Abstract
Stem and progenitor cells derived from adult marrow have been shown to regenerate vascular cells in response to injury. However, it is unclear whether the type of injury dictates the contribution of such cells to neovascularization and which subpopulations of cells contribute to vascular regeneration. To address these questions, we determined the extent that hematopoietic stem cells (HSC) contributed to blood vessel formation in response to two types of liver injury, partial hepatectomy (PH) and toxin-induced injury. Lac-Z-labeled HSC were engrafted into lethally irradiated, genetically matched recipients. After 14 d, we identified transplanted cells engrafted within the vascular endothelium of toxin-damaged liver, but not in the vasculature of liver regenerated in response to PH. Engraftment of HSC-derived cells occurred in a gradient fashion with the highest activity in the severely injured areas. Although HSC-derived cells contributed to both microvessels and large vessels, the large caliber vessels trended toward higher engraftment levels. Thus, the contribution of marrow-derived cells to hepatic neovascularization is dependent upon the type of injury sustained. Furthermore, following toxin-induced liver injury, engraftment rates trended higher in large vessels compared with capillaries, suggesting that remodeling of existing vessels is a predominant mechanism of repair, relative to the formation of new microvasculature.
Similar content being viewed by others
Main
All humans, from the preterm infant to the aging adult, would benefit from an ability to enhance tissue repair. Tissue regeneration and remodeling, such as in the liver (1,2) and heart (3), is dependent upon neovascularization of the injured tissues. Similarly, vascular development has been shown to play a critical role in early organ development (4). Thus, improving vascular regeneration should optimize healing and improve the survival and function of injured tissues for all age groups.
The precise origin of specific cells that support postnatal vascular regeneration and their relative contributions remains unclear. Recent studies have demonstrated that bone marrow–derived stem and progenitor cells contribute to vascular repair following tissue injury (5–8). Vascular progenitors reside within other nonmarrow adult tissues, including skeletal muscle (9,10), peripheral blood (11,12), and liver (13). However, there is evidence that circulating and tissue-resident vascular progenitor cells are primarily derived from and replenished by the marrow (9). The specific cellular origin of the most primitive vascular progenitor population within the marrow has yet to be delineated. Since hematopoietic stem cell (HSC)-derived cells have been shown to contribute to regeneration of the vascular endothelium following tissue injury (3,9–11), it is suggested that HSC are the source of circulating endothelial progenitor cells, which in turn differentiate into mature endothelial cells (EC). However, the differentiation of primitive HSC or their progeny toward an EC fate remains to be conclusively demonstrated.
Although there is evidence that marrow-derived cells contribute to vascular regeneration, the extent to which they contribute to blood vessel formation and remodeling widely vary in different studies (14,15). Nonetheless, injury appears to be an important stimulus, since engraftment of marrow-derived cells into blood vessels is rarely observed in uninjured tissues (15). To determine whether the type of injury dictates the relative contribution of marrow-derived cells to vascular repair, we measured levels of engraftment of marrow cells into the liver vasculature in response to two distinct types of injury, partial hepatectomy (PH) and toxin-induced injury. PH was selected because the procedure removes a large portion of the liver, necessitating tissue regeneration, yet also leaves a hepatic lobe containing resident stem and progenitor cells capable of local repair (16). In contrast, the toxin allyl alcohol (AA) induces a periportal (zone 1) liver injury, resulting in damage to hepatic vessels and subsequent ischemia, and is thought to destroy local endogenous stem and progenitor cells within the intact tissue (17).
To quantify the extent to which marrow-derived cells contribute to different types of injury-induced hepatic neovascularization, we used a HSC transplantation model to mark marrow-derived circulating cells. HSC were isolated as side population (SP) cells, a population shown in single-cell transplantation studies to be functionally equivalent to HSC isolated by cell surface marker expression (18,19). We selected HSC as the primary marrow population of interest because they have been proposed as the source of vascular stem and progenitor cells (9,20). To date, the contribution of marrow progenitor cells to damaged liver has focused on epithelial cell regeneration; few studies have evaluated hepatic vascular regeneration, which appears to be of substantial importance for overall tissue regeneration (1,2,21). This issue is addressed herein.
