Abstract
Rett syndrome (RTT) is an X-linked neurodevelopmental disorder caused by mutations in the MECP2 gene. In 49 female RTT children, aged 1.9–17 y, bone mass was assessed and correlated with clinical parameters and mutations involving the MECP2 gene. We also studied five adult females, aged 20–33 y, and one male child, aged 6 y. Lumbar spine bone mineral content (BMC) and bone mineral density (BMD) were correlated with weight, height, BMI, clinical severity, degree of scoliosis, use of anticonvulsants, and ambulatory status. L1-L4 BMD and BMC showed that 48.9% of them had BMD values >2 SD below age-related norms. BMD values were in the osteoporotic range in the five adult females with RTT. Eleven percent of the children and adults with RTT experienced fractures. Low bone mass was correlated with marginal significance to clinical severity and ambulation but not to scoliosis or anticonvulsant use. Lowest bone mass occurred in patients with T158M or R270X mutations but without statistical significance. Studies in a murine model of RTT confirmed low bone mass as an inherent component of this syndrome. MECP2 mutations and clinical parameters impact bone mass in RTT, but an association with a specific mutation was not demonstrable.
Similar content being viewed by others
Main
Rett syndrome (RTT) is an X-linked dominant neurodevelopmental disorder associated with mutations in MECP2 encoding methyl-CpG-binding protein 2 (1,2). The incidence is 1:22,000 live female births (3). The majority of patients are females. Males are more severely affected than females. The relationship of MECP2 mutations to the neurologic disease has been confirmed in humans and in murine models of the disorder (1,4,5).
Clinical features of RTT include early onset of neurologic signs with cognitive defects, deceleration of head growth, and stereotypic movements (6,7). Seizures are a common feature of the disorder, and the use of anticonvulsant drugs is frequent. Although a decrease in bone mass was first reported in patients with RTT in 1995, information is limited about the age-dependent pattern of bone loss, the fracture incidence, and the relationship of bone mass to associated clinical features (8). Neurological disability in patients with RTT evolves over several years with comorbidities that together limit the development of normal bone mass. These include limited weight bearing, poor nutrition because of swallowing difficulties, and the negative effects of anticonvulsant medications. Availability of the murine RTT model permits an assessment of the effects of the MECP2 mutations on bone mass independent of these associated factors.
METHODS
This study was approved by the Institutional Review Board, Johns Hopkins University. This is a cross-sectional observational study of the relation of lumbar spine bone mineral content (BMC) and bone mineral density (BMD) and related clinical parameters to X-linked MECP2 mutations in 49 girls with RTT syndrome, aged 1.9–17 y, one male child, aged 6 y, and five women, aged 20–33 y. Informed consent was obtained from the parent or guardian of the participants enrolled in the study. The diagnosis of RTT was made on the basis of criteria established by the RTT Diagnostic Criteria Work Group (6) and by MECP2 mutation analysis. Patients were scored for severity based on the Rett Syndrome Severity Scale (RSSS) as described in Hoffbuhr et al. (9). The z scores for weight for age (kg) and stature for age (cm) were obtained for gender and age using the Centers for Disease Control and Prevention ZWTAGE and ZSTATAGE charts for ages 2–20 y (cdcinfo@cdc.gov).
BMD measurements were performed at the Pediatric Clinical Research Unit of Johns Hopkins Hospital using a Hologic Discovery A Dual Energy Absorptiometer (DXA) equipped with pediatric software. The coefficient of variation (CV) for these DXA measurements was 3%. Lumbar L1-L4 BMC (gm) and areal BMD (gm/cm2) and z scores were obtained. The Hologic database provides z scores for ages ≥3 y. BMD z scores <−2.0 were considered significant (10). The z scores are not currently available for lumbar BMC measurements in this pediatric age group. Data are illustrated on modified Hologic machine printouts for five female adult patients. To compare RTT BMC measurements with normal, we used reference curves developed by Kalkwarf et al. (11) that report the 10th, 50th, and 90th percentile values for age, gender, and race for BMC in 1554 healthy girls aged 7–17 y. However, BMC values for children younger than 7 y are plotted in Figure 1 for reference only. For comparison of BMD with normal, we used the Hologic Discovery, a database that starts at the age of 3 y (Hologic, Inc., Bedford, MA). Patient lymphocytes, lymphoblasts, or fibroblast cell lines were used for identifying mutations in the MECP2 gene, as described by Hoffbuhr et al. (9). Serum 25(OH) vitamin D levels that were measured at baseline were performed by liquid chromatography/mass spectroscopy (Quest Diagnostics Nichols Institute, Chantilly, VA). Normal values are 20–100 ng/mL with CV of 10% ranging from 4 to 120 ng/mL. Serum total alkaline phosphatase was performed using the spectroscopic p-nitrophenol method at the Clinical Pathology Laboratory, Johns Hopkins Hospital. Normal values and CV for children and adolescent females are 100–320 with a 2.8% CV at a level of ∼80 U/L and 2.3% CV at a level of 360 U/L, respectively.
