Abstract
Children with stage IV neuroblastoma (NBIV) are often malnourished at time of diagnosis, observed as high as 50%. The emphasis of this study was to determine whether an increased resting energy expenditure (REE) is a causative factor. Our hypothesis was that children diagnosed with NBIV have an increased REE, which normalizes with cancer treatment. Changes in nutritional status from time of diagnosis in response to nutritional support were examined. REE and nutritional evaluation were obtained three times: at diagnosis before starting treatment, where tumor burden is expected to be highest; after two courses of chemotherapy, where some response to treatment is expected; and after surgical excision of the primary tumor, where there was presumably minimal residual disease. Ten subjects completed the study. Results showed that REE was not increased, and there was no significant difference between phases (p = 0.29). Fifty percent of our subjects were malnourished at diagnosis. Because REE is not increased in NBIV, it is concluded that malnutrition seen in NBIV is not due to increased energy needs, but is likely due to decreased intake because of the intra-abdominal mass and malignant malaise.
Similar content being viewed by others
Main
Children presenting with stage IV neuroblastoma (NBIV) are frequently cachectic. Cachexia occurs in the setting of uncontrolled growth of tumor, which places a demand on the host for essential nutrients. The mechanisms underlying cachexia are unclear (1). The typical clinical picture is one of weight loss, decreased muscle mass and diminished fat stores, anorexia, and fatigue. Several articles have indicated that an increase in energy expenditure and/or altered metabolism is a plausible explanation for this energy imbalance (2–4). In addition, tumor burden may be responsible for a multitude of metabolic abnormalities observed (2–4). Alterations in energy expenditure, protein, carbohydrate, and lipid metabolism have been reported (2–8). Factors such as fever, infection, stress, cold exposure, physical activity, and catecholamine release have been shown to elevate resting energy expenditure (REE) (9).
Several authors suggest that an increase in REE contributes to the weight loss seen in malignancy. Patients with malignant disease have been reported to have reduced normal or increased energy expenditure (4). Increases in REE have been reported in patients with sarcomas, lung/bronchial cancer, pancreatic cancer and gastric tumors, lymphoma, leukemia, whereas decreased or normal REE has been reported in other types of malignancy (10–16). Data describing the effect of cancer on energy expenditure of children are limited and mainly involve children with leukemia or lymphoma. Convincing data on the effect of the presence of a solid tumor on energy expenditure of children is lacking.
Neuroblastoma is a malignant solid tumor, which originates from the primitive neural crest cell that gives rise to the sympathetic nervous system (17). Neuroblastoma primary tumors can arise from anywhere where normal sympathetic nervous system structures are found (17). Approximately 90–95% of all patients with neuroblastoma will have increased excretion of urinary catecholamine metabolites (17–22). Knowing that this specific tumor originates from neural crest cells that are precursors of cells of the adrenal medulla and sympathetic nervous system, which produces catecholamines potentially causing an elevated heart rate (HR), it was reasoned that the malnutrition would likely be due to an increased REE. Therefore, the emphasis of this study was to determine whether an increased REE is a causative factor in the malnutrition frequently observed in children newly diagnosed with NBIV.
METHODS
Subjects.
Twelve subjects were enrolled in the study. All patients were admitted to the Hematology/Oncology inpatient unit at SickKids for diagnosis and therapy. Subjects were older than 6 mo and younger than 18 y of age. Subjects were excluded if the child was clinically unstable and needed immediate medical treatment. Approval for the study was obtained from the Research Ethics Board, at SickKids, and from the Scientific Advisory Research Board of the Division of Hematology/Oncology. Enrollment took place over a period of approximately 20 mo. Consecutive patients with newly diagnosed NBIV were recruited for the study. At the time of study entry, written informed consent was obtained from patients/parents/caregivers and an information (assent) form was reviewed with children more than 7 y of age.
Study design.