In these studies, we found the type of injury sustained is a key determinant of whether HSC-derived cells will contribute to vessel regeneration in the liver. That is, HSC-derived cells appeared to contribute to neovascularization when local stem and/or progenitors were ablated (AA injury), but not when intact endogenous stem and progenitor cells remained (PH injury). Furthermore, engraftment of HSC-derived cells into blood vessels occurred in a gradient fashion, with the highest engraftment in the most severely injured regions. HSC-derived cells tended to contribute more to the remodeling of large, existing vessels versus the formation of new microvasculature.
These results suggest that clinical therapies designed to optimize the contribution of marrow-derived cells to neovascularization may not be useful for all types of tissue injury. Therefore, when contemplating the use of marrow-derived cells for vascular regeneration, both type of injury and desired vascularization outcome should be considered.
METHODS
Marrow cell isolation and transplantation.
Animal studies were approved by Baylor College of Medicine institutional review board. Marrow was extracted from tibias and femurs of C57Bl/6-Rosa26-CD45.2 mice (Jackson Laboratories, Bar Harbor, ME) at 8–12 wk of age, suspended at 106 cells/mL in DMEM supplemented with 2% FBS/10 mmol HEPES, then stained with 5 μg/mL Hoechst 33342 (Sigma Chemical Co.-Aldrich, St. Louis, MO) for 90 min at 37°C, as previously described (5,19). Cells were resuspended in cold HBSS containing 2% FBS/10 mmol HEPES and 2 μg/mL propidium iodide. Analysis and collection of HSC (side population cells) were performed on a triple-laser MoFlow instrument (Cytomation Inc., Fort Collins, CO), as previously described. HSC accounted for 0.03–0.05% of unfractionated marrow.
C57Bl/6-CD45.1 mice (Jackson Laboratories) were lethally irradiated (10 Gy) and retro-orbitally injected with 2000 HSC isolated from C57Bl/6-Rosa26-CD45.2 mice. Eight weeks after transplantation, hematopoietic engraftment was determined by peripheral blood analysis using antibodies against CD45.2 (clone 104, BD Biosciences-PharMingen, San Jose, CA) followed by fluorescence-activated cell sorting analysis. Average hematopoietic engraftment was >80%. Isoflurane anesthesia was used during injections and phlebotomy. We chose the lacZ reporting system over GFP to avoid reported false positives (22).
PH.
Twelve weeks following HSC transplantation (n = 3), two hepatic lobes were surgically removed (23) under Avertin anesthesia. Survival was 100%. Mice were killed 2 wk after injury, at which time vascular regeneration is maximal (24). Regenerated livers were frozen and evaluated for incorporation of transplanted cells.
AA-induced liver injury.
For toxin-induced hepatic injury, we selected AA because it induces periportal (zone 1) injury, thereby maximizing the degree of vascular injury (17). Twelve weeks following HSC transplantation (n = 5), mice were injected with AA (Sigma Chemical Co.-Aldrich) in two intraperitoneal doses: 39 mg/kg on d –7, and 57 mg/kg on d 0 (17). Nontransplanted control C57Bl/6 mice (n = 2) were treated with the same regimen to evaluate for up-regulation of endogenous βgal activity. Survival was 100%. To monitor hepatic injury, serum transaminases and bilirubin levels were measured 2 wk before injury, and on d 1 and 8 following injection. An additional group of transplanted, AA-injured animals (n = 5) were used for transaminase and bilirubin measurements. Baylor Comparative Pathology Laboratory analyzed blood samples. Two weeks following injury, livers were frozen for immunohistochemical analysis.
Immunohistochemical analysis.
Frozen sections (7 μm) of experimental and control liver tissue were immunostained with antibodies against Ve-cadherin (BD Biosciences-PharMingen; 1:50) and ICAM-2 (BD Biosciences-PharMingen; 1:50) to identify ECs, and desmin (DAKO, Glostrup, Denmark; 1:500) and SM-α-actin (DAKO; 1:500) to identify pericytes and smooth muscle cells within vascular structures. For Ve-cadherin and ICAM-2, tissues were incubated at 4°C overnight with primary antibody, before fixation with 4% paraformaldehyde for 30 min at 4°C, as found optimal for this antiserum (25). For desmin and SM-α-actin immunostaining, tissue was fixed before primary antibody incubation. Anti-rat and anti-mouse Alexa 488 secondary antibodies (Invitrogen-Molecular Probes Inc., Carlsbad, CA) were used for Ve-cadherin or ICAM-2, and desmin or SM-α-actin, respectively. For mural markers, a Mouse-on-Mouse kit (Vector Labs, Burlingame, CA) was used. For CD45, tissues were fixed, incubated with anti-CD45 biotin-conjugated antibodies (BD Biosciences-PharMingen; 1:100) for 1 h, and then incubated with streptavidin-conjugated Alexa 488 or 594 (Invitrogen-Molecular Probes, Inc.; 1:500) for 30 min.