A, DXA L1–L4 BMC values compared with age-related normal. The 10th, 50th, and 90th percentiles for normal females are illustrated. The hollow circle shows BMC for the 6-y-old RTT male. B, BMC is plotted against age, ages 2–16 y. There is a linear relationship with a correlation coefficient of r = 0.617.
The murine RTT model lacking MECP2 as a result of deletion of exons 3 and 4 of the MECP2 gene was developed by Bird and coworkers (4) in C57BL/6 mice using Cre-loxP technology. Heterozygous female MECP2 tm1.1Bird mice (Jackson Laboratory, Bar Harbor, ME) on a C57BL/6 background were backcrossed with C57BL/6 males for at least nine generations. Radiographs of the mice were taken using a Faxitron MX20 Specimen Radiography System (Lincolnshire, IL).
Statistical analysis.
We examined relationships between BMC and BMD and age, and the relationships of age-corrected BMC and BMD to clinical severity, scoliosis, anticonvulsant use, and extent of ambulation. These were assessed by general linear model analysis using the R Statistical package (R version 2.10.1; 2009). The relationships of the mean BMC and BMD to various MECP2 mutations were analyzed using Mann-Whitney U tests and χ2 analysis.
RESULTS
Demographics of this RTT population.
This RTT cohort consisted of 49 girls aged 1.9–17 y (mean age, 7.6 ± 3.8 y), one male child aged 6 y, and five females aged 20–33 y.
BMC and BMD values for the lumbar spine (L1-L4).
We report BMC and BMD L1-L4 values but not total hip or whole body measurements because of the difficulty in positioning these patients who may have continuous upper extremity motion and/or lower extremity contractures. Total hip measurements were available in selected adult patients. Figure 1 shows age versus L1-L4 BMC for 44 RTT females and the single RTT male subject, using the 10th, 50th, and 90th percentile normative data for female children (9). BMC data plotted for children aged 1.9–6.11 y is not within the graph line as these data are not currently available for those younger ages. In 15 children aged 7–18 y, the BMC scores were <10th percentile, and in 10 other children of the same age, it was >10th percentile. Thus, 66% of the patients with RTT aged 7–18 y had BMC values that were <10th percentile for age (Fig. 1A). However, Figure 1B shows a linear relationship between BMC and age 2–16 y (r = 0.61).
Figure 2A presents BMD measurements plotted against normal female values as provided in the Hologic 4500 database. Although these are cross-sectional data, the distribution indicates low bone mass may occur at all ages and continues into adulthood. As with BMC measurements, BMD versus age shows a linear increase to age 16 y (r = 0.65; Fig. 2B). It is of interest that, because of the impact of patient size on DXA measurements, the correlation coefficient for BMC plotted against BMD in this population was 0.87 (data not shown).
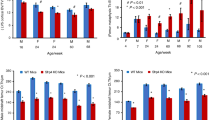
L1-L4 BMD z scores were <2.0 in 24 of 49 children (48.9%) aged 1.9–17 y. However, 11 of 49 children (22%) had z scores for the lumbar spine within normal reference range, between 0.0 and −1.5 SD. Partial DXA studies were obtained in five adult women because of previous spine fusion or hip surgery. L1-L4 BMD z scores for four of five adult females aged 20–33 y, whose BMD is shown in Figure 3 were −3.6, −4.4, and −2.3 but −0.2 in a patient with previous spine surgery. Total hip BMD z scores (age) available in four of five females were −3.4 (20 y), −3.0 (27 y), −1.9 (29 y), and −2.3 (33 y), respectively.
MECP2 mutations, BMC, and BMD.