This study was a prospective comparative study of patients newly diagnosed with NBIV, measuring REE by open-circuit indirect calorimetry and assessing nutritional status. REE and anthropometry measurements were obtained at three specific times by the same researcher (G.J.G.). Measurements were taken at diagnosis (phase 1), before starting high-risk neuroblastoma treatment protocol [Childrens Cancer Study Group (CCG)-3891 high-risk neuroblastoma protocol or Children's Oncology Group (COG) A3973 high-risk neuroblastoma protocol], when the tumor burden would be expected to be highest. They were repeated after two courses of chemotherapy (phase 2), when it would be reasonable to expect some response to treatment, and treatment protocols include tumor reimaging by CT/MRI of all areas of bulk tumor (primary and metastases) to monitor treatment response. The final measurement (phase 3) was performed 2 or more weeks after surgical excision of the primary tumor, when the child was fully recovered from surgery, and had been admitted for the sixth (final) cycle of chemotherapy, at which time it was presumed that there was minimal or no residual disease.
Study protocol.
Indirect calorimetry was performed using the Deltatrac II Metabolic Monitor (SensorMedics Corporation, Yorba Linda, CA). This is a computerized open-circuit indirect calorimetry system, which conducts continuous measurements of the concentrations of oxygen and carbon dioxide in inspired and expired air. Data are displayed in 1-min intervals on printout and/or computer screen formats as VO2, VCO2, respiratory quotient (RQ), and energy expenditure. All expired air is collected into this constant flow. Adjustments to the flow were predetermined by the investigator, according to the subject's age and body weight. The Deltatrac II Metabolic Monitor is calibrated every day, by an automatic gas calibration. The Deltatrac II Monitor is a portable system, so the calorimeter was brought to the patient's room. Patients were studied in a modified fasted state (minimum 4–6 h without any oral or enteral intake; i.v. fluids and nutrition support as needed). The subjects were at rest in the supine position on a bed or crib, or quietly reclining on mother's lap in a chair. A clear plastic canopy was placed over the child's head and air was drawn at a constant rate through the hood. The VO2 was monitored until a steady state was reached and maintained for approximately 15–20 min during which energy expenditure was measured. Carbon dioxide production VCO2 and oxygen consumption VO2 were calculated at 1-min intervals. REE was calculated by using the Weir (21) equation and expressed as kcal/d. The percentage predicted REE was calculated using World Health Organization equations (22) based on the Schofield (23) normative data for weight, age, and gender.
Nutritional evaluation was done at the time of diagnosis (phase 1) and at phases 2 and 3. The following criteria were used for diagnosing malnutrition: weight for height (WFH) <85%; serum albumin <32 g/L; and depleted fat stores, measured by triceps skin folds (TSF) <5 percentile for age and gender. A subject was considered at high risk for malnutrition if two or more of the above criteria were fulfilled (adapted from Rickard and Oguz) (24,25). Anthropometric evaluation consisted of weight (kg), height (cm), TSF measurements (mm), mid-arm circumference (MAC, mm), and calculation of mid-arm muscle circumference (MAMC, mm).
Body weight was measured with an electronic digital scale accurate to 0.1 kg (Scale-Tronix model 6006, Serial 6–1248, for children >2 y of age, and Scale-Tronix Pediatric Scale 4800, Serial 48–4442, for infants; Wheaton, IL). Standing height was measured to the nearest 0.1 cm with a wall-mounted stadiometer. Measured weight and height were plotted on the growth and development charts of Tanner et al, and %WFH was calculated (26,27). Percent WFH was calculated by dividing actual body weight (at time of study phases) by weight on the same percentile as the height. TSF was measured to the nearest 0.2 mm using Holtain calipers (Holtain Ltd., Crymch, UK) on all three measurement phases. TSF measurement was performed in triplicate and the average of the measurements was reported at each phase of study. The same examiner performed the measurement throughout the study to eliminate interexaminer error. A standard technique for measurement of TSF and MAC was used (28,29). MAMC is derived from measurements of TSF and MAC and was calculated using the following equation: MAMC = MAC − (π × TSF), where π = 3.14 (18). Arm anthropometric data were compared with published norms (29).
