Abstract
Suboptimal developmental environments program offspring to lifelong metabolic problems. We evaluated effects of maternal isocaloric low protein diet during pregnancy and/or lactation on milk quantity and leptin concentration at postnatal day 7, 14, and 21. Control mothers ate 20% casein (C) and restricted mothers (R) 10% casein to provide four groups: CC, RR, CR, and RC (first letter pregnancy and second lactation diet) to enable evaluation of effects influenced by maternal diet during pregnancy and lactation. Milk leptin was not a determinant of pup serum leptin. Pup serum leptin did not inhibit milk appetite at any postnatal age. Pup serum leptin did not correlate with pup adipose tissue. Finally, the normal postnatal leptin rise in pup serum was delayed by prenatal undernutrition. These data suggest that fetal nutrition modifies timing of neonatal leptin surge and may contribute to the development of altered appetite and metabolic disorders in later life.
Similar content being viewed by others
Main
Human epidemiologic (1) and experimental animal studies (2) have shown that suboptimal environments in the womb and during early neonatal life alter growth and predispose individuals to lifelong health problems. Effects of maternal nutrient restriction during pregnancy and/or lactation have been studied in many different models. A variety of growth, endocrine and cardiovascular phenotypes result from nutrient restriction in different developmental windows. Perinatal malnutrition predisposes to offspring obesity in adulthood by changes during the development of central neural pathways mediated by regulatory mechanisms including leptin (3). Rapid catch-up growth after early growth restriction increases the risk of developing obesity and cardiovascular disease in later life (4,5).
One of the most important neonatal factors involved in developmental programming is the adequacy of nutrition during the lactation period. Breast feeding decreases the risk of obesity in later childhood (6). Many factors including maternal milk composition, energy content, and quantity may influence future appetite control. Breast milk contains leptin (7) a hormone produced and secreted in a variety of tissues, predominantly by adipocytes (8) which regulates food intake and energy expenditure at the hypothalamic level in adult animals (8,9). Circulating levels of leptin correlate positively with the amount of fat stores (10). During the first days of postnatal life, leptin levels are higher than those observed later in development (11). Several studies have demonstrated a surge of leptin around postnatal days (PND) 10–14 in the rat (11). This surge has been correlated with maturation of the central nervous mechanisms that regulate appetite in later life. Leptin also seems to play a key role in programming the structural and functional development of hypothalamic orixigenic and anorexigenic centers in the early postnatal period.
We have demonstrated that maternal protein restriction in the rat during either pregnancy or lactation alters postnatal growth, appetitive behavior, and leptin concentrations at 110 d postnatal age (12). The developmental programming effects on pups are dependent on offspring sex and the period of exposure to undernutrition. Teixeira et al. have shown changes in leptin concentration, during lactation, in pups whose mothers were exposed to protein or energy restriction (13). In contrast with our reported data (12), the leptin serum concentrations after weaning were unchanged. This discrepancy could be explained by differences in the litter size retained during suckling in these studies.
Little attention has been given to how the various nutrient restriction regimens alter quantity and quality of milk available during lactation. Associations have been published between offspring weight, offspring growth, and milk leptin concentration (14). However, in altricial species where the early postnatal leptin surge plays a critical role in the maturation of appetitive centers in the brain, it is important to determine whether the leptin responsible for this key maturational function is of fetal or maternal origin.
We evaluated the effects of feeding an isocaloric low protein diet, during pregnancy and/or lactation, on milk quantity and milk leptin concentration available to pups at PND 7, 14, and 21 to determine whether there is any correlation between neonatal serum leptin concentrations with milk leptin concentrations and total milk leptin provided to the pups on neonatal life in the rat.
MATERIALS AND METHODS
Care and maintenance of animals.
All procedures were approved by the Animal Experimentation Ethics Committee of the “Intituto Nacional de Ciencias Médicas y Nutrición, Salvador Zubirán” (INCMNSZ), Mexico. Details of maternal diet, breeding, and management of the four groups of offspring have been published in detail (15). Briefly, mothers were virgin female albino Wistar rats aged 11 ± 1 wk and weighing 240 ± 20 g, obtained from INCMNSZ. Female rats with regular cycles were maintained on Purina Laboratory Chow 5001 and under controlled lighting (lights on from 7:00 am to 7:00 pm at 22–23°C).