Following immunohistochemistry, sections were stained for lacZ-positive cells as described previously (5). Coverslips were mounted using Vectashield containing DAPI (Vector Labs), and slides were viewed using a Zeiss epifluorescent microscope equipped with differential interference optics.
Quantification of vascular engraftment.
Sections of liver tissue were subclassified to reflect distinct injury zones based on gross and microscopic morphology, and engraftment into vascular cells was quantified for each zone. In AA injured tissues, four zones could be defined: an injury zone, the region of acute injury; a border zone, the area immediately adjacent to and contiguous with the injury zone, with a width equivalent to one high-powered microscope field (25 μm); a peri-injury zone, the area adjacent to the border zone and within the same liver lobe as the injured tissue; and a noninjury zone, a region in an uninjured lobe of the liver section. In PH tissues, we quantified areas adjacent and nonadjacent to the site of resection.
We analyzed engraftment of marrow cells into vessels of different sizes: capillaries were defined as 8–10 μm vessels comprised of <3 ECs; all other vessels were grouped as moderate to large vessels. For each vascular marker, we analyzed more than four random, nonserial tissue sections in each experimental and control liver. For each tissue section, we analyzed more than seven high-powered fields (100×) in each injury zone.
Statistical methods.
All data are presented as mean ± SEM. Intergroup comparisons were performed using an unpaired t test. Probability values of p < 0.05 were interpreted to denote statistical significance.
RESULTS
We assessed the contribution of HSC to liver neovascularization in response to two distinct types of liver injury, PH and toxin-induced injury (Fig. 1). The two different injury types were selected for their disparate mechanisms. PH necessitates tissue regeneration yet leaves a population of intact stem and/or progenitor cells in the remaining hepatic lobe (16). AA, on the other hand, induces an ischemic injury that damages the periportal region and ablates tissue-resident stem and/or progenitor cells (17,26).
Model of hematopoietic engraftment and liver injury. HSC were isolated and injected into recipients. After stable hematopoietic engraftment, livers were injured by either PH or AA injection. Regenerating liver tissues were assessed for engraftment of marrow-derived cells into the hepatic vasculature.
Serum transaminase levels serve as an indicator of liver injury.
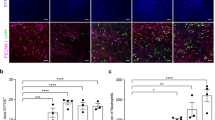
To monitor the degree of hepatic injury following AA injection, serum transaminases and total bilirubin levels were measured before and after injury. We found the alanine aminotransferase (ALT) and aspartate aminotransferase (AST) levels on d 1 after injury were dramatically elevated compared with baseline (2,000 to >14,000 U/L versus 25–50 U/L, respectively) (Fig. 2). By d 8 following injury, transaminase levels had returned to baseline levels (25–75 U/L). Control animals, which underwent irradiation and marrow cell transplantation but did not receive AA, exhibited no change in serum transaminase levels. Serum total bilirubin levels were measured, but did not correlate with AA-induced injury or tissue recovery (data not shown), and therefore, did not appear to be a sensitive measurement of hepatic tissue injury in these studies.
AA injury: transaminases. Serum transaminases in AA-injured and control mice were evaluated. (A) AST levels and (B) ALT levels were analyzed at serial time points. Baseline (black), d 1 following injury (dark gray), and d 8 following injury (light gray). 1–10 indicate transplanted, AA-injured animals. Controls 1 and 2 indicate HSC-transplanted, no injury controls. ↑ indicates values greater than 5000 U/L (ALT: 5,020–14,000 U/L; AST: 7,540–46,413 U/L).
HSC-derived cells contribute to vascular regeneration in response to AA injury, but not PH.
