND with clinical relevance due to its clear association with learning and behavioral disorders (18,19). Major neurologic dysfunction implies the presence of a distinct neurologic syndrome, such as cerebral palsy (17). We considered complex MND and major neurologic dysfunction as an adverse neurologic outcome. Specific types of dysfunction were mild fine motor dysfunction, mild gross motor dysfunction, mild visuomotor dysfunction, or a mild dysfunction in posture and muscle tone. At all ages, children were assessed by K.J.M. under supervision of M.H.-A., who were blind to mode of conception.
Statistical analysis.
In the preceding study on the efficiency of PGS to establish ongoing pregnancies (8), women were randomized into IVF with or without PGS. In this study, we continued to use women (or their pregnancies) as unit of analysis for the primary statistical analyses. In case of twins, a pregnancy was considered to have an adverse outcome when at least one of the children born had an adverse outcome. For descriptive purposes and secondary analyses, we also analyzed data at the child level, under the untested assumption that lack of independence among twins is negligible.
We used t test to analyze differences of means in continuous data. For categorical data, Fisher's exact test or Pearson's χ2 test was used when appropriate. Because group sizes were small and randomization had occurred at the level of the women before pregnancy and not on infant level, we a priori decided to adjust for factors known to effect neurodevelopmental outcome, i.e. gestational age, twins, and maternal age by means of logistic regression analysis. The p values of 5% and lower were considered statistically significant. Statistical analyses were performed with the use of SPSS for Windows, version 14.0.
RESULTS
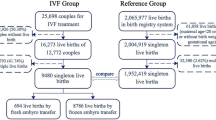
Between March 2004 and January 2006, the multicenter trial resulted in live birth of at least one child in 49 women after IVF treatment with PGS and 71 control women (8). Of these women, 25 and 32 gave birth after treatment at the University Medical Center Groningen. Twenty (80%) and 26 (81%) couples agreed to participate in the follow-up study with their child or children (Fig. 1). Reasons for nonparticipation were logistic (three study and four control couples), unwillingness to participate in a developmental study (two study and one control couples), or resistance against hospital visits due to fertility history (one control couple). The 20 couples of the PGS group participated with 25 children and the 26 couples of the control group with 31 children (Table 1). Couples with twin pregnancies participated either with both or none of the children. Postnatal attrition was low. One control child and one pair of study group twins were not assessed at the ages of respectively 2 wk and 3 and 4 mo. All included children were assessed at the ages of 4, 10, and 18 mo.
Demographic and perinatal characteristics of the PGS and the control group are shown in Table 1. Causes of subfertility were similar in the PGS and the control group. Both groups included five twin pregnancies. Testing age (corrected for preterm birth) was similar in study and control group at all follow-up ages.
Five pregnancies after IVF with PGS (25%) compared with four pregnancies in the control group (15%) resulted in the birth of at least one child with an adverse neurologic outcome at the age of 18 mo (adjusted odds ratio: 2.3 [0.4–12.0]; Table 2). Neurologic outcome at child level is presented in Table 3. Five PGS children (20%) and four controls (13%) showed complex MND. In addition, one child in the study group presented with a spastic diplegia. This condition resulted from myelum compression at the level of the medulla oblongata by an arteriovenous malformation diagnosed at the age of 1 y. Analysis of the specific types of dysfunction showed that PGS pregnancies tended to result more often in children with fine motor dysfunction (p = 0.08) and dysfunctional posture and muscle tone (p = 0.03; Table 2). Multivariate analyses were not possible because none of the children in the control group presented with these specific types of dysfunction. There were no differences in gross motor function, reflexes, and visuomotor function between the two groups. Neurodevelopmental outcome up to and including 10 mo of age was similar in children born after PGS and control children (data not presented).