Mutations in MECP2 were confirmed in all patients; three cases involved R106W, five subjects had R133C, two had R168X, 13 had T158M mutations, four had R255X, one had K266X, six had the R270X mutation, three had 294X, three had R306C, one had R306H, and one had 306P. Seven patients had a small deletion, and one patient had a large deletion. L1-L4 BMD values are plotted for specific mutations (Fig. 4). We did not find a statistically significant relationship between specific MECP2 mutations and the median lumbar spine BMC or BMD. However, the lowest BMD values were observed in patients with either the T158M or R270X mutations.
Contributory factors related to bone mass.
Poor nutrition and diminished growth are frequent among children with RTT (12). The average BMI was 16.4 ± 3.6 kg/m2 in children to age 18 y. Height and weight z scores adapted from CDC ZWTAGE and ZSTATAGE tables were obtained for age groups 1.9–7.0 y, 7.1–11 y, and >11 y. These CDC tables provide z scores only to <2.0, but they do not provide specific z score for values <−2.0. For the group with ages, 1.9–7 y, 4 of 22 children had both height and weight z scores <2.0. For girls with RTT ages, 7–11 y, 6 of 20 had height z scores < 2.0, and 2 of 20 had weight scores < 2.0. For children aged 11–18 y, 5 of 10 had height z scores <2.0, as did 5 of 10 had weight z scores >2 SD below the mean for age. However, height and weight z scores were not statistically related to each other or to BMC or BMD.
Baseline 25(OH)D levels were available in 26 children. The values ranged from <14 to 54 ng/mL (mean = 33. 8 ± 9.9 ng/mL). In 13 children, the serum 25(OH)D levels were within normal range, >32 ng/mL, 12 were between 20 and 31 ng/mL (D insufficiency), and 1 was <14 ng/mL or vitamin D deficient. Serum total alkaline phosphatase values ranged from 29.3 to 117.0 (41–134.6). Neither serum 25(OH)D nor alkaline phosphatase were related to BMD (data not shown).
The mean clinical RSSS score was 7.5 (range, 3–15) on a scale of 0–15. Severity scale scores were available for 45 patients aged <18 y. Fifteen patients had BMD z scores >2.0 and 20 patients had BMD z scores <2.0. The χ2 analysis showed a relationship of marginal significance between clinical severity score and BMD (p = 0.057).
In estimating the amount of independent ambulation required for the maintenance of lumbar bone mass, we defined the ability to walk independently for >2 consecutive h/d, as ambulatory. Thirty-four patients were defined as ambulatory, and 15 patients were considered nonambulatory. Of the nonambulatory patients, 6 of 15 (40%) had BMD z scores −2.0 SD or below the mean for age, whereas 21 of 34 (62%) of the ambulatory patients had BMD z scores below 2.0 SD. Figure 5 illustrates the difference in lumbar spine BMD versus age for ambulatory and nonambulatory patients.
Absolute BMC values were marginally different in the ambulatory patients (17.02 g/cm2) compared with the nonambulatory group (11.51 g/cm2; p = 0.0507). Similarly, absolute BMD values were significantly different in the ambulatory group (mean BMD = 0.498 ± 0.168 gm/cm2) compared with nonambulatory patients (mean BMD = 0.425 ± 0.104; p = 0.0423).
To evaluate scoliosis, the vertebral Cobb angle was measured in 49 patients. Scoliosis was graded 1–4 based on the following scales: no scoliosis = 0; 1 <20°; 2 <30°; 3 <40°; and 4 >40°. Scoliosis in excess of 20° was present in 30% of patients aged <18 y (13). No statistical relationship was observed between BMC or BMD and scoliosis severity grades 1 to 4 (BMC: p = 0.281; BMD: p = 0.133).
Current use of anticonvulsant drugs known to affect mineral metabolism was reported in 31% of 49 patients. These included phenytoin, carbamazepine, valproic acid, and phenobarbital (14). Five of 15 patients taking these anticonvulsants had a BMD z score <−2.0, whereas the remaining 10 had higher BMD values. Mean BMD z score for patients taking anticonvulsant drugs was −2.3 ± 1.65. Of the 34 patients taking no anticonvulsant medication, the mean BMD z score was −2.00 ± 2.21. Thus, no significant difference exists between the two groups (p = 0.266).