Two weeks following PH or AA injury, livers were harvested for immunohistochemical analysis to measure engraftment of HSC-derived cells into the regenerated vasculature. At the time of harvest of AA-injured livers, irregular areas of discoloration were noticeable upon gross inspection. These areas, which resulted from AA-induced injury and ischemia, were designated the most severely “injured zones.” No such changes were observed in the HSC-transplanted, but non-AA-treated controls.
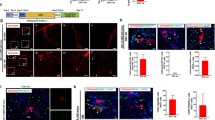
Liver sections were stained with X-gal to localize transplanted lacZ-expressing HSC-derived cells, and co-immunostained for endothelial and mural cell (pericyte and smooth muscle cell) specific proteins to determine whether HSC-derived cells engrafted and differentiated into vascular cells during neovascularization associated with tissue repair. CD45 immunostaining was performed to detect infiltrating blood cells; CD45-positive, lacZ-positive hematopoietic cells were eliminated from quantification. In the AA-injured livers, HSC-derived lacZ-positive cells co-localized only with the EC markers Ve-cadherin and ICAM-2 (Fig. 3), but not with mural cell markers SM-α-actin or desmin. In the adult, Ve-cadherin is constitutively expressed in all endothelial compartments, including arteries, veins, capillaries, and lymphatics (27); ongoing studies plan to address the contribution of HSC-derived cells to different vessel types, but is beyond the scope of this study. Thus, HSC-derived cells contribute specifically to vascular endothelial and not mural cell regeneration within 2 wk post ischemic liver injury. Importantly, liver tissue from transplanted and nontransplanted AA-injured mice was analyzed in a blinded fashion. The nontransplanted, toxin-injured livers showed no lacZ-positive staining, ensuring that neither marrow transplantation nor tissue injury resulted in endogenous βgal activity. Additionally, we did not observe any lacZ-positive hepatic cells.
Immunohistochemistry: AA injury. (A) βgal staining of AA-injured liver revealed several βgal-positive microvessels. (B) Higher magnification of microvessels shown in (A). (C) βgal-positive structures colocalized with (D) vascular EC marker Ve-cadherin, and (E) overlay of βgal and Ve-cadherin positive microvessels. (F) βgal-positive structures colocalized with (G) vascular EC marker ICAM-2, and (H) overlay of βgal and ICAM-2 positive microvessels. All images represent 7 μm sections, 100× magnification, and scale bars = 10 μm.
In contrast to AA-injured livers, no lacZ-positive vascular endothelial or mural cells were observed in the livers harvested and analyzed 2 wk following PH. Thus, HSC did not contribute to neovascularization during PH-induced vascular regeneration. Both AA and PH liver injury models were repeated in several independent experiments with consistent engraftment results; a representative group was analyzed in full detail. Similar analysis of tissue sections from HSC-transplanted, but uninjured, control mice revealed no lacZ-positive staining in the vasculature or other cell types.
Engraftment of HSC-derived cells occurs in a gradient fashion.
To accurately analyze the pattern and extent of HSC-derived cell engraftment into vascular cells, we divided the AA-injured liver tissues into distinct zones: injury zone, border zone, peri-injury zone, and noninjury zone, as discussed in Methods.
To quantify the incorporation of HSC-derived cells into EC, more than 400 Ve-cadherin-positive vessels were analyzed per zone in each experimental animal (Table 1). The highest levels of endothelial engraftment occurred in the injury zone, where HSC-derived cells comprised 7.6 ± 1.7% (mean ± SEM) of capillaries, and 16.7 ± 5.2% of larger vessels. Within capillaries, lacZ-positive EC often comprised the full vessel circumference, indicating formation of new microvessels. In moderate to large vessels, lacZ-positive cells represented approximately 10% of the cross-sectional EC population, indicating remodeling of existing structures. Lower engraftment levels were observed in the border zone, where HSC-derived cells contributed to 4.4 ± 1.2% of capillaries, and 2.7 ± 1.2% of larger vessels. Negligible levels of endothelial engraftment were seen in the peri-injury zone and none in the noninjured zone.
To determine incorporation of HSC-derived cells into mural cells, we analyzed liver sections stained for βgal expression and co-stained with antibodies to SM-α-actin or desmin. We analyzed >100 vessels per zone in each animal. However, following toxin-induced hepatic injury, we did not find co-localization of βgal and mural cell markers in any tissue zone.
Engraftment rates appear to be higher in large vessels compared with microvessels.
