Abstract
Intratracheal lipopolysaccharide (LPS) causes acute inflammation and injurious mechanical ventilation results in pulmonary and systemic inflammation. We aimed to determine in preterm lungs if continuous positive airway pressure (CPAP) protects against pulmonary and systemic inflammation, compared with conventional mechanical ventilation (CMV) after intratracheal LPS. Preterm fetuses were exposed to maternal betamethasone and Epostane 36 h before delivery at 133 d gestational age (term = 150 d). Lambs were intubated and randomized to receive gentle CMV (tidal volume 8 mL/kg) or CPAP with 8 cm H2O pressure. Surfactant (10 mg/kg) mixed with 1 mg LPS or saline was instilled into the trachea at 15 min. Blood gas status, ventilation variables, and arterial pressures were recorded for 3 h. Static pressure-volume curves and lung and systemic inflammation were assessed postmortem. CPAP lambs had elevated Paco2 and minute ventilation compared with the CMV lambs. Cytokine mRNA was increased in the lungs and liver of CPAP and CMV lambs relative to unventilated controls. Intratracheal LPS amplified the cytokine mRNA responses of IL-1β, IL-6, and IL-8 in the lung and liver. Blood neutrophils decreased similarly after LPS in CPAP and CMV groups. Cytokine markers of lung injury or the systemic response to intratracheal LPS were not decreased by CPAP relative to CMV, in preterm lambs.
Similar content being viewed by others
Main
Mechanical ventilation of lungs from volumes below the normal functional residual capacity (FRC) cause injury by repetitively opening collapsed lung units (1,2). Similarly, ventilation with lung volumes that approach or exceed total lung capacity will stretch the lungs and cause injury (3,4). The preterm fetal lung is particularly vulnerable to lung injury during the transition from a fluid-filled lung to airway breathing because surfactant deficiency increases the pressures required to open the lung, the lung aerates nonuniformly, and the delicate tissues of the preterm lung are easily stretched (5,6). The use of continuous positive airway pressure (CPAP) is being advocated for transitioning the preterm fetal lung to air breathing as a way to decrease lung injury (7,8). Although CPAP can cause lung injury in sepsis models (9), CPAP can decrease indicators of lung injury in preterm lambs (10) compared with conventional mechanical ventilation (CMV) and its use has been associated with decreased bronchopulmonary dysplasia (11).
Prior exposure of the adult lung to pro-inflammatory mediators such as endotoxin amplifies lung injury caused by ventilation from low lung volumes or elevated lung volumes (9,12,13), and the mediators can enter the systemic circulation and cause systemic inflammatory responses (14,15). Term-ventilated lambs do not leak endotoxin from the airspaces into the systemic circulation unless the lungs are over-stretched (16). In contrast, preterm lungs leak endotoxin or IL-1 with conventional ventilation (16,17). Chorioamnionitis is associated with many preterm deliveries before 32 wk gestational age, and inflammatory mediators are present in the fetal lung (18,19). We hypothesized that spontaneous breathing with CPAP would protect the preterm lung and minimize a systemic inflammatory response to intratracheal lipopolysaccharide (LPS) in comparison with CMV.
METHODS
The investigations were approved by the Animal Ethics Committees of the University of Western Australia and Cincinnati Children's Hospital Medical Center. Twin bearing Merino ewes were treated with betamethasone (0.5 mg/kg, Celestone Chronodose, Schering-Plough, New South Wales, Australia) and Epostane (20 mg V, Sanobi-Synthelabo, Malvern, PA) about 36 h before euthanasia by penetrating captive bolt and exsanguination at 133 d (term 150 d). The pretreatment with corticosteroids and the progesterone inhibitor is necessary to stimulate breathing after delivery of preterm lambs (10,20). The lambs were randomized to treatment groups before delivery. The lambs were delivered rapidly, intubated, and received either gentle CMV or CPAP using 8 cm H2O pressure (21). The initial ventilation settings for the CMV lambs were a peak pressure of 30 cm H2O, a positive end-expiratory pressure (PEEP) of 5 cm H2O, and a rate of 40 breaths/min using heated and humidified 40% oxygen with an inspiratory time of 0.7 s. Peak inspiratory pressures were adjusted in CMV groups to target Paco2 at 50 to 60 mm Hg with no changes in other ventilator settings. CPAP was delivered via a disposable bubble CPAP device provided by Fisher and Paykel Healthcare (Auckland, New Zealand) using heated, humidified 40% oxygen and with the CPAP set to 8 cm H2O. A loading dose (20 mg/kg IV) of caffeine was given within the first 5 min to CMV and CPAP lambs to stimulate spontaneous breathing in CPAP lambs. FiO2 was adjusted for all lambs to target Pao2 at 40 to 100 mm Hg.