Urinary catecholamine metabolites were measured and recorded at diagnosis. A random specimen of urine was taken to determine vanillylmandelic acid (VMA) and homovanillic acid (HVA) levels. Levels are measured quantitatively based on ion-exchange cleanup and using HPLC. (VMA and HVA by HPLC testing; Bio-Rad, October 1985).
Sample size.
Based on our laboratory observations in healthy control subjects, which showed a variance in REE of around 10% (between subjects ±10%, 2SD) (30), an REE of more than 15% is regarded as clinically significant (31). We hypothesized that we would see a 25–30% elevation at baseline measurement, with a decrease at each subsequent measurement point. With these estimates and setting alpha, i.e., type I error 0.01, and with the estimate of the sample size adjusted for multiple testing, the sample size was calculated to be a minimum of eight patients.
Statistical analysis.
Data are presented as mean ± SD. Repeated measures analysis of variance was used to compare the effect of treatment on REE and HR. Regression and correlation analysis was used to determine relationship between variables.
RESULTS
Twelve subjects were enrolled in the study. There were two dropouts from the study. One patient withdrew before the start of the study, another patient was medically unstable during the course of treatment and subsequently died. Ten subjects newly diagnosed with NBIV (6 males and 4 females) completed the study.
Summary of urinary catecholamine metabolite levels at presentation are shown in Table 1. Mean age at diagnosis was 3.8 (±1.5) y, 10 patients completed the study (6 males, 4 females). Five subjects were shown to be malnourished at time of diagnosis, three remained malnourished at phase 2, and two at post-tumor excision (phase 3). Effect of treatment response on nutritional characteristics and HR is summarized in Table 2.
An HR measurement was retrospectively obtained. After preliminary calorimetry, results from the study were noted not to be increased. The HR was obtained from the subjects' vital sign recording sheets, and an average was calculated from 3 consecutive days within each phase (day before study, day of study, and day after study) to account for any major fluctuations. Results showed that the HR (beats/min) was elevated upon presentation of disease and decreased in response to treatment. The mean HR was 124 ± 23.5 at phase 1, 109 ± 18.7 at phase 2, and 98 ± 16.1 at phase 3 (p = 0.003). The mean HR was obtained at diagnosis for 9 of 10 subjects. In seven patients at diagnosis, HR was elevated (range, 73–145 beats/min) whereas two had normal values for age. The HR of four patients in phase 2 was likewise elevated (range, 80–135 beats/min), and five had normal values for age. The HR of one patient in phase 3 was elevated, and the remaining patients had normal values for age (normative HR value for age: 80–110 beats/min for 6–12 mo, 70–110 beats/min for 1–3 y, and 65–110 beats/min for 3–6 y) (32).
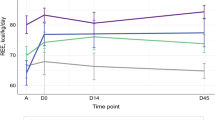
Measurements of REE and response to treatment are presented in Table 3. There was no increase observed in metabolic rate compared with predicted values of REE in all phases. Mean RQ's were 0.87 ± 0.05 (0.82–1.00) in phase 1, 0.88 ± 0.06 (0.79–0.94) in phase 2, and 0.88 ± 0.05 (0.82–0.96) in phase 3.
To assess if there was any relationship between variables, linear regression and correlation analysis was performed. Correlation between the REE and the HR is presented in Figure 1A–C. An unexpected inverse correlation between REE and HR was observed (r = −0.73, p = 0.03).
Phase 1 (A): Correlation between resting energy expenditure (REE, kcal/min) and heart rate (HR, beats/min) is presented. A significant inverse correlation between REE and HR is observed. Correlation coefficient = −0.732, r2 = 0.54, p = 0.03. Phase 2 (B): Correlation between REE (kcal/min) and HR (beats/min) is presented. An inverse relationship between REE and HR is observed. Correlation coefficient = −0.703, r2 = 0.49, p = 0.04. Phase 3 (C): Correlation between REE (kcal/min) and HR (beats/min) is presented. There was no significant correlation between REE and HR in phase 3 of study. Correlation coefficient = 0.170, r2 = 0.03, p = NS. Each ▪ symbol represents an individual measurement (all panels).
