Abstract
Mucolipidosis type IV (MLIV) is a neurodegenerative channelopathy that is caused by the deficiency of TRPML1 activity, a nonselective cation channel. TRPML1 is a lysosomal membrane protein, and thus, MLIV is a lysosomal storage disorder. The basic, specific function of TRPML1 has not been yet clarified. A recent report (Soyombo AA, Tjon-Kon-Sang S, Rbaibi Y, Bashllari E, Bisceglia J, Muallem S, Kiselyov K: J Biol Chem 281:7294–7301, 2006) indicated that TRPML1 functions as an outwardly proton channel whose function is the prevention of overacidification of these organelles. Thus, in MLIV the lysosomal pH is lower than normal. Furthermore, attempts by these investigators to increase slightly the lysososmal pH with either Nigericin or Chloroquine suggested corrective effect of the abnormal storage in MLIV cells. We investigated this approach using these agents with cultured fibroblasts from severely affected and milder patients. Our data indicated that there was no reduction in the total number of storage vesicles by either agent, although Nigericin resulted in a change in the nature of the storage materials, reducing the presence of lamellated substances (lipids) so that the storage vesicles contained predominantly granulated substances. On the other hand, transfection with the normal MCOLN1 cDNA (the gene coding for TRPML1) resulted in the removal of almost all the storage materials.
Similar content being viewed by others
Main
Mucolipidosis type IV (MLIV, MIM-252650), a neurodegenerative lysosomal storage disease, is caused by mutations in the MCOLN1 gene (1,2). The disease is found in increased frequency in the Ashkenazi Jewish (AJ) population with two MCOLN1 founder mutations comprising 95% of the MLIV alleles in this population and heterozygote frequency of 1:100 (3). In addition, over 15 other mutations were identified among Jewish and non Jewish patients with MLIV (2). The protein encoded by MCOLN1—mucolipin1 was characterized to be a member of the transient receptor potential (TRP/TRPL) superfamily of cation channels, mostly calcium channels (4–6), thus the protein is also referred to as TRPML1. The basic function of TRPML1 is still under investigation, particularly the nature of the cation that is primarily involved in the channel activity. These studies are complicated by the fact that TRPML1 is located almost exclusively in late endosomes/lysosome vesicles (7–9), which poses technical difficulties in performing direct electrophysiological activity measurements of this channel. Thus, most of the data obtained hitherto involved mistargeting of TRPML1 to the plasma membrane in heterologous systems by overexpressing this protein; it is uncertain whether these systems indeed are suitable for these evaluations, representing the natural microenvironment of this protein in the lysosomal membrane. It was suggested that TRPML1 is a nonselective cation channel active with mono and divalent cations and is regulated by pH and calcium changes (10,11). On the other hand, there is no consensus yet for the primary function of TRPML1. Various studies indicated it to function as a calcium channel (10,12–14), whereas others suggested its function as an iron exporter from the lysosomal milieu, (15) and other studies indicated it to be a proton channel whose function is to regulate intralysosomal pH (16). The latter report demonstrated decreased pH values compared with normal controls in the lysosomal vacuole of cells from patients with MLIV (16). It was suggested that normally TRPML1 prevents overacidification in the lysosomes resulting from the catabolic activity, and thus, overacidification is caused by the malfunction of TRPML1 in MLIV. The lower acidic environment may result in the malfunction of various lysosomal hydrolases, leading to substrates accumulation. To restore the normal pH, agents that can slightly increase the lysosomal pH [Nigericin and Chloroquine (CQ)] were added to the cells with the aim to restore the normal acidity and thus correct the abnormal storage. These studies indicated that this treatment corrected the storage (16).
In this study, we evaluated the possibility to correct, or at least reduce, the number of the storage vesicles in MLIV using Nigericin and CQ for this purpose. Our data indicated that these treatments, although lead to some changes in the nature of the stored materials, did not result in a significant reduction in the number of the abnormal vesicles in the diseased cells.
MATERIALS AND METHODS
Materials.