A higher percentage of large vessels in the injury zone exhibited engraftment of HSC-derived cells into the endothelium when compared with small vessel engraftment rates. In mice transplanted with HSC, large vessel EC engraftment rates were 16.7 ± 5.2 (mean ± SEM) (Table 1). Capillary EC engraftment rates were 7.6 ± 1.7, suggesting a trend toward a higher degree of remodeling of existing vessels compared with formation of new microvasculature.
In summary, we found that injury mechanism plays an important role in determining engraftment levels of HSC-derived cells into the regenerating liver vasculature. HSC-derived cells contribute to vascular repair only when local endogenous stem and/or progenitor cells are ablated (AA injury). Furthermore, the vascular engraftment of HSC-derived cells occurs in a gradient, with highest levels of engraftment in the most severely injured regions. Vascular EC engraftment rates are high in larger vessels, suggesting that the remodeling of existing vessels is a predominant mechanism in the repair of ischemic-injured tissue.
DISCUSSION
The presence of injury was previously shown to promote vascular engraftment of marrow-derived cells, but it was not known whether the specific type of injury played any role (15). Herein, we used a liver model to assess the contribution of marrow-derived cells to neovascularization induced in response to different types of injury. We demonstrate that injury mechanism is a key determinant of overall contribution of marrow-derived cells to vessel repair, and HSC are the cell source of vascular progenitors in the marrow. Thus, not all types of injury elicit the same regenerative response.
In our studies, although HSC-derived cells contributed to AA-induced neovascularization, they did not contribute to neovascularization following PH. This parallels patterns observed during liver epithelial cell regeneration: following PH, the liver epithelium regenerates through proliferation of endogenous cells, including hepatocytes and differentiated intrahepatic cells, rather than through recruitment of stem and/or progenitor cells from the blood or bone marrow (16). During liver epithelial cell regeneration, extrahepatic progenitor cells contribute only in situations in which endogenous liver cell proliferation is precluded, as seen when PH is combined with a chemical hepatic injury (16), resulting in the destruction of tissue-resident stem and/or progenitor cells. Based on our studies, we propose that PH-induced neovascularization is mediated by the proliferation of endogenous endothelial and mural cells, or intact tissue resident progenitors. Thus, following PH, a procedure that does not damage tissue-resident stem and/or progenitor cells in the remaining hepatic lobe, HSC-derived cells do not contribute to vessel formation.
In contrast to the local regenerative response observed following PH, AA-induced hepatic injury enabled the contribution of HSC-derived cells to neovascularization. AA induces a periportal (zone 1) injury, the region in which a large percentage of the hepatic vasculature, as well as endogenous stem and/or progenitor cells are localized (28). AA causes direct EC damage and secondary ischemia due to interruption of blood circulation, acting through oxidation of AA to acrolein, with subsequent glutathione depletion and lipid peroxidation leading to tissue injury (17,26). Such injury is thought to impair or destroy local cells, which would include mature vascular cells and tissue-resident stem and/or progenitors, thereby necessitating contribution of marrow-derived, circulating progenitor pools to vascular repair.
These results parallel findings demonstrated in ischemic-injury models in other organs including the heart and hind limb (3,5,29). Tissue-resident stem and/or progenitor cells reside in specific microenvironments or niches, as described in organ systems such as the testis, skin, and gut crypts (30), and are damaged in response to local injury. AA-induced injury to the periportal region, where mature vessels and endogenous stem and/or progenitor cells reside, likely results in vacant niches and enables engraftment of marrow-derived endothelial progenitor populations. In our studies, engraftment occurred in a gradient fashion, with the highest levels observed in the most severely injured regions. This supports the concept that types of tissue injury that deplete local stem and/or progenitor cells enable engraftment of circulating marrow-derived cells; whereas, uninjured tissue regions do not allow incorporation of such progenitor populations because there are no vacancies in the tissue architecture.
Following AA-induced liver injury, HSC-derived cells contributed to the remodeling and expansion of moderate to large existing vessels at high levels. These results support the idea that early restoration of blood flow in acutely injured tissue is significantly mediated by collateralization, or arteriogenesis, which involves the enlargement and remodeling of preexisting vessels to improve perfusion and enhance tissue recovery, as opposed to vasculogenesis, or the de novo formation of new microvessels (31,32). The exact cellular and molecular mechanisms driving arteriogenesis are not completely understood. Marrow-derived and circulating monocytes appear to play an essential role in the process of collateralization, exerting paracrine-signaling effects to promote vessel growth, and either incorporating into EC themselves, or recruiting other marrow-derived progenitor populations (33,34).