DISCUSSION
To our knowledge, this is the first study that longitudinally investigated the REE of children with NBIV from diagnosis and in response to treatment. The study was undertaken to attempt to understand why these children are often malnourished at time of diagnosis. The prevalence of malnutrition in childhood cancer can range from 6 to 50% (33), the degree of which is associated with the type of tumor and the extent of metastatic disease (33). Children with NBIV have been recognized as a high nutritional risk patient population (33). It was postulated from a review of the literature that the malnutrition would be due to hypermetabolism. To our surprise, despite the presence of extensive disease the children were not hypermetabolic, although ∼50% were malnourished at diagnosis (consistent with the literature). Furthermore, measured mean REE was initially 13% lower than predicted REE. There was no effect on REE with cancer treatment.
Published pediatric metabolic studies in cancer patients are varied. Bond et al. (33) studied basal metabolic rate of children with either acute lymphoblastic leukemia during maintenance chemotherapy or solid tumors, having no evidence of active disease, compared with healthy controls. Both groups exhibited values for basal metabolic rate that were comparable with predicted (33). Stallings et al. (31) used open-circuit indirect calorimetry to investigate the effect of tumor burden in children newly diagnosed with acute lymphoblastic leukemia on REE. Patients were divided into two groups according to tumor burden at diagnosis. Only patients with a larger tumor burden had increased REE compared with standard predicted values (31). Repeated measurement of REE showed normalization after response to 2 wk of chemotherapy in the high tumor burden group (31). Den Broeder et al. (34) investigated basal metabolic rate in children with solid tumors. In contrast to our data, they reported that basal metabolic rate was higher than the estimated reference in all patients at diagnosis, and decreased during treatment. Picton (35) reported the energy expenditure in children with leukemia and solid tumors. The children with solid tumors, who became cachectic during therapy, had significantly elevated sleeping energy expenditure at presentation compared with noncachectic patients and controls (35). Leukemic children showed a small but significant raise in metabolic rate at presentation compared with controls and a reduction of metabolic rate at remission (35). These findings are consistent with those of Stallings et al. (31). The results of these cited studies might not be comparable with our study because of the heterogeneity of patient populations examined (acute lymphoblastic leukemia, Hodgkin lymphoma, osteosarcoma, rhabdomyosarcoma, and B-cell lymphoma). An additional consideration could be whether any of the medications given could effect REE, and a careful review of the literature revealed that they do not (36).
The paradox in the present study is that the children had a tumor, which secretes large amounts of catecholamines, which would be expected to increase REE and HR. HR was elevated at phase 1 and was significantly lower by phase 3 of study (p = 0.003). Pathophysiologically, we can reason that the elevated HR corresponds to the elevated urinary catecholamine metabolites, and was reflective of untreated, active neuroblastoma. At phase 3, HR was much lower, at a point where there was minimal residual disease. Unfortunately, in our subjects measurements of urinary catecholamine metabolites were only obtained at time of diagnosis, as treatment response at our center is ascertained primarily by oncological evaluation (i.e., follow-up CT scans, MRI, and bone marrow aspirate). Nonetheless, because others have shown that urinary catecholamine metabolites normalize with response to treatment (18,19,37–39), we reason that circulated catecholamine metabolites would have reverted toward normal by phase 3; and this is supported by the fall in HR toward normal (Table 2).
An expected finding with an elevated HR would be a corresponding increased REE particularly at phase 1 of our study. Hyltander et al. (16) demonstrated that there was a strong positive relationship between REE and HR, and that HR was the most powerful factor for prediction of elevated REE, with cancer patients, having a significantly steeper slope between HR and REE than controls. However, we observed an inverse relationship with REE in both phases 1 and 2. The cause of the paradoxical inverse relationship observed between REE and HR is unclear.