Chloroquine and Na+ Nigericin were from Sigma-Aldrich.
Cultured fibroblasts.
All the cultured cells studied in this work are anonymous without any indication for the identity of a specific patient, only the relevant clinical data and the identity of the relevant mutations are at hand. As required by the Israeli law for patients' genetic counseling and diagnosis, an oral consent and agreement has been obtained from each patient or their parents. Cultured skin fibroblasts from two patients with MLIV were used in this study. The first patient, AJ is severely affected, was found to be a compound heterozygote for the AJ founder mutation del EX1-EX7 (14,17) and c.1210insT. The second patient, a mild patient, also AJ, bearing two MCOLN1 mutations, the major AJ founder mutation and an in-frame amino acid deletion (ΔF408, c.1221-1223 del CTT). Two normal lines served as controls.
All the experiments were performed with cultures that were not older than the eighth passage, because older normal fibroblasts begin to exhibit nonspecific cellular abnormalities, primarily lysosomal storage, observed by electron microscopy (EM) (18). The cultures were grown and maintained as previously described (19).
Electron microscopy.
Cells were harvested by trypsin (19) and then were washed three times with PBS and fixed for 4 h in 2.5% glutaraldehyde at room temperature. The cells were washed with 0.1 M sodium cacodylate buffer (pH 7.4) and postfixed with 2% osmium tetroxide for 1 h. Then cells were dehydrated in a graded ethanol series and embedded in araldite epon that was allowed to polymerize for 48 h at 60°C. Samples were cut into ultrathin sections (70 nm), mounted on copper grids, and stained with uranyl acetate followed by lead citrate and examined in Philips CM12 transmission electron microscope.
cDNA transfection.
Full length MCOLN1 cDNA, cloned in pEYFPN-1 vector (Clontech, Mountain View, CA) was transfected into cultured fibroblasts with Fugene 6 (Roche, Mannheim, Germany) according to the manufacturer instructions.
RESULTS
A slight increase in the lysosomal pH can be achieved by either Nigericin, a monovalent antiporter, or by CQ, a weak base (20,21). CQ in a concentration of 1 μM was shown to be sufficient to cause an increase of 0.5 pH unit in the intralysosomal pH, whereas 2.6 μM Nigericin was shown to raise intralysosomal pH by a 0.8 pH unit (21). We investigated the effect of these compounds on the abnormal storage in the diseased cells. For each treatment, the average number of storage bodies was calculated based on counting the cytoplasmic storage vacuoles in 100 cells.
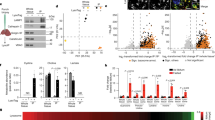
Nigericin concentrations higher than 0.01 μM were found to be toxic to the cells. Figure 1 demonstrate the effect of incubating cultured cells immediately after their passage in the presence of 0.01 μM Nigericin for 5 d before EM. It is clear that the nature of the stored materials has been changed by this treatment so that there is a reduction in the content of lamellated bodies, which represent amphiphilic lipoid materials, and there are now predominantly storage vesicles containing granulated materials, which represent water-soluble substances (22–25). The total number of storage vesicles has not changed. Before the treatment in the cells of the mild patient, there are on the average (±SD) 35 ± 14 storage vesicles/cell, whereas in the severe patient, the number of storage vesicles is on average 50 ± 19/cell and these quantities remained unchanged after the treatment (Table 1). Although, after the treatment the nature of the stored materials partially changed, particularly in the mild patient where a significant reduction in the content of lamellated structures was observed, the cells contained the same number of storage vesicles primarily with granulated materials, whereas in the severely affected patient more lamellated structures still remained. Similar observations were obtained after 10 d incubation with this compound (data not shown). Nigericin had no apparent effect on cellular structures in MLIV, other than the lysosomal storage. Incubating normal controls with this compound under identical conditions did not result in any observable cellular changes. It should be noted that incubations with higher Nigericin concentrations than those used here resulted in vacuolization of normal cells in previous reports (26). Incubations of MLIV cells with lower Nigericin concentrations 0.005 μM, 0.001 μM, and 0.5 nM had no effect at all on either the number or nature of the storage vesicles.