At 15 min of age, lambs received 10 mg/kg intratracheal surfactant (Curosurf, Chiesi, Parma, Italy) mixed at a concentration of 5 mg/mL with either 1 mg LPS (Escherichia coli 055:B5, Sigma-Aldrich) or saline (Sal). The surfactant was given to distribute the endotoxin to the lungs (22). The lambs continued on CPAP or ventilation to 3 h of age. The lambs had umbilical arterial and venous catheters placed soon after birth, and CMV lambs were anesthetized throughout the ventilation procedures with a continuous umbilical venous infusion of Remifentanil (0.05 μg/kg/h; Ultiva, Glaxo Smith Kline Ltd., Victoria, Australia) and Propofol (0.1 mg/kg/h: Repose, Norbrook Laboratories Ltd., Victoria, Australia).
Blood-gas status, ventilation variables, and arterial pressures were recorded regularly. VT values were measured continuously with Florian respiratory monitors (Acutronic Medical Systems, Hirzel, Switzerland) (20). Oxygen index (OI) was calculated as FiO2 × 100 × mean airway pressure/PaO2. The animals were ventilated with 100% oxygen for 3 min at 3 h of age then heavily sedated with pentobarbital (20 mg/kg) before clamping of the tracheal tube to facilitate lung collapse by oxygen absorption. After 3 min, the lambs received a lethal dose of pentobarbital (100 mg/kg). A group of eight unventilated euthanized lambs were studied for comparison with the CMV and CPAP groups.
Measurements.
After opening the chest, a deflation pressure-volume curve was measured following gas inflation to 40 cm H2O pressure (10). Three pooled saline lavages of the left lung were used to recover bronchoalveolar lavage fluid (BALF). Protein (23), and cell counts and differentials using cytospins were measured from the BALF. Total RNA was isolated from lung and liver, and 10 μg of total RNA was used for IL-1β, IL-6, IL-8, and TLR-4 quantitation using RNAse protection assays (24,25). LPS was measured in plasma collected after 3 h of ventilation using a Limulus amebocyte lysate assay (Bio Whittaker, Walkersville, MD) (16).
Data analysis and statistics.
Results are shown as mean (SEM). Statistics were analyzed using SigmaStat 3.5 (Systat Software Inc., San Jose, CA). For normally distributed data, a two-way or three-way ANOVA with the Holm-Sidak multiple comparison procedure was used for comparisons between ventilation groups using time, treatment, and ventilation strategy as variables. Significance was accepted as p < 0.05.
RESULTS
Ventilation and blood pressure.
The animals randomized to each group had similar body weights and cord blood gas values (Table 1). The respiratory status of the LPS and saline-exposed lambs on CPAP was worse than for the CMV lambs. The CPAP lambs had higher Paco2 values and somewhat lower VT (Fig. 1). However, the CPAP lambs had higher respiratory rates such that minute ventilation at 120 min of age for the CPAP lambs was 395 ± 44 mL/kg compared with 250 ± 25 mL/kg for the CMV lambs (p = 0.04). This high minute ventilation in the face of elevated Paco2 indicates significant lung immaturity, inefficient ventilation, and respiratory failure in the CPAP lambs. The peak inspiratory pressures for the CMV lambs were 21.1 ± 1.7 cm H2O at 120 min. All lambs survived to the 3 h study. Pao2 was not different between groups throughout the ventilation protocol. Average values were CPAP/SAL: 69.9 ± 8.3 mm Hg; CPAP/LPS: 64.1 ± 8.1 mm Hg; CMV/SAL: 54.4 ± 8.4 mm Hg; and CMV/LPS: 72.8 ± 15.9 mm Hg.