Neuroblastoma is a metabolically active tumor derived from neural crest cells. Increased production of catecholamines [dopamine, norepinephrine (NE), and epinephrine] and their metabolites (VMA and HVA) is distinctive to neuroblastoma (19). Circulating NE causes increased activity of the heart, as does epinephrine; however, epinephrine has a greater effect on cardiac activity than NE (40). Our subjects had a significantly higher HR that involves primarily the cardiac receptor β1, but with a corresponding low REE in which β2 receptor activity is principally involved. It is plausible that prolonged elevated catecholamine production may have altered the adrenergic state in our subjects. It could be reasoned that this inverse relationship is a result of a diminished physiologic response that occurs after prolonged exposure of an effector tissue to an adrenergic agonist. This occurrence is known as tachyphylaxis or desensitization (40). Contrary to our results, Hyltander et al. (16) reasoned that their findings of elevated HR and REE are suggestive that whole-body catecholamine sensitivity and production may be upregulated in cancer-bearing hosts, but not from our results, in children with neuroblastoma.
Zambrano and Reyes-Mugica (19) correlated urinary NE, dopamine, VMA, and HVA levels with stage, age at diagnosis, histopathological features, and N-myc status of patients with neuroblastoma. Their findings show that aggressive neuroblastoma is associated with higher urinary catecholamine levels, and that stage IV disease with unfavorable histology shows a blockade in metabolism of dopamine (19). Similarly, all our subjects had stage IV disease with unfavorable histopathology. Ninety percent of our subjects had elevated urinary catecholamine metabolite levels either spot vanillyl mandelic (SVM)/VMA or HVA at time of diagnosis.
LaBrosse et al. (18) review of catecholamine metabolism in neuroblastoma found that there was excess production and excretion of the NE precursors, dihydroxyphenylalanine (DOPA), and dopamine, and that catecholamines were largely inactivated by intracellular metabolism in the tumor cells. Interestingly, Sato et al. (41) found that endogenous dopamine overload in neuroblastoma inhibits NE production. The main blockade in the catecholamine pathway may be where dopamine is converted to NE. It has been further suggested that the enzyme converting NE to epinephrine is lacking in neuroblastoma cells (42). Lack of epinephrine may be an additional reason for the finding of an elevated HR but no increase in REE.
CONCLUSION
The results of this study indicate that despite extensive disease at diagnosis children with NBIV are not hypermetabolic. Furthermore, there was no effect on REE with cancer treatment. However, there was an inverse relationship observed between HR and REE. Possibly because of tachyphylaxis effecting the β2 adrenergic receptor or an alteration in catecholamine metabolism. An inhibitory effect on the conversion of dopamine to NE may result in an increase in dopamine level but no marked increase in NE or epinephrine levels and hence an increased HR but no stimulation of REE. Further research needs to be done to elucidate the potential underlying mechanisms related to metabolic control of REE in neuroblastoma. Thus, the malnutrition observed in this patient population at diagnosis seems to be due to poor dietary intake, related to the intra-abdominal mass and anorexia rather than an increased REE.