Four PGS pregnancies (20%) and three control pregnancies (12%) resulted in admission of at least one child to neonatal intensive care (adjusted odds ratio: 10.2 [0.4–235.2]; Table 2). Reasons for neonatal intensive care admission were prematurity (four PGS twin children and two control twin children), respiratory insufficiency (two PGS twin children), short-term transitional problems after birth (one PGS singleton), and sepsis (one control singleton). In total, seven PGS children (28%) and three control children (10%) were admitted to neonatal intensive care (Table 3). As condition at birth, i.e. the requirement of neonatal intensive care, might be an important mediator of neurologic condition at 18 mo, we explored this relationship. Only one of the 10 children who had been admitted to neonatal intensive care presented with adverse neurologic outcome at 18 mo, indicating no statistically significant association (p = 0.67). In addition, we found no relation between neonatal intensive care admission and the number of dysfunctional clusters present (p = 0.84) or the presence of any specific type of MND.
In four PGS pregnancies (20%) and one control pregnancy, at least one child presented with a major malformation at the age of 18 mo (adjusted odds ratio: 4.7 [0.4–51.2]; Table 2). As the presence of congenital malformations is associated with an increased risk of neurologic dysfunction, we explored this relationship. We found an evident relation between major malformations and adverse neurologic outcome at 18 mo (p = 0.007). Children with a major malformation showed more dysfunctional clusters at the age of 18 mo (p < 0.001) and presented more often with fine motor impairment (p = 0.05), gross motor impairment (p = 0.007), and mild dysregulation of posture and muscle tone (p = 0.003). Congenital malformations of any kind (major or minor) were observed in respectively seven (35%) and five (19%) pregnancies (adjusted odds ratio: 2.1 [0.5–9.1]; Table 2). These malformations were not related to adverse neurologic outcome (p = 0.11) or the presence of any specific type of MND, however, children with any kind of malformation had more clusters of dysfunctions (p = 0.04) than children without malformation.
DISCUSSION
In this prospective, assessor-blinded randomized follow-up study, we found similar rates of adverse neurologic outcome, neonatal intensive care admission, and congenital malformations in pregnancies resulting from IVF with or without PGS. Mild fine motor dysfunction and mildly dysfunctional posture and muscle tone tended to be present more frequently after PGS pregnancies. At child level, results were slightly more unfavorable for children born after PGS than for control children. Therefore, an increased risk for a less favorable neurologic outcome in children born after PGS cannot be excluded.
The main limitation of our study is the relatively small sample size, which was caused by the fact that the power analysis of the PGS study was based on the number of women needed to detect an increase in ongoing pregnancy rates and not on the number of pregnancies necessary for follow-up (8). This means that this study has an explorative nature and that our findings should be interpreted with caution. From an infant developmental perspective, the fact that randomization had occurred at the level of future mothers and not at infant level might also be regarded as a limitation of the study. We addressed the problem of minor differences in perinatal adversity between the groups by including multivariate statistics. Further minor limitations of the study are the 19–20% loss to follow-up and the relatively limited duration of follow-up. For practical reasons, outcome at the age of 18 mo is frequently the end point in developmental studies. However, it should be realized that the majority of minor developmental disabilities first emerge at school age (18). Therefore, long-term neurodevelopmental follow-up of children born after IVF with PGS is urgently needed.
The strengths of our study are prospective follow-up, blinding of the assessor, and application of longitudinal detailed, standardized neurologic assessments. Furthermore, randomization of couples to IVF treatment with or without PGS contributed to the comparability of study and control group.
Many studies have investigated neurodevelopmental outcome of children born after assisted reproduction (reviewed in Refs. 20–22). Results from two recent systematic reviews suggest that IVF is associated with an increased risk for cerebral palsy. At least partly, this association can be explained by the increased risk of multiple births and preterm delivery after IVF (21,22). Little is known about the association of assisted reproduction and MND (21), although these so-called minor neurodevelopmental disorders may have a major impact on daily life of the child and his or her family (19,23). Even less is known on outcome of children born after PGS. So far, data on two nonrandomized controlled trials have been reported. Banerjee et al. (12) reported similar developmental and behavioral scores in children born after PGS or natural conception. In addition, a Belgian group found no adverse effect of embryo biopsy on growth, congenital malformations, neonatal intensive care admissions, behavior, and mental and psychomotor development assessed with the Bayley Scales in singletons at 2 y (10,11,24).