Female rats were mated overnight with proven male breeders and the day on which spermatozoa were present in a vaginal smear was designated as the day of conception, day 0. Only rats which were pregnant within 5 d of introduction of the male were retained in the study. Pregnant rats were transferred to individual metabolic cages and allocated at random to one of two groups to be fed either a 20% casein (control diet—C) or 10% casein isocaloric diet (restricted diet—R) (16). Food and water were available ad libitum.
Food was provided in the form of flat biscuits, which were retained behind a grill through which the rats nibbled the food. On day 20 postconception, pregnant rats were transferred to normal cages to optimal conditions for delivery, which occurred in the early daylight hours between 9.00 am and noon on postconceptual day 22. Day of delivery was considered as day 0 of postnatal life.
Maternal weight, litter size, and pup weight were recorded at birth. Ano-genital distance was measured with calipers. According to our published data (12), sex was judged according to whether the ano-genital distance was less (female) or greater than (male) 2.5 mm. To ensure homogeneity of study subjects, litters of over 14 pups, or less than 12 pups, were not included in the study. Litters of 12–14 pups were adjusted to 12 pups for each mother while maintaining as close to a 1:1 sex ratio as possible. Four groups were established: CC in which mothers that received the control diet during pregnancy continued to be fed the control diet during lactation; RR in which mothers that had received the restricted diet during pregnancy continued to receive the restricted diet during lactation; CR in which mothers that received the control diet during pregnancy received the restricted diet during lactation; and RC in which mothers that received the restricted diet during pregnancy were provided with the control diet during lactation. After weaning (PND 21), all pups were fed with C diet.
Experiment 1.
Twenty-two mothers/litters: CC (n = 5: 5.6 ± 0.4 male/litter, 6.4 ± 0.4 female/litter), RR (n = 6: 5.5 ± 0.5 male/litter, 6.5 ± 0.5 female/litter), CR (n = 6: 5.3 ± 0.7 male/litter, 6.7 ± 0.7 female/litter), and RC (n = 5: 6.2 ± 0.2 male/litter, 5.8 ± 0.2 female/litter) were studied for maternal milk production and pup milk intake. Randomly two male and two female pups per litter were used to determinate carcass components. Preliminary analysis for differences according to the sex of the pup revealed no difference, and thus all data were pooled.
Experiment 2.
Eighty mothers and litters, n = 5 for group per age were used to study maternal milk and pup serum leptin concentrations. Randomly, two male and two female pups/litter per age were used for serum leptin determination with the exception of offspring from PND 2 in which all the pups for each sex per litter (n = 4–8 pups/sex) were used and the samples polled. No sex differences were found in serum leptin at this age, therefore, we averaged male and female data.
Measurement of maternal milk production and pup milk intake.
At 7:00 am on PND 7, 14, and 21, pups were removed from the mothers for 4 h during which mothers ate ad libitum whereas the pups did not feed. Pups were labeled on the tail with a permanent marker. Mothers were weighed at the beginning and end of the 4-h period. Pups were weighed individually immediately before they were returned to the mothers and again 1 h later.
As discussed below, the excellent correlation between the increase in maternal and pup weight was used to justify approximation of maternal weight gain to milk production. Relative milk intake was calculated from the absolute food intake/pup body weight.
Measurement of milk leptin concentration.
Milk was obtained on PND 7, 14, and 21. Pups were removed from the mothers for 4 h after which the mothers received 0.8 U oxytocin (ip) and milk expressed 15 min later. Samples were vortexed, divided in aliquots, and frozen at −20°C until analysis. Milk samples were thawed at 37°C and vortexed vigorously before pipetting to ensure sample uniformity. Milk leptin concentration was determined by RIA using a commercial rat kit from Linco Research, Inc. with a detection limit 0.5 ng/mL using 100 μL samples size. Milk samples were assayed in duplicate.
Measurement of pup serum leptin concentrations.