Abbreviations
- DOPA:
-
dihydroxyphenylalanine
- HR:
-
heart rate
- HVA:
-
homovanillic acid
- MAC:
-
mid-arm circumference
- MAMC:
-
mid-arm muscle circumference
- NBIV:
-
stage IV neuroblastoma
- NE:
-
norepinephrine
- REE:
-
resting energy expenditure
- SVM:
-
spot vanillylmandelic acid
- TSF:
-
triceps skin fold
- VMA:
-
vanillylmandelic acid
- WFH:
-
weight for height
References
Elhasid R, Laor A, Lischinsky S, Postovsky S, Arush MW 1999 Nutritional status of children with solid tumours. Cancer 86: 119–125
Albrecht JT, Canada TW 1996 Cachexia and anorexia in malignancy. Hematol Oncol Clin North Am 10: 791–800
Matthys P, Billiau A 1997 Cytokines and cachexia. Nutrition 13: 763–770
Tisdale MJ 1997 Cancer cachexia: metabolic alterations and clinical manifestations. Nutrition 13: 1–7
Laviano A, Meguid MM 1996 Nutritional issues in cancer management. Nutrition 12: 358–371
Lind DS, Cendan JC, Copeland EM 1995 Nutrition for the cancer patient. Contemp Surg 47: 17–23
Puccio M, Nathanson L 1997 The cancer cachexia syndrome. Semin Oncol 24: 277–287
Shike M 1996 Nutrition therapy for the cancer patient. Hematol Oncol Clin North Am 10: 221–234
Das SK, Roberts SB 2001 Energy metabolism. In: Bowman BA, Russell RM (eds) Present Knowledge in Nutrition. 8th ed. ILSI Press, Washington, DC, pp 3–12
Mulligan K, Bloch AS 1998 Energy expenditure and protein metabolism in human immunodeficiency virus infection and cancer cachexia. Semin Oncol 25: 82–91
Knox LS, Crosby LO, Feurer ID, Buzby GP, Miller CL, Mullen JL 1983 Energy expenditure in malnourished cancer patients. Ann Surg 197: 152–162
Arbeit JM, Lees DE, Corsey R, Brennan MF 1984 Resting energy expenditure in controls and cancer patients with localized and diffuse disease. Ann Surg 199: 292–298
Macfie J, Burkinshaw CO, Holmfield JH, Hill GL 1982 The effect of gastrointestinal malignancy on resting metabolic expenditure. Br J Surg 69: 443–446
Dempsey DT, Feurer ID, Knox LS, Crosby LO, Buzby GP, Mullen JL 1984 Energy expenditure in malnourished gastrointestinal cancer patients. Cancer 53: 1265–1273
Hansell DT, Davies JW, Burns HJ 1986 The effects on resting energy expenditure of different tumour types. Cancer 58: 1739–1744
Hyltander A, Drott C, Korner U, Sandstrom R, Lundholm K 1991 Elevated energy expenditure in cancer patients with solid tumours. Eur J Cancer 27: 9–15
Caron H, Pearson A 1998 Neuroblastoma. In: Voute PA, Kalifa C, Barrett A (eds) Cancer in Children: Clinical Management. 4th ed. Oxford University Press, New York, pp 274–289
LaBrosse EH, Comoy E, Bohuon C, Zucker JM, Schweisguth O 1976 Catecholamine metabolism in neuroblastoma. J Natl Cancer Inst 57: 633–638
Zambrano E, Reyes-Mugica M 2002 Hormonal activity may predict aggressive behavior in neuroblastoma. Pediatr Dev Pathol 5: 190–199
Goldsby RE, Matthay KK 2004 Neuroblastoma: evolving therapies for a disease with many faces. Pediatr Drugs 6: 107–122
Weir JB 1949 New methods for calculating metabolic rate with special reference to protein metabolism. J Physiol 109: 1–9
1985 Energy and protein requirements. Report of a joint FAO/WHO/UNU Expert Consultation,. Geneva, World Health Organ Tech Rep Ser, 724: 1–206
Schofield WN 1985 Predicting basal metabolic rate, new standards and review of previous work. Hum Nutr Clin Nutr 39: 5–41
Rickard KA, Detamore CM, Coates TD, Grosfeld JL, Weetman RM, Matchett N, Provisor AJ, Boxer LA, Loghmani ES, Oei TO, Yu PL, Baehner RL 1983 Effect of nutrition staging on treatment delays and outcome in stage IV neuroblastoma. Cancer 52: 587–598
Oguz A, Karadeniz C, Pelit M, Hasanoglu A 1999 Arm anthropometry in evaluation of malnutrition in children with cancer. Pediatr Hematol Oncol 16: 35–41