EM of cultured fibroblasts from patients with MLIV after Nigericin treatment. A, B: Severely affected patient with MLIV cells without treatment. A, (×3400); B, (×5600); C–E, mild patient cells after treatment with 0.01 μM Nigericin for 5 d; C, D, (×4400); E, (×5600); and F, severely affected patient cells after treatment with 0.01 μM Nigericin for 5 d (×4400). Lamellar body (LB), granular vesicle (GV), and nucleus (N).
Chloroquine.
This was the second compound tested to reduce the acidity in MLIV lysosomes. Fibroblasts were incubated with concentrations lower than 5 μM CQ, which was found by us and others (27) to be toxic for MLIV fibroblasts, whereas these concentrations had no effect on normal culture fibroblasts. We have no clear explanation for this toxic effect. Cultures, immediately after their passage, were incubated for 5 d with CQ concentrations ranging from 50 pM to 5 μM.
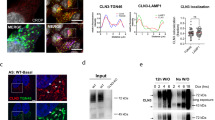
There was no significant changes either in the number of storage vesicles or in the nature of the storage in either the severe or the mild patients' cells in concentrations range 10 nM–5 μM, whereas there was a slight reduction by 50 pM CQ in the number of lipid storage vesicles only in the mild patient but no change in the total storage vesicles (Fig. 2). After treatment with CQ concentrations higher than 1 μM (1–5 μM), an increase in the total number of storage vesicles was observed, including both the lamellated and the granulated substances (Table 2, Fig. 3). There was no other apparent effect on cellular structures. CQ in these concentrations had no effect on the normal control cells. High concentrations of CQ (higher than 10 μM) that cause marked reduction of the lysosomal lumen acidity are known to induce vacuolization of the cytoplasm and swelling of lysosomes (28).
EM of cultured fibroblasts from patients with MLIV after treatment with different CQ concentrations for 5 d: 50 pM (A, mild patient) (×5600), 10 nM (B, mild patient) (×5600), 200 nM (C, mild patient) (×3400), and 200 nM (D, severely affected patient) (×3400). Lamellar body (LB), granular vesicle (GV), nucleus (N).
Effect of CQ on the storage in MLIV fibroblasts. The average number of storage bodies in MLIV cells after treatment with different concentrations of CQ (0–5 μM) for 5 d. Dark columns—mild MLIV patient cells, bright columns—severe MLIV patient cells. For each treatment, the average number of storage bodies ±SD was calculated based on counting of the cytoplasmic vacuoles in 100 cells.
MCOLN1 transfection.
Transfecting MLIV cells with the normal MCOLN1 cDNA (Fig. 4) resulted in a significant reduction of the total storage organelles in MLIV. The transfection yield in these cells was 30%, as calculated using the EYFP fluorescent probe, and indeed, in 30% of the cells after the cDNA transfection, the content of storage organelles were dramatically reduced from an average of 50 ± 19/cell to less than 10 ± 3 in the severe patient's cells and from 35 ± 14 to 6 ± 2 in the mild patient (Fig. 5). Many cells were indistinguishable from normal controls. Thus, correction of the storage here involved a clear reduction of the abnormal lysosomes and a return to normal cell structure. Mock transfection with the YFP construct alone did not affect at all the cellular structure of the affected cells.