There were no differences between the LPS and saline-exposed CMV groups for Paco2, VT, or oxygenation index. The CPAP group exposed to LPS had higher Paco2 values than the saline-exposed lambs on CPAP, but VT and oxygenation index were similar over the 3 h study. Blood pressures tended to decrease after birth in all groups, but the mean blood pressures were not different between CMV and CPAP groups or with LPS exposure (Fig. 2). Heart rates were consistently higher for the CPAP lambs than for control lambs with mean values of 237 ± 8 beats/min for CPAP lambs and 149 ± 7 beats/min for CMV lambs at 120 min (p < 0.001). LPS did not change heart rates. There were also no differences in the deflation volumes of the pressure-volume curves measured after the 3 h of CMV or CPAP (Fig. 3). Therefore, the intratracheal dose of 1 mg LPS had minimal effects on the respiratory status of the preterm lambs.
Inflammation—lung.
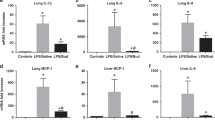
The total protein in BALF was increased for the CMV and CPAP groups relative to the unventilated comparison lambs, but there was no increased protein because of LPS exposure (Table 2). The LPS exposure did increase the numbers of inflammatory cells in the BALF with CMV and CPAP. Most of these cells were monocytes and few granulocytes were present. Cytokine mRNA for IL-1β, IL-6, and IL-8 increased similarly in the lungs of the CPAP and CMV groups. The LPS exposure amplified the increase in mRNA for the three pro-inflammatory cytokines. The mRNA for the TLR-4 receptor also increased more with the LPS exposures than with CMV or CPAP alone. The indications of mild lung injury were increased with the LPS exposure similarly in the CMV and CPAP lungs.
Inflammation—systemic.
The neutrophils in the blood did not change with CMV or CPAP at 1.5 or 3 h relative to cord blood neutrophil numbers (Fig. 4). In contrast, the blood neutrophil numbers decreased similarly in CMV and CPAP lambs exposed to intratracheal LPS. The mRNA expression for the pro-inflammatory cytokine IL-1β increased in liver with CMV or CPAP and LPS exposure further increased IL-1β mRNA in the CPAP group.
The number of neutrophils in the blood in lambs given either intratracheal saline (IT Sal) or LPS (IT LPS) at 15 min during ventilation with either CMV or CPAP. Samples were taken from cord blood (baseline; black bars) and at 1.5 h (white bars) and 3 h (hatched bars). Data are shown as mean ± SEM *p < 0.05 from baseline value.
IL-6 mRNA also was increased in liver in the LPS exposed CMV and CPAP groups. There were only small changes in expression of TLR-4 mRNA in the liver. LPS was not detected in the plasma at 3 h of age in the LPS exposed lambs.
DISCUSSION
The preterm surfactant deficient lung is easily injured with CMV during the transition at birth from a fluid-filled lung to an aerated lung (26,27). The initiation of ventilation with birth can cause nonuniform inflation and small airway stretch (5,6). Although the use of PEEP can improve oxygenation (28), it may not protect the lungs from injury (29). Negative pressure ventilation improves oxygenation and decreases injury relative to CMV in rabbits made surfactant deficient by lung lavage (30). Similarly, high frequency ventilation using sufficient mean airway pressure can protect the preterm and surfactant deficient adult lung from injury (31,32). CPAP combines airspace distention with spontaneous ventilation and can decrease lung injury relative to CMV in preterm lambs (10,21). Contrary to our hypothesis and previous studies, we did not find a decrease in indicators of lung injury or systemic inflammatory responses in preterm lambs supported on CPAP relative to CMV. Intratracheal LPS amplified the indicators of lung injury and systemic responses modestly without differential effects between the CPAP and CMV groups.