Tanner JM, Whitehouse RH, Takaishi M 1966 Standards from birth to maturity for height, weight, height velocity, and weight velocity: British Children, 1965 I. Arch Dis Child 41: 454–471
Tanner JM, Whitehouse RH, Takaishi M 1966 Standards from birth to maturity for height, weight, height velocity, and weight velocity: British Children, 1965 II. Arch Dis Child 41: 613–635
Owen G 1997 Measurement, recording, and assessment of skinfold thickness in childhood and adolescence: report of a small meeting. Am J Clin Nutr 35: 629–638
Frisancho AR 1981 New norms of upper limb fat and muscle areas for assessment of nutritional status. Am J Clin Nutr 34: 2540–2545
Azcue MP, Pencharz PB, Zello GA 1991 Are the FAO/WHO/UNU predictive equations for resting metabolic rate accurate?. Am J Clin Nutr 54: 613–614
Stallings VA, Vaisman N, Chan HS, Weitzman SS, Hahn E, Pencharz PB 1989 Energy metabolism in children with newly diagnosed acute lymphoblastic leukemia. Pediatr Res 26: 154–157
Hazinski MF 1992 Neurologic disorders. In: Hazinski MF (ed) Nursing Care of the Critically Ill Child. 2nd ed. Mosby-Year Book, Inc, St. Louis, MO, pp 521–628
Bond SA, Han AM, Wootton SA, Kohler JA 1992 Energy intake and basal metabolic rate during maintenance chemotherapy. Arch Dis Child 67: 229–232
Den Broeder E, Oeseburg B, Lippens RJ, van Staverent WA, Sengers RC, van't Hof MA, Tolboom JJ 2001 Basal metabolic rate in children with a solid tumour. Eur J Clin Nutr 55: 673–681
Picton SV 1998 Aspects of altered metabolism in children with cancer. In J Cancer Suppl 11: 62–64
Dorr RT, Von Hoff DD 1994 Part III Drug monographs In: Dorr RT, Von Hoff DD (eds) Cancer Chemotherapy Handbook. 2nd ed. Appleton & Lange, East Norwalk, CT, pp 131–978
Nakagawara A, Ikeda K, Higashi K, Saszuki T 1990 Inverse correlation between N-myc amplification and catecholamine metabolism in children with advanced neuroblastoma. Surgery 107: 43–49
Laug WE, Siegel SE, Shaw KF, Landing B, Baptista J, Gutenstein M 1978 Initial urinary catecholamine metabolite concentrations and prognosis in neuroblastoma. Pediatrics 62: 77–83
Imashuku S, Takada H, Sawada T, Nakamura T, LaBrosse EH 1975 Studies on tyrosine hydroxylase in neuroblastoma, in relation to urinary levels of catecholamine metabolites. Cancer 36: 450–457
Landsberg L, Young JB 1991 Physiology and pharmacology of the autonomic nervous system. In: Wilson JD, Braunwald E, Isselbacher KJ, Petersdorf R, Martin JB, Fauci AS, Root RK (eds) Harrison's Principles of Internal Medicine. 12th ed. McGraw-Hill, Inc, New York, pp 380–392
Sato T, Minami S, Igarashi N, Miyagawa K 1989 Suppression of norepinephrine secretion by dopamine in children with neuroblastoma: evidence for precursor inhibition in catecholamine pathway. Endocrinol Jpn 36: 595–603
Brodeur GM, Maris JM 2002 Neuroblastoma. In: Pizzo PA, Poplack DG (eds) Principles and Practice of Pediatric Oncology. 4th ed. Lippincott Williams & Wilkins, Philadelphia, PA, pp 895–899
Acknowledgements
We thank Karen Chapman and Helen Richards of the Clinical Investigations Unit for their help with using the Deltrac II Metabolic Monitor and Marhroukh Rafi for her statistical assistance. To the families and patients, we greatly appreciate your cooperation.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Green, G., Weitzman, S. & Pencharz, P. Resting Energy Expenditure in Children Newly Diagnosed With Stage IV Neuroblastoma. Pediatr Res 63, 332–336 (2008). https://doi.org/10.1203/PDR.0b013e318163a2d4
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1203/PDR.0b013e318163a2d4
This article is cited by
-
Glomerular hyperfiltration in children with cancer: prevalence and a hypothesis
Pediatric Radiology (2017)
-
Favorable survival and metabolic outcome for children with diencephalic syndrome using a radiation-sparing approach
Journal of Neuro-Oncology (2014)