DISCUSSION
The report by Soyombo et al. (16) suggesting the correction of the abnormal storage in MLIV by decreasing slightly intralysosomal acidity might have been a hopeful approach for this devastating disease. At present there is no alternative tool to treat or cure this syndrome in which the CNS is primarily affected. Using agents that might cross the blood-brain barrier is thus of utmost importance. The basis of this approach was the finding that the intralysosomal milieu in MLIV cells are more acidic than normal as a result of the malfunctioning of TRPML1 that was indicated to be a proton pump functioning as a lysosomal pH regulator by exporting the excess H+ ions out from the lysosomal milieu (16). It should be noted that other reports were not in agreement with this finding (29). Furthermore, the basic defect in MLIV is not resolved. Although various studies demonstrated a defect in the biogenesis process of lysosomes as the basic defect in MLIV, leading to a block of the transport of membrane components in the final stages of the endocytosis process and thus it is suggested that the heterogeneous storage in this disease occurs in prelysosomal vesicles and not in the mature lysosome (14,29–33). These final stages are dependant on the efflux of calcium ions that play an important role in the trafficking process of late endosomes to multivesicular bodies and to lysosomes (33). Indeed, it was also suggested that TRPML1 functions primarily as a calcium channel (12). Soyomabo et al. (16) on the other hand suggested the defect to underlie in the mature lysosomes and TRPML1 functions as a H+ channel. The investigators suggested that by slightly increasing the intralysosomal pH with appropriate agents (Nigericin and CQ), added exogenously to cultures cell medium, will correct the defect and thus prevent the accumulation process. Using the same compounds, we studied the fate of the storage in MLIV cells. The data obtained in this report do not support this approach. Nigericin is a carboxylic channel functioning as an antiporter to monovalent/H+ ions exchanging Na/K ions with H+ leading thus to decreased acidity without affecting the membrane potential (34). Nigericin is lipophilic and thus easily endocytosed into cells (35). The second compound used was CQ, a weak base that also easily enters cells and localize in acidic compartments (36). Using these compounds under optimal conditions did not abolish the storage, although the nature of the storage has been changed by Nigericin, primarily in the mild patient, so that it contained predominantly storage vesicles with granulated substances. However, the total number of storage vesicles remained unchanged. With the severe patient, who represents most of the patients with MLIV diagnosed hitherto, this effect was less remarkable. It is difficult at present to assess whether the membraneous, lipoid materials were indeed removed by this treatment although it did not affect the granulated water soluble substances stored in MLIV or whether this treatment resulted in a new cellular pathology that is not related anymore to the basic disease. The latter alternative is more likely; MLIV is a monogenic channelopathy in which the defect in the function of a protein leads to the simultaneous storage of both lipids and water-soluble substances. Thus, it is unlikely that a corrective effect will remove one group of substances and not the other. Furthermore, previous reports indicated that the correction of lysosomal storage of glycosaminoglycans in mucopolysaccharidoses, which are also the major stored water-soluble substances in MLIV (19), by the deficient enzyme is easily achieved, whereas the correction of lipidic materials is much more difficult to achieve.
The use of CQ in this study had only very mild corrective effect and basically it showed an effect of the storage in lower concentrations only with the mild patient, whereas higher concentrations (1–5 μM) increased the content of storage vesicles. The severe patient barely showed any effect. We have no clear explanations why CQ did not change the storage pattern at concentrations higher than 50 pM.
On the other hand, the addition of the normal gene (MCOLN1 cDNA) resulted in clear correction of the overall storage and many cells became indistinguishable from normal controls.
Thus, the data here indicate that attempts to decrease lysosomal acidity is apparently not feasible for meaningful correction of the abnormal storage, whereas a gene therapy approach might be principally useful to successfully treat this disease.
The outcome of these experiments cannot yet answer whether the storage in MLIV stems from the abnormal endocytosis process and the biogenesis of lysosomes or whether the defect indeed underlies in the regulation of pH in mature lysosomes.