We asked if CPAP would protect the newborn from the pro-inflammatory mediator LPS in the lungs because many preterm infants are born with inflamed lungs (33). Ventilation of inflamed adult lungs can release inflammatory mediators to the systemic circulation and cause multi-system organ failure (34). In term lambs, short-term gentle ventilation of lungs exposed to intratracheal LPS (10 mg) caused lung inflammation, but minimal systemic responses (16). In contrast, high tidal volume ventilation of term lambs or gentle ventilation of preterm lambs exposed to a 100-fold lower dose of intratracheal LPS (0.1 mg) or IL-1α (50 μg/kg) caused lung inflammation and systemic responses with detection of LPS in the plasma (17). In many centers, the initial respiratory care of preterm infants now is focused on the use of CPAP and avoidance of CMV (8), as a less invasive and potentially less injurious treatment. A large, randomized, controlled trial reported early benefits to CPAP relative to CMV despite an increase in pneumothorax rate, but no differences in death or BPD at 36 wk (8). One benefit could be decreased systemic effects if the inflamed preterm lung were not ventilated. We find no support for a protective effect of CPAP in these lambs exposed to intratracheal LPS at 15 min of age.
There are a number of caveats and limitations to the study. These lambs were exposed to betamethasone as fetuses to prepare them to breathe (10,20). This is clinically relevant as most women at risk of preterm delivery receive antenatal corticosteroids (35). Fetal exposure to betamethasone will thin the mesenchyme of the lungs and increase the potential lung gas volume, but not increase surfactant pools within the 36 h interval between maternal treatment and delivery used here (36,37). Such an effect is suggested because the gas volume measured with 40 cm H2O distending pressure was about 70 mL/kg in these 133 d gestational age lambs, whereas lambs bred concurrently for a different study and not exposed to betamethasone had lung volumes of about 50 mL/kg (29). We did not measure the amount of surfactant because the animals received 10 mg/kg surfactant to help distribute the LPS to the distal lung. However, the saturated phosphatidylcholine in the lambs studied in parallel by us was 2 to 3 μmol/kg, a very low surfactant amount (21). The lambs used for this study were surfactant deficient based on the pressures required to ventilate the CMV lambs, the inspired oxygen requirement of all groups, and the high Paco2 and minute ventilation values resulting from spontaneous ventilation by the CPAP lambs. Fetal betamethasone exposure also decreases epithelial permeability of the lungs (38). In contrast to a previous report (16), LPS was not detected in plasma of the CMV lambs, possibly because of the betamethasone effect on epithelial permeability. The inflammatory cell recruitment to the BALF also was minimal with LPS exposure, and maternal betamethasone treatments will initially decrease monocyte, and presumably neutrophil, responses in sheep (39). However, pro-inflammatory cytokine mRNA levels were similar in these lungs to studies without antenatal betamethasone exposures (29). Our results demonstrating systemic effects of LPS from the airspaces suggest that antenatal corticosteroids will not protect the systemic circulation from inflamed lungs. Also, the injury caused within the first 15 min before LPS administration may have confounded the results, however, this seems unlikely due to the similarity of inflammation markers in the saline control groups of both CPAP and CMV lambs.
It is worth emphasizing that spontaneous ventilation with 8 cm H2O CPAP did not decrease the indicators of lung injury relative to the gentle CMV in the groups not exposed to LPS. The difference between this study and our previous reports with preterm lambs demonstrating subtle differences between CPAP and CMV is that the CPAP lambs in the current study had high Paco2 values and respiratory distress indicated by tachypnea with ineffective breathing and high minute ventilation (10,21). It is possible that the difference in Paco2 between CMV and CPAP lambs may have confounded the results, however, the Paco2 levels are within levels widely observed clinically. The clinical correlate is that CPAP may not protect the lung from injury when significant lung immaturity/surfactant deficiency is present. The low 10 mg/kg dose of surfactant seemed to have no effect on respiratory distress or Paco2 values in these lambs.