Murine models of RTT permits an initial assessment of the putative contribution of MECP2 mutations independent of confounding factors such as the amount of ambulation and anticonvulsant use. Figure 6 illustrates the difference in bone mass between wild-type mice and the male null animal in which exons 3–4 of the MECP2 gene have been deleted (4). Both cortical thickness and medullary mineralization seem decreased in these animals.
DISCUSSION
This study used DXA scans to assess bone mass in children and young adults with mutation-confirmed RTT and to examine the relationships of BMC and BMD to MECP2 mutations. In addition, we examined the relationship of bone mass to associated clinical factors such as anticonvulsant use, scoliosis, ambulation, and RSSS.
Obtaining an accurate DXA measurement in patients with RTT is difficult because of the movement disorders, joint contractures, and scoliosis that limit proper positioning and the presence of surgical hardware in the spine or hip. Despite these impediments, L1-L4 DXA measurements confirm the presence of decreased BMC and BMD in approximately one half of the RTT females as early as at the age of 3 y. The 6-y-old nonambulatory male child also had significantly lower BMC and BMD. Each of the five adults also had lumbar spine or total hip z scores that were in the osteopenic/osteoporotic range.
An age-related decrease in bone mass has been previously reported in RTT. Leonard et al. (8) reported that in their survey of 17 RTT cases, 86% showed reduced hand bone density. Subsequent studies in 137 Australian girls with RTT demonstrated mean metacarpal cortical thickness z scores of −1.94 compared with −0.38 for healthy children (p < 0.001) (15). Haas et al. (16) observed that spine BMC and BMD were significantly reduced in 20 subjects with RTT syndrome. Haas et al. (16) did not find an association between BMC and ambulatory status when in a small cohort of subjects with RTT compared with those with cerebral palsy and normal controls. In this cohort, 34% had impaired ambulation, walking <2 h each day. Impaired ambulation was marginally associated with a decreased lumbar spine BMC or BMD in this cohort. Decreased bone mass is frequently observed at the hip and distal femur sites when normal weight bearing is impaired but lumbar spine BMD is usually preserved in nonambulatory children who spend hours daily in wheelchairs. Here, diminished lumbar BMC and BMD suggest that factors other than impaired mobility were contributing to the low bone mass.
Patients with RTT in our cohort had markedly decreased height and weight z scores. Similarly, Schwartzman et al. (12) reported a height-to-age deficit in 48.1% of girls with RTT, which was greater in those more severely affected (stage IV). Although the availability of BMC standards for young girls is limited to >7 y, BMC was measured because girls with RTT are small for age. Smaller L1-L4 vertebral bone size induces an artifact that may cause areal BMD (gm/cm2) values to seem lower than they actually are when measured as BMC (gm). However, both BMC and BMD confirm the significant association of low bone mass.
Motil et al. (17) indicate that although BMD and BMC increased with age, z scores were significantly lower in older compared with younger females with RTT. We did not observe this relationship as BMC and BMD values in this cross-sectional study were low in children, tended to increase with age in a linear manner, but remained in the osteoporotic range in the five older patients. Figure 5, where BMD versus age is compared for ambulatory versus nonambulatory children, illustrates this age-related trend to increased BMD in ambulatory individuals. Larger numbers of patients may be required to define changes in BMD with increasing age.
Low bone mass is associated with increased risk of fracture. Here, 11% of the patients had a history of fracture, all of which occurred before the age of 12, which is similar to the 14% incidence reported by Loder et al. (18). Leonard et al. (15) reported fractures in one third of 137 Australian cases and estimated that >40% of girls would sustain a fracture by the age of 15 y. A more recent questionnaire survey of 234 girls in the Australian RTT syndrome database revealed a fracture incidence of 43.3 episodes per 1000 person years, nearly four times greater than the population rate (19). It is not clear why the incidence of fractures in the Australian patients is significantly greater than in our cohort.
We observed that clinical severity as defined by the RSSS is marginally related to BMD (p = 0.057). The scale that includes scoliosis, seizure frequency, and ambulation also includes factors such as head growth and respiratory irregularity, which would not be expected to influence lumbar bone mass. A larger data set is required to confirm a relationship between RSSS and BMD.