Abbreviations
- CQ:
-
chloroquine
- MLIV:
-
mucolipidosis type IV
- TRP:
-
transient receptor potential cation channels (protein family)
References
Bach G 2001 Mucolipidosis type IV. A review. Mol Genet Metab 73: 197–203
Bach G 2005 Mucolipin 1: endocytosis and cation channel—a review. Pflugers Arch 451: 313–317
Bach G, Webb MB, Bargal R, Zeigler M, Ekstein J 2005 The frequency of mucolipidosis type IV in the Ashkenazi Jewish population and the identification of 3 novel MCOLN1 mutations. Hum Mutat 26: 591–596
Bargal R, Avidan N, Ben Asher E, Olender Z, Zeigler M, Frumkin A, Raas Rothschild A, Glusman G, Lancet D, Bach G 2000 Identification of the gene causing Mucolipidosis type IV. Nat Genet 26: 118–123
Montell C 2005 The TRP superfamily of cation channels. Sci STKE 2005: re3
Nilius B, Voets T 2005 TRP channels: a TR(I)P through a world of multifunctional cation channels. Pflugers Arch 451: 1–10
Pryor PR, Reimann F, Gribble FM, Luzio JP 2006 Mucolipin-1 is a lysosomal membrane protein required for intracellular lactosylceramide traffic. Traffic 7: 1388–1398
Manzoni M, Monti E, Bresciani R, Bozzato A, Barlati S, Bassi MT, Borsani G 2004 Overexpression of wild-type and mutant mucolipin proteins in mammalian cells: effects on the late endocytic compartment organization. FEBS Lett 567: 219–224
Kiselyov K, Chen J, Rbaibi Y, Oberdick D, Tjon-Kon-Sang S, Shcheynokov N, Muallem S, Soyombo A 2005 TRP-ML1 is a lysosomal monovalent cation channel that undergoes proteolytic cleavage. J Biol Chem 280: 43218–43223
Cantiello HF, Montalbetti N, Goldmann WH, Raychowdhury MK, Gonzalez-Perrett S, Timpanaro GA, Chasan B 2005 Cation channel activity of mucolipin-1: the effect of calcium. Pflugers Arch 451: 304–312
Raychowdhury MK, Gonzalez-Perrett S, Montalbetti N, Timpanaro GA, Chasan B, Goldmann WH, Stahl S, Cooney A, Goldin E, Cantiello HF 2004 Molecular pathophysiology of mucolipidosis type IV: pH dysregulation of the mucolipin-1 cation channel. Hum Mol Genet 13: 617–627
LaPlante JM, Falardeau J, Sun M, Kanazirska M, Brown EM, Slaugenhaupt SA, Vassilev PM 2002 Identification and characterization of the single channel function of human mucolipin-1 implicated in mucolipidosis type IV, a disorder affecting the lysosomal pathway. FEBS Lett 532: 183–187
Zhang F, Jin S, Yi F, Li PL 2008 TRP-ML1 functions as a lysosomal NAADP-sensitive Ca(+2) release channel in coronary myocytes. J Cell Mol Med, Epub ahead of print
Luzio JP, Bright NA, Pryor PR 2007 The role of calcium and other ions in sorting and delivery in the late endocytic pathway. Biochem Soc Trans 35: 1088–1091
Dong XP, Cheng X, Mills E, Delling M, Wang F, Kurz T, Xu H 2008 The type IV mucolipidosis-associated protein TRPML1 is an endolysosomal iron release channel. Nature 455: 992–996
Soyombo AA, Tjon-Kon-Sang S, Rbaibi Y, Bashllari E, Bisceglia J, Muallem S, Kiselyov K 2006 TRP-ML1 regulates lysosomal pH and acidic lysosomal lipid hydrolytic activity. J Biol Chem 281: 7294–7301
Bargal R, Avidan N, Olender T, Ben Asher E, Zeigler M, Raas-Rothschild A, Frumkin A, Ben-Yoseph O, Friedlender Y, Lancet D, Bach G 2001 Mucolipidosis type IV: novel MCOLN1 mutations in Jewish and non-Jewish patients and the frequency of the disease in the Ashkenazi Jewish population. Hum Mutat 17: 397–402
Arnon J, Ornoy A, Bach G 1988 Culture conditions found to minimize false positive diagnosis of lysosomal storage disorders. In Vitro Cell Dev Biol 24: 1159–1164