At 7:00 am on PND 7, 14, and 21 pups were removed from the mothers for 4 h during which pups did not feed. We obtained all samples from rats in the fasted state to avoid leptin variation. Offspring of 2 d of age were not subjected to fasting. Between 11:00 am and noon, pups were rapidly killed by decapitation using a rodent guillotine (Thomas Scientific, NJ). Trunk blood was collected into polyethylene tubes and allowed to clot at 4°C for 1 h. Blood samples were centrifuged at 1500 g for 15 min at 4°C. Serum samples were kept at −20°C until assayed. Pup serum leptin concentrations were determined by RIA using a commercial rat kit from Linco Research, Inc. Each serum sample was assayed in duplicate.
Carcass components (fat-to-protein ratio).
At 25 d of age, rats were rapidly killed as described above and kept frozen in small plastic bags until they were analyzed. Head and gastrointestinal tract were discarded from pups and not included in the analysis. After weighing (wet weight), each carcass was chopped into small pieces, placed in a tared beaker and dried at 60°C to constant weight. The weight lost is considered to be water weight. The dried carcasses were ground-up and aliquots were taken. Fat was determined gravimetrically, evaporating the solvent from an aliquot of the hexane-isopropanol extract by Soxhlet method and total nitrogen by the Kjeldahl method (17).
Statistical analysis.
Data from animals within the litter were averaged and analysis performed only on number of litters. All data are presented as the means ± standard error of the mean. Statistical analysis was performed using ANOVA followed by Tukey's test. Unpaired t test was also used to compare male and female data. Maternal milk production, pup milk intake, maternal and pup serum leptin, and pup body weight correlations were calculated using a Pearson correlation. p < 0.05 was considered significant.
RESULTS
Maternal body weight.
Table 1 shows maternal weight. There were no differences between the groups on the day of delivery. The maternal weight data on the day of birth lack precision because of the irregular timing of delivery relative to the time at which the dams were weighed, therefore, data at birth are provided to give an approximate weight at the start of lactation. Although maternal weight showed a tendency to be lower in RR and CR compared with CC, the difference was only significant on PND 14.
Milk production and leptin concentration.
Maternal milk production was lower in RR and CR and was higher in RC at PND 14 and 21 (Table 2). CC milk leptin concentration was not different at the three ages studied. RR milk leptin concentration was higher at PND 14 than at PND 7. CR milk leptin concentration was higher at PND 21 than at 14 and higher at PND 14 than 7 and milk leptin concentration in the RC group was higher at PND 21 than PND 7 (Table 2). Milk leptin concentration was higher in the CR group than in the CC, RR, and RC groups at PND 14 and 21, and higher in the RC group than in the CC group at PND 21 (Table 2).
Pup serum leptin.
For CC and CR groups, the highest pup leptin concentration was at PND 14. In contrast, in RR and RC the highest was observed at PND 21, indicating a delay in the leptin peak occurrence in animals which were undernourished prenatally (Fig. 1). At PND 2, there were no differences among groups in pup serum leptin. At PND 14, RR and RC had lower leptin values in comparison with those in CC. At PND 21, leptin values in RR were higher than in CC and CR, and at PND 25, pup leptin concentration for RC was higher than for CC and CR.
There was no correlation between milk and pup serum leptin concentrations at any age either in the individual groups (p > 0.05) or when all the groups and the different days were pooled (r = 0.04, p = 0.7). Milk leptin-to-pup serum leptin ratio was higher in CR than in CC at all ages (p < 0.05) as were RR and RC at PND 14 (Table 2).
Pup body weight and milk intake.
There were no important offspring sex differences for pup body weight in the first 25 PND (Table 3). At PND 7, RC pup body weight was higher than the weight in RR and CR. Both male and female offspring in the groups restricted during lactation had lower body weights in comparison with the weights in CC and RC from PND 14 to 25.
Absolute food intake by the pups increased in all groups with age (Table 4). Pups in the RC group had a higher milk intake at PND 7 in comparison with those in CC and CR. At PND 14, the RC group had the highest and CR the lowest absolute food intake. At PND 21, the absolute food intake in the RR group was lower than the intake in CC and RC. The only differences in relative food intake were at PND 14 when RC pups had a higher relative food intake than those at RR and CR (Table 4).