Considerations and conclusions.
Invasive assisted reproductive techniques such as embryo biopsy carry potential risks for further development of the embryo. Biologically, it is plausible that the use of laser or chemicals for opening of the zona pellucida may induce thermal, mechanical or mutagenic side effects (25). Likewise, intuition suggests that removal of up to one quarter of the embryo's cell mass might have developmental consequences. In addition, a different selection of embryos, on the basis of ploidy-status instead of morphologic criteria of the embryo, might hypothetically result in children with less favorable neurologic outcome.
At the RCT level of pregnancies, no adverse effect of PGS could be demonstrated. However, at child level, PGS was associated with a higher rate of MND. Contrary to our expectations, adverse neurologic outcome was not associated with neonatal intensive care admission. Adverse neurologic outcome was, however, strongly associated with major congenital malformations. Previous studies that found an association between minor congenital malformations and impaired neurodevelopmental outcome, in particular impaired fine motor function, have suggested that the link points to an early ontogenetic origin of the neural dysfunctions (26,27). The absence of a relation between neonatal intensive care admission and adverse neurodevelopmental outcome in this study might be due to the small sample size.
It is important to continue neurologic follow-up at least up to school age because children may grow into an adverse neurologic outcome. This is a well-known developmental phenomenon. Developmental changes in the infant brain strongly affect the age-specific expression of dysfunction. For instance, early signs of dysfunction in infants may disappear because of restorative mechanisms in the brain, but dysfunction may also be uncovered by developmental progress. Cases in point of the latter are development of dyslexia or the time needed for a full expression of cerebral palsy (15).
Embryo biopsy is also applied in preimplantation genetic diagnosis, however, the indication for this procedure is fundamentally different from PGS (28). In preimplantation genetic diagnosis, blastomeres obtained with embryo biopsy are analyzed for specific heritable disorders, such as cystic fibrosis. There is a steady rise in centers practicing preimplantation genetic diagnosis, and the technique is used for more and more indications (29). The increase in application of embryo biopsy in combination with the findings of this study demonstrates the need for determination of its safety.
In conclusion, in this prospective, assessor-blinded randomized follow-up study, we found similar rates of adverse neurologic outcome, neonatal intensive care admission, and congenital malformations in PGS and control pregnancies. Because at child level, rates of adverse outcome were higher in children born after PGS than in control children, an increased risk for a less favorable neurologic outcome in children born after PGS cannot be excluded by this study. Biologic plausibility, i.e. the possibility that interference with the very first steps of ontogeny may give rise to impaired neurologic development, is reason for concern. The safety of new assisted reproductive techniques, such as PGS, should be carefully evaluated in follow-up studies preceding large-scale implementation of these techniques.