Based on this study, CPAP will not protect the moderately preterm lung from injury in spontaneously breathing lambs with significant respiratory distress. CPAP also will not protect the lung or the body from effects of pro-inflammatory mediators in the lungs. Variables that no doubt modulate these findings are the amount of lung maturation, the exposure history to corticosteroids, and the techniques used to ventilate the preterm lung.
Abbreviations
- CMV:
-
conventional mechanical ventilation
- CPAP:
-
continuous positive airway pressure
- FRC:
-
functional residual capacity
- LPS:
-
lipopolysaccharide (Escherichia coli)
- OI:
-
oxygenation index
- PEEP:
-
positive end-expiratory pressure
References
Bjorklund LJ, Ingimarsson J, Curstedt T, John J, Robertson B, Werner O, Vilstrup CT 1997 Manual ventilation with a few large breaths at birth compromises the therapeutic effect of subsequent surfactant replacement in immature lambs. Pediatr Res 42: 348–355
Tsuchida S, Engelberts D, Peltekova V, Hopkins N, Frndova H, Babyn P, McKerlie C, Post M, McLoughlin P, Kavanagh BP 2006 Atelectasis causes alveolar injury in nonatelectatic lung regions. Am J Respir Crit Care Med 174: 279–289
Dreyfuss D, Saumon G 1998 Ventilator-induced lung injury: lessons from experimental studies. Am J Respir Crit Care Med 157: 294–323
Tremblay LN, Slutsky AS 1998 Ventilator-induced injury: from barotrauma to biotrauma. Proc Assoc Am Physicians 110: 482–488
Clark RH, Gerstmann DR, Jobe AH, Moffitt ST, Slutsky AS, Yoder BA 2001 Lung injury in neonates: causes, strategies for prevention, and long-term consequences. J Pediatr 139: 478–486
Sinclair SE, Molthen RC, Haworth ST, Dawson CA, Waters CM 2007 Airway strain during mechanical ventilation in an intact animal model. Am J Respir Crit Care Med 176: 786–794
Ammari A, Suri M, Milisavljevic V, Sahni R, Bateman D, Sanocka U, Ruzal-Shapiro C, Wung JT, Polin RA 2005 Variables associated with the early failure of nasal CPAP in very low birth weight infants. J Pediatr 147: 341–347
Morley CJ, Davis PG, Doyle LW, Brion LP, Hascoet JM, Carlin JB 2008 Nasal CPAP or intubation at birth for very preterm infants. N Engl J Med 358: 700–708
Tsuchida S, Engelberts D, Roth M, McKerlie C, Post M, Kavanagh BP 2005 Continuous positive airway pressure causes lung injury in a model of sepsis. Am J Physiol Lung Cell Mol Physiol 289: L554–L564
Jobe AH, Kramer BW, Moss TJ, Newnham JP, Ikegami M 2002 Decreased indicators of lung injury with continuous positive expiratory pressure in preterm lambs. Pediatr Res 52: 387–392
Avery ME, Tooley WH, Keller JB, Hurd SS, Bryan MH, Cotton RB, Epstein MF, Fitzhardinge PM, Hansen CB, Hansen TN, Hodson WA, James LS, Kitterman JA, Nielsen HC, Poirier TA, Truog WE, Wung JT 1987 Is chronic lung disease in low birth weight infants preventable? A survey of eight centers. Pediatrics 79: 26–30
Muscedere JG, Mullen JB, Gan K, Slutsky AS 1994 Tidal ventilation at low airway pressures can augment lung injury. Am J Respir Crit Care Med 149: 1327–1334
Tremblay L, Valenza F, Ribeiro SP, Li J, Slutsky AS 1997 Injurious ventilatory strategies increase cytokines and c-fos m-RNA expression in an isolated rat lung model. J Clin Invest 99: 944–952
Chiumello D, Pristine G, Slutsky AS 1999 Mechanical ventilation affects local and systemic cytokines in an animal model of acute respiratory distress syndrome. Am J Respir Crit Care Med 160: 109–116