Scoliosis with onset at a young age occurs frequently in patients with RTT. The early onset of RTT symptoms, decreased mobility at 10 mo, and nonambulatory status have been identified as predictors of scoliosis in RTT (20). Moreover, low BMD has been reported in non-RTT adolescents with scoliosis (21). In agreement with Motil et al. (17), we found that patients with scoliosis in excess of 20° were more likely to have BMC and BMD z scores <−2.0, but this relationship was not statistically significant.
Bone loss and fractures are common in children with seizure disorders (22). The use of phenobarbital, phenytoin, carbamazepine, and valproic acid may alter bone mass. We found that BMD z scores were not decreased with anticonvulsant drug use compared with nonusers (14). Leonard et al. (15) reported a decrease in cortical thickness and cortical area in girls with RTT using anticonvulsants. Motil et al. (17) reported no relationship to current anticonvulsant use but did find lower BMC/BMD in individuals reporting previous use of anticonvulsants. A review by Gissel et al. (22) of 14 published reports on the effect of anticonvulsant drugs on BMD in children is of interest. Six studies found no effect of carbamazepine on bone density, and equivocal results were reported for valproate effects on bone density (23). This suggests that multiple factors may influence the effects of anticonvulsants on bone metabolism: seizure frequency affecting dosage, vitamin D status, or combinations of medications differing from those used in this cohort could influence the impact of anticonvulsants on bone mass.
Hypovitaminosis D is common in children with seizure disorders (24). Here, 42% had serum 25(OH)D levels in the D-insufficient or D-deficient range. Although possibly related to anticonvulsant use, low serum vitamin D levels may be caused by a lack of sunlight exposure and the difficulty of administering oral vitamin supplements. Bone biopsies in girls with RTT and mouse models of RTT confirm the impact of MECP2 mutations on bone (25,26). Figure 6 illustrates the extent to which bone mass is decreased in 6-wk-old null (male) C57BL/6 RTT mice compared with control. Additional studies in our laboratory have demonstrated that the male homozygote shown in Figure 6 has decreased bone density, decreased bone mass, lower trabecular number, and greater trabecular separation (data not shown; Shapiro JR et al., Rett Syndrome: Bone Mass in Girls and Mice, 2009 ASBMR Meeting, April 27–29, 2009, Bethesda, MD, Abstract 22: S499). Iliac crest bone biopsies in 5 RTT children, 9.5–14 y, showed decreased bone volume, decreased osteoclast surface and number, and reduced rate of bone formation suggesting decreased osteoblast function (25). O'Connor et al. (26) have reported histology in the male null mouse model MECP2 −/yBird, which at postnatal d 60, had reduced skeletal size compared with wild type, decreased mineral apposition rate in trabecular but not cortical bone, and decreased cortical and trabecular bone volume. The numbers of calvarial osteoblasts/bone surface were decreased suggesting that osteoblast dysfunction was probably the primary cause of reduced bone volume in RTT syndrome.
In this series, the lowest values for BMC/BMD occurred in children with T158M and R270X MECP2 mutations (Fig. 4). However, this did not reach statistical significance for either mutation and is in keeping with the lack of relationship between MECP2 mutations and BMC or BMD observed by Motil et al. (17). A relation of BMD to the T158M mutation may lie in the observation that individuals with T158M mutations are more neurodevelopmentally affected than those with other mutations (27). In the cohort of 238 patients participating in research studies at the Kennedy Krieger Institute, mutations consisting of T158M occurred in 12% of the group. In these, seizures were frequent (60%), 53% were not ambulatory, and 44% had scoliosis. A marginal relationship of clinical severity to BMD was observed here suggesting that a larger number of observations are required to clarify the role of these mutations. Downs et al. (19) reported a positive relationship between the R270X mutation and fracture risk in Australian individuals with RTT. We suggest that although factors such as decreased ambulation, anticonvulsants, and scoliosis may account for a portion of the decrease in bone mass, the deficiency seen in RTT bone mass may be the result of direct effects of the MECP2 mutations on osteoblast metabolism as suggested in human biopsy studies and observations in mutant mice (25,26).