Abbreviations
- GMs:
-
general movements
- IVF:
-
in vitro fertilization
- MND:
-
minor neurological dysfunction
- PGS:
-
preimplantation genetic screening
References
Andersen AN, Goossens V, Ferraretti AP, Bhattacharya S, Felberbaum R, de Mouzon J, Nygren KG, European IVF-monitoring (EIM) Consortium European Society of Human Reproduction and Embryology (ESHRE) 2008 Assisted reproductive technology in Europe, 2004: results generated from European registers by ESHRE. Hum Reprod 23: 756–771
Wright VC, Chang J, Jeng G, Macaluso M, Centers for Disease Control and Prevention (CDC) 2008 Assisted reproductive technology surveillance—United States, 2005. MMWR Surveill Summ 57: 1–23
Wilton L 2002 Preimplantation genetic diagnosis for aneuploidy screening in early human embryos: a review. Prenat Diagn 22: 512–518
Staessen C, Platteau P, Van Assche E, Michiels A, Tournaye H, Camus M, Devroey P, Liebaers I, Van Steirteghem A 2004 Comparison of blastocyst transfer with or without preimplantation genetic diagnosis for aneuploidy screening in couples with advanced maternal age: a prospective randomized controlled trial. Hum Reprod 19: 2849–2858
Jansen RP, Bowman MC, de Boer KA, Leigh DA, Lieberman DB, McArthur SJ 2008 What next for preimplantation genetic screening (PGS)? Experience with blastocyst biopsy and testing for aneuploidy. Hum Reprod 23: 1476–1478
Schoolcraft WB, Katz-Jaffe MG, Stevens J, Rawlins M, Munne S 2009 Preimplantation aneuploidy testing for infertile patients of advanced maternal age: a randomized prospective trial. Fertil Steril 92: 157–162
Staessen C, Verpoest W, Donoso P, Haentjens P, Van der Elst J, Liebaers I, Devroey P 2008 Preimplantation genetic screening does not improve delivery rate in women under the age of 36 following single-embryo transfer. Hum Reprod 23: 2818–2825
Mastenbroek S, Twisk M, Echten-Arends J, Sikkema-Raddatz B, Korevaar JC, Verhoeve HR, Vogel NE, Arts EG, de Vries JW, Bossuyt PM, Buys CH, Heineman MJ, Repping S, van der Veen F 2007 In vitro fertilization with preimplantation genetic screening. N Engl J Med 357: 9–17
Hardarson T, Hanson C, Lundin K, Hillensjo T, Nilsson L, Stevic J, Reismer E, Borg K, Wikland M, Bergh C 2008 Preimplantation genetic screening in women of advanced maternal age caused a decrease in clinical pregnancy rate: a randomized controlled trial. Hum Reprod 23: 2806–2812
Nekkebroeck J, Bonduelle M, Desmyttere S, Van den Broeck W, Ponjaert-Kristoffersen I 2008 Mental and psychomotor development of 2-year-old children born after preimplantation genetic diagnosis/screening. Hum Reprod 23: 1560–1566
Nekkebroeck J, Bonduelle M, Desmyttere S, Van den Broeck W, Ponjaert-Kristoffersen I 2008 Socio-emotional and language development of 2-year-old children born after PGD/PGS, and parental well-being. Hum Reprod 23: 1849–1857
Banerjee I, Shevlin M, Taranissi M, Thornhill A, Abdalla H, Ozturk O, Barnes J, Sutcliffe A 2008 Health of children conceived after preimplantation genetic diagnosis: a preliminary outcome study. Reprod Biomed Online 16: 376–381
Bonduelle M, Liebaers I, Deketelaere V, Derde MP, Camus M, Devroey P, Van Steirteghem A 2002 Neonatal data on a cohort of 2889 infants born after ICSI (1991–1999) and of 2995 infants born after IVF (1983–1999). Hum Reprod 17: 671–694
Hadders-Algra M 2005 The neuromotor examination of the preschool child and its prognostic significance. Ment Retard Dev Disabil Res Rev 11: 180–188
Hadders-Algra M, Mavinkurve-Groothuis AM, Groen SE, Stremmelaar EF, Martijn A, Butcher PR 2004 Quality of general movements and the development of minor neurological dysfunction at toddler and school age. Clin Rehabil 18: 287–299
Touwen BC 1976 Neurological Development in Infancy. Mac Keith Press, London, pp 1–150
Hadders-Algra M 2003 Developmental coordination disorder: is clumsy motor behavior caused by a lesion of the brain at early age?. Neural Plast 10: 39–50