Correlation studies.
There was a positive correlation between milk production and milk intake when all groups and days were pooled (Fig. 2). No correlation was found for PND 7, but there was a high positive correlation on PND 14 (r = 0.8 p < 0.001) and approaching significance at PND 21 (r = 0.4 p = 0.08) (Fig. 2).
There was no correlation between pup serum leptin and body weight gain after 1 h of feeding (milk intake) in any individual group on the 3 days of measurement. However, when all groups and ages were pooled, milk intake and pup serum leptin were significantly positively correlated (r = 0.3, p = 0.01) (Fig. 3).
There was a positive correlation between absolute milk intake (g/h) and pup body weight (g) in CC, CR, and RC groups at PND 7, 14, and 21 (p < 0.05). No correlation was found for the RR group (r = 0.3, p = 0.3) (Fig. 4). Relative food intake expressed as weight gain/h/g of body weight was positively correlated with pup weight in CC and CR. No correlation was observed in the RR and RC groups. It is interesting to note that these groups were those that presented a delay in serum leptin peak occurrence.
Pup body weight (A) as a function of absolute milk intake: CC (r = 0.76, p = 0.001), RR (r = 0.28, p = 0.3), CR (r = 0.9, p < 0.001), and RC (r = 0.66, p = 0.006) or (B) as a function of relative milk intake: CC (r = 0.7, p = 0.005), RR (r = 0.08, p = 0.8), CR (r = 0.8, p < 0.001), and RC (r = 0.16, p = 0.55) on PND 7, 14, and 21. Mean ± SEM, n = 5–6 litters.
Pup body composition at 25 d of age.
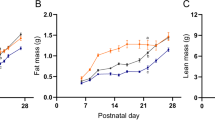
At PND 25, body fat-to-protein ratio was higher in RC than RR male offspring and higher in RC than in CC, RR, and CR female pups (Fig. 5).
DISCUSSION
In comparison with the literature on postnatal growth and function of offspring of rats submitted to various nutritional challenges during pregnancy and lactation there are few data on how various nutrient restriction regimens alter quantity and quality of milk available to the pups. Consideration of the ways in which milk leptin may affect pup development has received less attention than the effects of experimental leptin administration in the neonatal period. The present study was conducted to determine the effects of maternal protein restriction during pregnancy and/or lactation on offspring milk availability and intake, milk leptin concentration and total milk leptin provided to the pups, pup serum leptin and pup growth, and their relationship to offspring body composition at weaning.
Maternal weight showed a tendency to be lower in RR and CR, with not statistical differences among them. However, the subsequent weight gain during lactation of RR was 24 g whereas CR was 3 g. We do not have an explanation for this difference, but because the mothers in RR group were restricted during pregnancy they probably had a greater drive to eat during lactation than those in the CR group.
Our finding of similar maternal weight at birth and similar pup weight at birth correlates very well. As in many other studies, protein restriction at these levels is not enough to make any difference in maternal and pup weight at birth (18). In the developmental programming field, it is now generally considered that weight is a very poor measure of changes. Important modifications occur in the pups without a change in the overall weight.
Analysis of the data for maternal weight gain over a period of 4 h (milk production) revealed interesting differences in the four groups. Although weight at all the three ages studied was similar in CC and RC, the maternal weight gain in RC was 50% greater than in that in CC. Because maternal weight over the whole period did not differ in these two groups we have made the assumption that increased weight gain in the 4 h reflects increased milk production. This conclusion is supported by the greater increase in pup weight gain after 1 h with the mother (milk intake) in the RC compared with CR group at PND 7 and 14. In contrast, mothers in RR and CR exhibited decreased milk production. Maternal and offspring suckling factors are both likely to be involved in the increased milk production in a setting of increased pup milk consumption. There are data in the literature that indicate that pup consumption affects milk production as well as data showing that milk production has an influence on pup consumption (19,20). One way to determine the relative influences of these two possibilities in future studies would be to reduce the number of pups suckling the dam.