Murphy DB, Cregg N, Tremblay L, Engelberts D, Laffey JG, Slutsky AS, Romaschin A, Kavanagh BP 2000 Adverse ventilatory strategy causes pulmonary-to-systemic translocation of endotoxin. Am J Respir Crit Care Med 162: 27–33
Kramer BW, Ikegami M, Jobe AH 2002 Intratracheal endotoxin causes systemic inflammation in ventilated preterm lambs. Am J Respir Crit Care Med 165: 463–469
Mulrooney N, Jobe AH, Ikegami M 2004 Lung inflammatory responses to intratracheal interleukin-1alpha in ventilated preterm lambs. Pediatr Res 55: 682–687
Andrews WW, Goldenberg RL, Faye-Petersen O, Cliver S, Goepfert AR, Hauth JC 2006 The Alabama Preterm Birth study: polymorphonuclear and mononuclear cell placental infiltrations, other markers of inflammation, and outcomes in 23- to 32-week preterm newborn infants. Am J Obstet Gynecol 195: 803–808
de Dooy J, Colpaert C, Schuerwegh A, Bridts C, van der Planken M, Ieven M, de Clerck L, Stevens W, Mahieu L 2003 Relationship between histologic chorioamnionitis and early inflammatory variables in blood, tracheal aspirates, and endotracheal colonization in preterm infants. Pediatr Res 54: 113–119
Pillow JJ, Hillman N, Moss TJ, Polglase G, Bold G, Beaumont C, Ikegami M, Jobe AH 2007 Bubble continuous positive airway pressure enhances lung volume and gas exchange in preterm lambs. Am J Respir Crit Care Med 176: 63–69
Mulrooney N, Champion Z, Moss TJ, Nitsos I, Ikegami M, Jobe AH 2005 Surfactant and physiologic responses of preterm lambs to continuous positive airway pressure. Am J Respir Crit Care Med 171: 488–493
Charon A, Taeusch W, Fitzgibbon C, Smith GB, Treves ST, Phelps DS 1989 Factors associated with surfactant treatment response in infants with severe respiratory distress syndrome. Pediatrics 83: 348–354
Lowry OH, Rosebrough NJ, Farr AL, Randall RJ 1951 Protein measurement with the Folin phenol reagent. J Biol Chem 193: 265–275
Kallapur SG, Willet KE, Jobe AH, Ikegami M, Bachurski CJ 2001 Intra-amniotic endotoxin: chorioamnionitis precedes lung maturation in preterm lambs. Am J Physiol Lung Cell Mol Physiol 280: L527–L536
Hillman NH, Moss TJ, Nitsos I, Kramer BW, Bachurski CJ, Ikegami M, Jobe AH, Kallapur SG 2008 Toll-like receptors and agonist responses in the developing fetal sheep lung. Pediatr Res 63: 388–393
Hillman NH, Moss TJ, Kallapur SG, Bachurski C, Pillow JJ, Polglase GR, Nitsos I, Kramer BW, Jobe AH 2007 Brief, large tidal volume ventilation initiates lung injury and a systemic response in fetal sheep. Am J Respir Crit Care Med 176: 575–581
Wada K, Jobe AH, Ikegami M 1997 Tidal volume effects on surfactant treatment responses with the initiation of ventilation in preterm lambs. J Appl Physiol 83: 1054–1061
Polglase GR, Morley CJ, Crossley KJ, Dargaville P, Harding R, Morgan DL, Hooper SB 2005 Positive end-expiratory pressure differentially alters pulmonary hemodynamics and oxygenation in ventilated, very premature lambs. J Appl Physiol 99: 1453–1461
Polglase GR, Hillman N, Pillow JJ, Cheah FC, Nitsos I, Moss TJ, Kramer BW, Ikegami M, Kallapur SG, Jobe AH 2008 Positive end-expiratory pressure and tidal volume during initial ventilation of preterm lambs. Pediatr Res 64: 517–522
Grasso F, Engelberts D, Helm E, Frndova H, Jarvis S, Talakoub O, McKerlie C, Babyn P, Post M, Kavanagh BP 2008 Negative-pressure ventilation: better oxygenation and less lung injury. Am J Respir Crit Care Med 177: 412–418
Hamilton PP, Onayemi A, Smyth JA, Gillan JE, Cutz E, Froese AB, Bryan AC 1983 Comparison of conventional and high-frequency ventilation: oxygenation and lung pathology. J Appl Physiol 55: 131–138