In summary, our studies demonstrate that injury mechanism is an important factor regulating the nature of the regenerative response. It appears that tissue injuries that leave resident stem and progenitor cells intact do not rely on marrow-derived circulating cells for neovascularization. In contrast, ischemic injury associated with ablation of local endogenous stem and/or progenitor cells enables engraftment of such cells into the tissue architecture. This is further supported by the observation that, following ischemic injury, vascular engraftment occurs in a gradient fashion: higher levels of HSC-derived cell engraftment are seen in the most heavily injured regions that have the highest vacancy of endogenous stem and/or progenitor niches. Additionally, engraftment levels are high in moderate to large vessels, suggesting that the process of collateralization plays a significant role in vascular repair. Several mechanisms, including differentiation and cellular fusion, have been implicated in the engraftment of marrow-derived cells into injured tissues (35,36). Notably, however, fusion is not thought to be a mechanism of vascular EC engraftment (37,38).
Further understanding of the mechanisms involved in vascular regeneration will enable us to better harness the potential of exogenous progenitors, as well as promote endogenous neovascularization to aid in tissue perfusion and organ regeneration. We cannot optimize the vascular regeneration required to repair tissue injury unless we are aware of the type of neovascularization normally involved following that specific injury mechanism: incorporation of HSC-derived, circulating cells, versus proliferation and expansion of resident vasculature. As advances in medicine continue to extend life on both ends of the spectrum, from the extremely preterm infant to the aging adult, the ability to enhance tissue repair has infinite therapeutic potential.
Abbreviations
- AA:
-
allyl alcohol
- ALT:
-
alanine aminotransferase
- AST:
-
asparate aminotransferase
- EC:
-
endothelial cell
- HSC:
-
hematopoietic stem cell
- PH:
-
partial hepatectomy
References
Marsden ER, Hu Z, Fujio K, Nakatsukasa H, Thorgeirsson SS, Evarts RP 1992 Expression of acidic fibroblast growth factor in regenerating liver and during hepatic differentiation. Lab Invest 67: 427–433
Drixler TA, Vogten MJ, Ritchie ED, van Vroonhoven TJ, Gebbink MF, Voest EE, Borel Rinkes IH 2002 Liver regeneration is an angiogenesis-associated phenomenon. Ann Surg 236: 703–711 discussion 711–712.
Kocher AA, Schuster MD, Szabolcs MJ, Takuma S, Burkhoff D, Wang J, Homma S, Edwards NM, Itescu S 2001 Neovascularization of ischemic myocardium by human bone-marrow-derived angioblasts prevents cardiomyocyte apoptosis, reduces remodeling and improves cardiac function. Nat Med 7: 430–436
Matsumoto K, Yoshitomi H, Rossant J, Zaret KS 2001 Liver organogenesis promoted by endothelial cells prior to vascular function. Science 294: 559–563
Jackson KA, Majka SM, Wang H, Pocius J, Hartley CJ, Majesky MW, Entman ML, Michael LH, Hirschi KK, Goodell MA 2001 Regeneration of ischemic cardiac muscle and vascular endothelium by adult stem cells. J Clin Invest 107: 1395–1402
Bailey AS, Jiang S, Afentoulis M, Baumann CI, Schroeder DA, Olson SB, Wong MH, Fleming WH 2004 Transplanted adult hematopoietic stems cells differentiate into functional endothelial cells. Blood 103: 13–19
Jin H, Aiyer A, Su J, Borgstrom P, Stupack D, Friedlander M, Varner J 2006 A homing mechanism for bone marrow-derived progenitor cell recruitment to the neovasculature. J Clin Invest 116: 652–662