Leptin is present in the milk of many different species (21–23), including humans (7). Nursing rats can transfer leptin to the neonates via the milk (24). Milk leptin concentration is determined by a number of factors, the major ones being mammary gland leptin secretion and total volume of milk produced. There were no clear associations between milk leptin concentration at the different postnatal ages and the four dietary regimens. This finding of constancy in milk leptin in mothers with different dietary histories is in keeping with the view that milk composition is protected under a wide range of maternal metabolism (25). This protection does not however extend to the quantity of milk produced as we show here.
The milk production and pup milk intake in RC group is significantly higher than in the CC group, but pup body weights between these two groups are similar. This observation demonstrates the complexity of just evaluating postnatal development in terms of weight. Clearly other factors such as metabolic rate, activity, and heat loss will all play a role. The results observed in the RC group (milk intake and body composition) are of importance for determination of optimal postdelivery and preweaning feeding of human neonates undernourished in utero due to causes other than poor maternal nutrition such as uterine vascular disease when the fetus is undernourished but the neonate is not exposed to poor maternal nutrition during lactation. The changes we have observed in pup serum leptin and in body growth postnatally in the RC group, may be those for which this type of newborn is most at risk. Rapid catch-up growth during the early months of life during lactation or feeding with artificial formulas can result in problems in later life (26).
Serum leptin concentrations are higher in neonates than in adults (27,28) despite the presence of a lower proportion of body fat immediately after birth (28). We observed a delay in the leptin peak (PND 21) in RR and RC. This observation suggests that the common factor is the restriction during pregnancy and is of considerable interest in the light of the work from several laboratories that indicates that the normal rat postnatal serum leptin surge that takes place around PND 10–14 plays a central role in the maturation of the hypothalamic appetitive centers (29). Advancing the leptin peak by exogenous subcutaneous leptin administration leads to obesity in later life (3), however, the opposite effects have been reported by others (30). There are no data on the effects of a delayed serum leptin surge such as we describe in the RR and RC groups. We have reported that the offspring of RC mothers become very obese whereas offspring of RR mothers have the lowest weight gain of all four groups in adulthood. These observations apply to both males and females (12,31). These findings indicate that the effects of developmental influences during pregnancy are further modified by the level of nutrition available in lactation. There is currently much interest in an altered timing of the leptin surge in predicting future appetite and phenotype of offspring. Our findings indicate that there are many factors determining eventual phenotype in addition to the timing of the leptin surge.
When body composition was evaluated at PND 25, the highest leptin and body fat were seen in the RC group. However, body fat did not correlate with pup leptin in the other three groups. Further studies are needed to evaluate both the source of pup serum leptin and the mechanism by which the leptin surge is delayed in the offspring of mothers restricted during pregnancy and how changes in appetite brought about by this alteration interact with other factors such as activity and altered metabolic rate in the genesis of obesity.
We also sought to determine whether the leptin in fetal serum is of fetal or maternal origin. Our findings indicate that there is no correlation between milk leptin intake and pup serum leptin. This finding is of importance in relation to the potential role of pup leptin in inhibiting pup suckling behavior. It is well known that in adults the hormone leptin inhibits food intake. However, the correlation between pup serum leptin and milk intake was very weak and showed no significance in any individual group. This finding is consistent with previous observations that leptin administration is unable to influence body weight, fat pad, and milk intake (28). In addition, exogenous leptin did not modify milk intake in neonatal mice at PND 7–10 (28). In contrast, rat studies have shown that physiologic oral doses of leptin administered during lactation exert anorexigenic effects without affecting body weight gain (23). Our findings are not surprising in view of the very different timing of maximal pup serum leptin values in the four groups. In fact the poor correlation of pup serum leptin and milk intake over the full study was positive showing no indication of an inhibitory effect of leptin on milk appetite in the neonatal pups.
In conclusion, we would make four major observations from our evaluation of changes in milk and pup leptin. First, our findings indicate that milk leptin is unlikely to be a major determinant of pup serum leptin. Secondly, we could find no evidence that pup serum leptin inhibits milk appetite at this age. Thirdly, pup adipose tissue may not be the only neonatal source of leptin in pup serum. Finally, the normal postnatal rise in leptin was delayed in animals undernourished prenatally. These data are compatible with the view that the timing of neonatal leptin surge is influenced by fetal nutrition and may play a role in altered appetitive regulation and contribute to the development of metabolic disorders in later life.