Yoder BA, Siler-Khodr T, Winter VT, Coalson JJ 2000 High-frequency oscillatory ventilation: effects on lung function, mechanics, and airway cytokines in the immature baboon model for neonatal chronic lung disease. Am J Respir Crit Care Med 162: 1867–1876
Watterberg KL, Demers LM, Scott SM, Murphy S 1996 Chorioamnionitis and early lung inflammation in infants in whom bronchopulmonary dysplasia develops. Pediatrics 97: 210–215
Plotz FB, Slutsky AS, van Vught AJ, Heijnen CJ 2004 Ventilator-induced lung injury and multiple system organ failure: a critical review of facts and hypotheses. Intensive Care Med 30: 1865–1872
Roberts D, Dalziel S 2006 Antenatal corticosteroids for accelerating fetal lung maturation for women at risk of preterm birth. Cochrane Database Syst Rev 3: CD004454
Ikegami M, Polk DH, Jobe AH, Newnham J, Sly P, Kohan R, Kelly R 1996 Effect of interval from fetal corticosteriod treatment to delivery on postnatal lung function of preterm lambs. J Appl Physiol 80: 591–597
Willet KE, Jobe AH, Ikegami M, Newnham J, Brennan S, Sly PD 2000 Antenatal endotoxin and glucocorticoid effects on lung morphometry in preterm lambs. Pediatr Res 48: 782–788
Ikegami M, Polk DH, Jobe AH, Newnham J, Sly P, Kohen R, Kelly R 1995 Postnatal lung function in lambs after fetal hormone treatment. Effects of gestational age. Am J Respir Crit Care Med 152: 1256–1261
Kramer BW, Ikegami M, Moss TJ, Nitsos I, Newnham JP, Jobe AH 2004 Antenatal betamethasone changes cord blood monocyte responses to endotoxin in preterm lambs. Pediatr Res 55: 764–768
Acknowledgements
We gratefully acknowledge the assistance of JRL Hall & Co. for the supply of the sheep, particularly Ross Wales, Sara Ritchie, and Fiona Hall. We also thank Dr Jenni Henderson, Ms Carryn McLean, Mr David Cruise, and Ms Marina Modric for their assistance in delivering and caring for the lambs and performing postmortem tissue preparations and analyses.
Author information
Authors and Affiliations
Corresponding author
Additional information
Supported by NICHD 12714 and the Women and Infants Research Foundation, NHMRC and NHFA Fellowship (G.R.P.), and Sylvia and Charles Viertel Senior Medical Research Fellowship (J.J.P.). Grant support, CPAP circuits, humidifiers, and radiant warmer beds were provided by Fisher and Paykel Healthcare (NZ).
Rights and permissions
About this article
Cite this article
Polglase, G., Hillman, N., Ball, M. et al. Lung and Systemic Inflammation in Preterm Lambs on Continuous Positive Airway Pressure or Conventional Ventilation. Pediatr Res 65, 67–71 (2009). https://doi.org/10.1203/PDR.0b013e318189487e
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1203/PDR.0b013e318189487e
This article is cited by
-
Preterm lung and brain responses to mechanical ventilation and corticosteroids
Journal of Perinatology (2023)
-
Metabolic-endocrine disruption due to preterm birth impacts growth, body composition, and neonatal outcome
Pediatric Research (2022)
-
Aerosol drug delivery to spontaneously-breathing preterm neonates: lessons learned
Respiratory Research (2021)
-
Budesonide with surfactant decreases systemic responses in mechanically ventilated preterm lambs exposed to fetal intra-amniotic lipopolysaccharide
Pediatric Research (2021)
-
Dose of budesonide with surfactant affects lung and systemic inflammation after normal and injurious ventilation in preterm lambs
Pediatric Research (2020)