Hadders-Algra M 2002 Two distinct forms of minor neurological dysfunction: perspectives emerging from a review of data of the Groningen Perinatal Project. Dev Med Child Neurol 44: 561–571
Batstra L, Neeleman J, Hadders-Algra M 2003 The neurology of learning and behavioural problems in pre-adolescent children. Acta Psychiatr Scand 108: 92–100
Sutcliffe AG, Ludwig M 2007 Outcome of assisted reproduction. Lancet 370: 351–359
Middelburg KJ, Heineman MJ, Bos AF, Hadders-Algra M 2008 Neuromotor, cognitive, language and behavioural outcome in children born following IVF or ICSI-a systematic review. Hum Reprod Update 14: 219–231
Hvidtjørn D, Schieve L, Schendel D, Jacobsson B, Svaerke C, Thorsen P 2009 Cerebral palsy, autism spectrum disorders, and developmental delay in children born after assisted conception: a systematic review and meta-analysis. Arch Pediatr Adolesc Med 163: 72–83
Gillberg IC, Gillberg C 1989 Children with preschool minor neurodevelopmental disorders. IV: behaviour and school achievement at age 13. Dev Med Child Neurol 31: 3–13
Desmyttere S, De Schepper J, Nekkebroeck J, De Vos A, De Rycke M, Staessen C, Liebaers I, Bonduelle M 2009 Two-year auxological and medical outcome of singletons born after embryo biopsy applied in preimplantation genetic diagnosis or preimplantation genetic screening. Hum Reprod 24: 470–476
Kanyo K, Konc J 2003 A follow-up study of children born after diode laser assisted hatching. Eur J Obstet Gynecol Reprod Biol 110: 176–180
Largo RH, Pfister D, Molinari L, Kundu S, Lipp A, Duc G 1989 Significance of prenatal, perinatal and postnatal factors in the development of AGA preterm infants at five to seven years. Dev Med Child Neurol 31: 440–456
Soorani-Lunsing RJ, Hadders-Algra M, Huisjes HJ, Touwen BC 1993 Minor neurological dysfunction after the onset of puberty: association with perinatal events. Early Hum Dev 33: 71–80
Sermon K, Van Steirteghem A, Liebaers I 2004 Preimplantation genetic diagnosis. Lancet 363: 1633–1641
Goossens V, Harton G, Moutou C, Scriven PN, Traeger-Synodinos J, Sermon K, Harper JC 2008 ESHRE PGD Consortium data collection VIII: cycles from January to December 2005 with pregnancy follow-up to October 2006. Hum Reprod 23: 2629–2645
Acknowledgements
We thank all parents and infants who participated in this study for their enthusiastic cooperation. Additionally, we kindly acknowledge the help of Marjolein Pereboom and Michiel Schrier for their technical assistance, Loes de Weerd for scheduling the assessments, Sebastiaan Mastenbroek for constructive comments on an earlier version of the manuscript, and Sjoerd Repping for his contribution in the design of the study. We are indebted to Jannie van Echten-Arends, Sebastiaan Mastenbroek, and Moniek Twisk for the use of their data system.
Author information
Authors and Affiliations
Corresponding author
Additional information
Supported by a grant of the University Medical Center Groningen, project-number 754510. The PGS trial was supported by a grant of the Netherlands Organisation of Health Research and Development (ZonMw; 945-03-013 follow-up).
Rights and permissions
About this article
Cite this article
Middelburg, K., Heineman, M., Haadsma, M. et al. Neurological Condition of Infants Born After In Vitro Fertilization With Preimplantation Genetic Screening. Pediatr Res 67, 430–434 (2010). https://doi.org/10.1203/PDR.0b013e3181d2273e
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1203/PDR.0b013e3181d2273e
This article is cited by
-
Clinical application of noninvasive chromosomal screening for elective single-blastocyst transfer in frozen-thawed cycles
Journal of Translational Medicine (2022)
-
Preimplantation Genetic Screening with Spent Culture Medium/Blastocoel Fluid for in Vitro Fertilization
Scientific Reports (2018)
-
Intracytoplasmic sperm injection for male infertility and consequences for offspring
Nature Reviews Urology (2018)
-
Pregnancy and child developmental outcomes after preimplantation genetic screening: a meta-analytic and systematic review
World Journal of Pediatrics (2018)