Abbreviations
- PND:
-
postnatal day
- C:
-
control
- R:
-
restricted
References
Strauss RS 1997 Effects of the intrauterine environment on childhood growth. Br Med Bull 53: 81–95
Nathanielsz PW 2006 Animal models that elucidate basic principles of the developmental origins of adult diseases. ILAR J 47: 73–82
Yura S, Itoh H, Sagawa N, Yamamoto H, Masuzaki H, Nakao K, Kawamura M, Takemura M, Kakui K, Ogawa Y, Fujii S 2005 Role of premature leptin surge in obesity resulting from intrauterine undernutrition. Cell Metab 1: 371–378
Gluckman PD, Cutfield W, Hofman P, Hanson MA 2005 The fetal, neonatal, and infant environments-the long-term consequences for disease risk. Early Hum Dev 81: 51–59
Anguita RM, Sigulem DM, Sawaya AL 1993 Intrauterine food restriction is associated with obesity in young rats. J Nutr 123: 1421–1428
von Kries R, Koletzko B, Sauerwald T, von Mutius E, Barnert D, Grunert V, von Voss H 1999 Breast feeding and obesity: cross sectional study. BMJ 319: 147–150
Houseknecht KL, McGuire MK, Portocarrero CP, McGuire MA, Beerman K 1997 Leptin is present in human milk and is related to maternal plasma leptin concentration and adiposity. Biochem Biophys Res Commun 240: 742–747
Campfield LA, Smith FJ, Guisez Y, Devos R, Burn P 1995 Recombinant mouse OB protein: evidence for a peripheral signal linking adiposity and central neural networks. Science 269: 546–549
Pelleymounter MA, Cullen MJ, Baker MB, Hecht R, Winters D, Boone T, Collins F 1995 Effects of the obese gene product on body weight regulation in ob/ob mice. Science 269: 540–543
Ostlund RE Jr, Yang JW, Klein S, Gingerich R 1996 Relation between plasma leptin concentration and body fat, gender, diet, age, and metabolic covariates. J Clin Endocrinol Metab 81: 3909–3913
Rayner DV, Dalgliesh GD, Duncan JS, Hardie LJ, Hoggard N, Trayhurn P 1997 Postnatal development of the ob gene system: elevated leptin levels in suckling fa/fa rats. Am J Physiol 273: R446–R450
Zambrano E, Bautista CJ, Deas M, Martinez-Samayoa PM, Gonzalez-Zamorano M, Ledesma H, Morales J, Larrea F, Nathanielsz PW 2006 A low maternal protein diet during pregnancy and lactation has sex- and window of exposure-specific effects on offspring growth and food intake, glucose metabolism and serum leptin in the rat. J Physiol 571: 221–230
Teixeira C, Passos M, Ramos C, Dutra S, Moura E 2002 Leptin serum concentration, food intake and body weight in rats whose mothers were exposed to malnutrition during lactation. J Nutr Biochem 13: 493–498
Dundar NO, Anal O, Dundar B, Ozkan H, Caliskan S, Buyukgebiz A 2005 Longitudinal investigation of the relationship between breast milk leptin levels and growth in breast-fed infants. J Pediatr Endocrinol Metab 18: 181–187
Zambrano E, Rodriguez-Gonzalez GL, Guzman C, Garcia-Becerra R, Boeck L, Diaz L, Menjivar M, Larrea F, Nathanielsz PW 2005 A maternal low protein diet during pregnancy and lactation in the rat impairs male reproductive development. J Physiol 563: 275–284
Zambrano E, Martinez-Samayoa PM, Bautista CJ, Deas M, Guillen L, Rodriguez-Gonzalez GL, Guzman C, Larrea F, Nathanielsz PW 2005 Sex differences in transgenerational alterations of growth and metabolism in progeny (F2) of female offspring (F1) of rats fed a low protein diet during pregnancy and lactation. J Physiol 566: 225–236
AOAC 2002 Official Methods of Analysis of AOAC International No. 920.05, 920.39
Langley SC, Browne RF, Jackson AA 1994 Altered glucose tolerance in rats exposed to maternal low protein diets in utero. Comp Biochem Physiol Physiol 109: 223–229