Bach G, Zeigler M, Kohn G, Cohen MM 1977 Mucopolysaccharide accumulation in cultured skin fibroblasts derived from patients with mucolipidosis IV. Am J Hum Genet 29: 610–618
Reijngoud DJ, Oud PS, Tager JM 1976 Effect of ionophores on intralysosomal pH. Biochim Biophys Acta 448: 303–313
Poole B, Ohkuma S 1981 Effect of weak bases on the intralysosomal pH in mouse peritoneal macrophages. J Cell Biol 90: 665–669
Berman ER, Livni N, Shapira E, Merin S, Levij IS 1974 Congenital corneal clouding with abnormal systemic storage bodies: a new variant of mucolipidosis. J Pediatr 84: 519–526
Merin S, Livni N, Berman ER, Yatziv S 1975 Mucolipidosis IV: ocular, systemic and ultrastructural findings. Invest Ophthalmol 14: 437–448
Goebel HH, Kohischutter A, Lenard HJ 1982 Morphological and chemical biopsy findings in Mucolipidosis IV. Clin Neuropathol 1: 73–82
Tellez-Nagel I, Rapin I, Iwamoto T, Johnson AA, Norton WT, Nitowsky H 1976 Mucolipidosis IV: clinical, ultrastructural, histochemical and chemical studies of a case, including a brain biopsy. Arch Neurol 33: 828–835
Ohkuma S, Poole B 1978 Fluorescence probe measurement of the intralysosomal pH in living cells and the perturbation of pH by various agents. Proc Natl Acad Sci USA 75: 3327–3331
Goldin E, Cooney A, Kaneski CR, Brady RO, Schiffmann R 1999 Mucolipidosis IV consists of one complementation group. Proc Natl Acad Sci USA 96: 8562–8566
Ohkuma S, Poole B 1981 Cytoplasmic vacuolation of mouse peritoneal macrophages and the uptake into lysosomes of weakly basic substances. J Cell Biol 90: 656–664
Bach G, Chen CS, Pagano RE 1999 Elevated lysosomal pH in Mucolipidosis type IV cells. Clin Chim Acta 280: 173–179
Kopitz J, Gerhard C, Holfer P, Cantz M 1994 [14C]Methylamine accumulation in cultured human skin fibroblasts—a biochemical test for lysosomal storage and lysosomal diseases. Clin Chim Acta 227: 121–133
Bargal R, Bach G 1997 Mucolipidosis type IV: abnormal transport of lipids to the lysosomes. J Inherit Metab Dis 20: 625–632
Chen CS, Bach G, Pagano RE 1998 Abnormal transport along the lysosomal pathway in Mucolipidosis type IV disease. Proc Natl Acad Sci USA 95: 6373–6378
Pryor PR, Mullock BM, Bright NA, Gray SR, Luzio JP 2000 The role of intra-organellar Ca(+2) in late endosome-lysosome heterotypic fusion and in the reformation of lysosomes from hybrid organelles. J Cell Biol 149: 1053–1062
Choi YO, Park JH, Song YS, Lee W, Moriyama Y, Choe H, Leem CH, Jang YJ 2007 Involvement of Vesicular H+-ATPase in Insulin-stimulated glucose transport in 3T3–F442A Adipocytes. Endocr J 54: 733–743
Kucejova B, Kucej M, Petrezselyova S, Abelovska L, Tomaska L 2005 A screen for Nigericin-resistant yeast mutants revealed genes controlling mitochondrial volume and mtochondrial cation homeostasis. Genetics 171: 517–526
Weber SM, Levitz SM, Harrison TS 2000 Chloroquine and the fungal phagosome. Curr Opin Microbiol 3: 349–353
Acknowledgements
The authors thank David Zeevi for the preparation of the MCOLN1 plasmid.
Author information
Authors and Affiliations
Corresponding author
Additional information
Supported by the Rita Altura Foundation for lysosomal storage diseases research.
Rights and permissions
About this article
Cite this article
Kogot-Levin, A., Zeigler, M., Ornoy, A. et al. Mucolipidosis Type IV: The Effect of Increased Lysosomal pH on the Abnormal Lysosomal Storage. Pediatr Res 65, 686–690 (2009). https://doi.org/10.1203/PDR.0b013e3181a1681a
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1203/PDR.0b013e3181a1681a