Recent studies involving leptin and neuropeptide Y2 have disclosed unrecognized interactions between the CNS, peripheral neurotransmitters, and osteoblasts (28,29). In contrast to agents acting through intermediates such as the sympathetic nervous system or membrane receptors, MECP2 acts as a transcriptional repressor affecting the neural tissue (2). Neuronal tissue studies suggest that MECP2 could affect osteoblast function by altering osteoblast chromatin structure as observed with brain chromatin or by altering cell maturation as observed in RTT neuronal tissues (25,30). Other CNS neurotransmitters altered in patients with RTT could potentially influence osteoblast growth and maturation (31). Thus, the recognition of osteoporosis in RTT syndrome adds yet another dimension to our understanding of the possible role MECP2 and other neural factors might play in the regulation of bone metabolism.
Abbreviations
- BMC:
-
bone mineral content
- BMD:
-
bone mineral density
- CV:
-
coefficient of variation
- DXA:
-
dual energy absorptiometer
- MECP2 :
-
methyl-CpG-binding protein 2
- RTT:
-
Rett syndrome
- RSSS:
-
Rett Syndrome Severity Scale
References
Kozinetz CA, Skender ML, MacNaughton N, Almes MJ, Schultz RJ, Percy AK, Glaze DG 1993 Epidemiology of Rett syndrome: a population-based registry. Pediatrics 91: 445–450
Bird A 2008 The methyl-CpG binding protein MeCP2 and neurological disease. Biochem Soc Trans 36: 575–583
RettBASE IRSF MECP2 Variation Database. Available at: http://mecp2.chw.edu.au. Accessed June 9, 2010
Guy J, Hendrich B, Holmes M, Martin JE, Bird A 2001 A mouse MeCP2-null mutation causes neurological symptoms that mimic Rett syndrome. Nat Genet 27: 322–326
Amir RE, Van den Veyver IB, Wan M, Tran CQ, Francke U, Zoghbi HY 1999 Rett syndrome is caused by mutations in X-linked MECP2, encoding methyl-CpG-binding protein 2. Nat Genet 23: 185–188
Hagberg B, Hanefeld F, Percy A, Skjeldal O 2002 An update on clinically applicable diagnostic criteria in Rett syndrome. Comments to Rett Syndrome Clinical Criteria Consensus Panel Satellite to European Paediatric Neurology Society Meeting, Baden, Germany, 11 September 2001. Eur J Pediatr Neurol 6: 293–297
Trevathan E, Naidu S 1988 The clinical recognition and differential diagnosis of Rett syndrome. J Child Neurol 3: S6–S16
Leonard H, Thomson M, Bower C, Fyfe S, Constantinou J 1995 Skeletal abnormalities in Rett syndrome: increasing evidence for dysmorphogenetic defects. Am J Med Genet 58: 282–285
Hoffbuhr KC, Moses LM, Jerdonek MA, Sirianni N, Scacheri C, Giron J, Schuette J, Innis J, Marino M, Philippart M, Narayanan V, Umansky R, Kronn D, Hoffman EP, Naidu S 2002 Associations between MeCP2 mutations, X-chromosome inactivation, and phenotype. Ment Retard Dev Disabil Res Rev 8: 99–105
Baim S, Leonard M, Bianchi ML, Hans DB, Kalkwarf H, Langman C, Rauch F 2008 Official positions of the international Society for clinical Densitometry and Executive summary of the 2008 ISCD Pediatric Position Development Conference. J Clin Densitom 11: 6–21
Kalkwarf HJ, Zemel BS, Gilsanz V, Lappe JM, Horlick M, Oberfield S, Mahboubi S, Fan B, Frederick MM, Winer K, Shepherd JA 2007 The bone mineral density in childhood study: bone mineral content and density according to age, sex, and race. J Clin Endocrinol Metab 92: 2087–2099
Schwartzman F, Vitolo MR, Schwartzman JS, Morais MB 2008 [Eating practices, nutritional status and constipation in patients with Rett syndrome]. Arq Gastroenterol 45: 284–289
Tan KJ, Moe MM, Vaithinathan R, Wong HK 2009 Curve progression in idiopathic scoliosis: follow-up study to skeletal maturity. Spine 34: 697–700
Chou IJ, Lin KL, Wang HS, Wang CJ 2007 Evaluation of bone mineral density in children receiving carbamazepine or valproate monotherapy. Acta Paediatr Taiwan 48: 317–322