McDonald JM, Nielsen MK 2006 Correlated responses in maternal performance following divergent selection for heat loss in mice. J Anim Sci 84: 300–304
Morag M 1970 Estimation of milk yield in the rat. Lab Anim 4: 259–272
McFadin EL, Morrison CD, Buff PR, Whitley NC, Keisler DH 2002 Leptin concentrations in periparturient ewes and their subsequent offspring. J Anim Sci 80: 738–743
Whitley NC, Walker EL, Harley SA, Keisler DH, Jackson DJ 2005 Correlation between blood and milk serum leptin in goats and growth of their offspring. J Anim Sci 83: 1854–1859
Sanchez J, Oliver P, Miralles O, Ceresi E, Pico C, Palou A 2005 Leptin orally supplied to neonate rats is directly uptaken by the immature stomach and may regulate short-term feeding. Endocrinology 146: 2575–2582
Casabiell X, Pineiro V, Tome MA, Peino R, Dieguez C, Casanueva FF 1997 Presence of leptin in colostrum and/or breast milk from lactating mothers: a potential role in the regulation of neonatal food intake. J Clin Endocrinol Metab 82: 4270–4273
Jenness R 1974 Proceedings: biosynthesis and composition of milk. J Invest Dermatol 63: 109–118
Singhal A, Lanigan J 2007 Breastfeeding, early growth and later obesity. Obes Rev 8: 51–54
Devaskar SU, Ollesch C, Rajakumar RA, Rajakumar PA 1997 Developmental changes in ob gene expression and circulating leptin peptide concentrations. Biochem Biophys Res Commun 238: 44–47
Mistry AM, Swick A, Romsos DR 1999 Leptin alters metabolic rates before acquisition of its anorectic effect in developing neonatal mice. Am J Physiol 277: R742–R747
Grove KL, Smith MS 2003 Ontogeny of the hypothalamic neuropeptide Y system. Physiol Behav 79: 47–63
Vickers MH, Gluckman PD, Coveny AH, Hofman PL, Cutfield WS, Gertler A, Breier BH, Harris M 2005 Neonatal leptin treatment reverses developmental programming. Endocrinology 146: 4211–4216
Guzman C, Cabrera R, Cardenas M, Larrea F, Nathanielsz PW, Zambrano E 2006 Protein restriction during fetal and neonatal development in the rat alters reproductive function and accelerates reproductive ageing in female progeny. J Physiol 572: 97–108
Author information
Authors and Affiliations
Corresponding author
Additional information
This work was partially supported by CONACyT-48839 (México) and the NIH HD21350.
Rights and permissions
About this article
Cite this article
Bautista, C., Boeck, L., Larrea, F. et al. Effects of a Maternal Low Protein Isocaloric Diet on Milk Leptin and Progeny Serum Leptin Concentration and Appetitive Behavior in the First 21 Days of Neonatal Life in the Rat. Pediatr Res 63, 358–363 (2008). https://doi.org/10.1203/01.pdr.0000304938.78998.21
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1203/01.pdr.0000304938.78998.21
This article is cited by
-
Developmental Programming in Animal Models: Critical Evidence of Current Environmental Negative Changes
Reproductive Sciences (2023)
-
Different Protein Sources in the Maternal Diet of the Rat during Gestation and Lactation Affect Milk Composition and Male Offspring Development during Adulthood
Reproductive Sciences (2021)
-
Protein-restricted diet during pregnancy after insemination alters behavioral phenotypes of the progeny
Genes & Nutrition (2017)
-
Thrifty metabolic programming in rats is induced by both maternal undernutrition and postnatal leptin treatment, but masked in the presence of both: implications for models of developmental programming
BMC Genomics (2014)
-
Short- and long-term reproductive effects of prenatal and lactational growth restriction caused by maternal diabetes in male rats
Reproductive Biology and Endocrinology (2011)