Leonard H, Thomson M, Glasson E, Fyfe S, Leonard S, Ellaway C, Christodoulou J, Bower C 1999 Metacarpophalangeal pattern profile and bone age in Rett syndrome: further radiological clues to the diagnosis. Am J Med Genet 83: 88–95
Haas RH, Dixon SD, Sartoris DJ, Hennessy MJ 1997 Osteopenia in Rett syndrome. J Pediatr 131: 771–774
Motil KJ, Ellis KJ, Barrish JO, Caeg E, Glaze DG 2008 Bone mineral content and bone mineral density are lower in older than in younger females with Rett syndrome. Pediatr Res 64: 435–439
Loder RT, Lee CL, Richards BS 1989 Orthopedic aspects of Rett syndrome: a multicenter review. J Pediatr Orthop 9: 557–562
Downs J, Bebbington A, Woodhead H, Jacoby P, Jian L, Jefferson A, Leonard H 2008 Early determinants of fractures in Rett syndrome. Pediatrics 121: 540–546
Ager S, Fyfe S, Christodoulou J, Jacoby P, Schmitt L, Leonard H 2006 Predictors of Scoliosis in Rett Syndrome. J Child Neurol 21: 809–813
Lee WT, Cheung CS, Tse YK, Guo X, Qin L, Lam TP, Ng BK, Cheng JC 2005 Association of osteopenia with curve severity in adolescent idiopathic scoliosis: a study of 919 girls. Osteoporos Int 16: 1924–1932
Gissel T, Poulson CS, Vestergaard P 2007 Adverse effects of antiepileptic drugs on bone mineral density in children. Expert Opin Drug Saf 6( 3): 267–278
Gissel T, Poulson CS, Vestergaard P 2007 Adverse effects of epileptic drugs on bone mineral density in children. Expert Opin Drug Saf 6: 267–278
Nettekoven S, Strohle A, Trunz B, Wolters M, Hoffmann S, Horn R, Steinert M, Brabant G, Lichtinghagen R, Welkoborsky HJ, Tuxhorn I, Hahn A 2008 Effects of antiepileptic drug therapy on vitamin D status and biochemical markers of bone turnover in children with epilepsy. Eur J Pediatr 167: 1369–1377
Budden SS, Gunness ME 2003 Possible mechanisms of osteopenia in Rett syndrome: bone histomorphometric studies. J Child Neurol 18: 698–702
O'Connor RD, Zayzafoon M, Farach-Carson MC, Schanen NC 2009 MeCP2 deficinecy decreases bone formation and reduces bone volume in a rodent model of Rett syndrome. Bone 45: 346–356
Archer H, Evans J, Leonard H, Colvin L, Ravine D, Christodoulou J, Williamson S, Charman T, Bailey M, Sampson J, Klerk N, Clarke A 2007 Correlation between clinical severity in patients with Rett syndrome with a p.R168X or p.T158M MECP2 mutation, and the direction and degree of skewing of X-chromosome inactivation. J Med Genet 44: 148–152
Allison SJ, Baldock PA, Herzog H 2007 The control of bone remodeling by neuropeptide Y receptors. Peptides 28: 320–325
Elefteriou F 2008 Regulation of bone remodeling by the central and peripheral nervous system. Arch Biochem Biophys 473: 231–236
Chadwick LH, Wade PA 2007 MeCP2 in Rett syndrome: transcriptional repressor or chromatin architectural protein?. Curr Opin Genet Dev 17: 121–125
Johnston MV, Blue ME, Naidu S 2005 Rett syndrome and neuronal development. J Child Neurol 20: 759–763
Author information
Authors and Affiliations
Corresponding author
Additional information
This study was funded by grants from NICHD PO1-24448, and the Institute for Clinical and Translational Research, grants UL1RR025005 and FD-R-002408 [to S.N.].
Rights and permissions
About this article
Cite this article
Shapiro, J., Bibat, G., Hiremath, G. et al. Bone Mass in Rett Syndrome: Association With Clinical Parameters and MECP2 Mutations. Pediatr Res 68, 446–451 (2010). https://doi.org/10.1203/PDR.0b013e3181f2edd2
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1203/PDR.0b013e3181f2edd2
This article is cited by
-
MiR-422a promotes adipogenesis via MeCP2 downregulation in human bone marrow mesenchymal stem cells
Cellular and Molecular Life Sciences (2023)
-
Methyl-CpG-binding protein 2 (MECP2) mutation type is associated with bone disease severity in Rett syndrome
BMC Medical Genetics (2020)