Taguchi A, Soma T, Tanaka H, Kanda T, Nishimura H, Yoshikawa H, Tsukamoto Y, Iso H, Fujimori Y, Stern DM, Naritomi H, Matsuyama T 2004 Administration of CD34+ cells after stroke enhances neurogenesis via angiogenesis in a mouse model. J Clin Invest 114: 330–338
Majka SM, Jackson KA, Kienstra KA, Majesky MW, Goodell MA, Hirschi KK 2003 Distinct progenitor populations in skeletal muscle are bone marrow derived and exhibit different cell fates during vascular regeneration. J Clin Invest 111: 71–79
Tamaki T, Akatsuka A, Ando K, Nakamura Y, Matsuzawa H, Hotta T, Roy RR, Edgerton VR 2002 Identification of myogenic-endothelial progenitor cells in the interstitial spaces of skeletal muscle. J Cell Biol 157: 571–577
Asahara T, Murohara T, Sullivan A, Silver M, van der Zee R, Li T, Witzenbichler B, Schatteman G, Isner JM 1997 Isolation of putative progenitor endothelial cells for angiogenesis. Science 275: 964–966
Cogle CR, Wainman DA, Jorgensen ML, Guthrie SM, Mames RN, Scott EW 2004 Adult human hematopoietic cells provide functional hemangioblast activity. Blood 103: 133–135
Cherqui S, Kurian SM, Schussler O, Hewel JA, Yates JR 3rd, Salomon DR 2006 Isolation and angiogenesis by endothelial progenitors in the fetal liver. Stem Cells 24: 44–54
Krause DS, Theise ND, Collector MI, Henegariu O, Hwang S, Gardner R, Neutzel S, Sharkis SJ 2001 Multi-organ, multi-lineage engraftment by a single bone marrow-derived stem cell. Cell 105: 369–377
Wagers AJ, Sherwood RI, Christensen JL, Weissman IL 2002 Little evidence for developmental plasticity of adult hematopoietic stem cells. Science 297: 2256–2259
Fausto N, Campbell JS 2003 The role of hepatocytes and oval cells in liver regeneration and repopulation. Mech Dev 120: 117–130
Lee JH, Ilic Z, Sell S 1996 Cell kinetics of repair after allyl alcohol-induced liver necrosis in mice. Int J Exp Pathol 77: 63–72
Camargo FD, Green R, Capetanaki Y, Jackson KA, Goodell MA 2003 Single hematopoietic stem cells generate skeletal muscle through myeloid intermediates. Nat Med 9: 1520–1527
Goodell MA, Brose K, Paradis G, Conner AS, Mulligan RC 1996 Isolation and functional properties of murine hematopoietic stem cells that are replicating in vivo. J Exp Med 183: 1797–1806
Olmsted-Davis EA, Gugala Z, Camargo F, Gannon FH, Jackson K, Kienstra KA, Shine HD, Lindsey RW, Hirschi KK, Goodell MA, Brenner MK, Davis AR 2003 Primitive adult hematopoietic stem cells can function as osteoblast precursors. Proc Natl Acad Sci U S A 100: 15877–15882
Taniguchi E, Kin M, Torimura T, Nakamura T, Kumemura H, Hanada S, Hisamoto T, Yoshida T, Kawaguchi T, Baba S 2006 Endothelial progenitor cell transplantation improves the survival following liver injury in mice. Gastroenterology 130: 521–531
Jackson KA, Snyder DS, Goodell MA 2004 Skeletal muscle fiber-specific green autofluorescence: potential for stem cell engraftment artifacts. Stem Cells 22: 180–187
Higgins GM, Anderson RM 1931 Experimental pathology of the liver. I. Restoration of the white rat following partial surgical removal. Arch Pathol 12: 186–202
Ross MA, Sander CM, Kleeb TB, Watkins SC, Stolz DB 2001 Spatiotemporal expression of angiogenesis growth factor receptors during the revascularization of regenerating rat liver. Hepatology 34: 1135–1148
Drake CJ, Fleming PA 2000 Vasculogenesis in the day 6.5 to 9.5 mouse embryo. Blood 95: 1671–1679
Yavorkovsky L, Lai E, Ilic Z, Sell S 1995 Participation of small intraportal stem cells in the restitutive response of the liver to periportal necrosis induced by allyl alcohol. Hepatology 21: 1702–1712
Alva JA, Zovein AC, Monvoisin A, Murphy T, Salazar A, Harvey NL, Carmeliet P, Iruela-Arispe ML 2006 VE-Cadherin-Cre-recombinase transgenic mouse: a tool for lineage analysis and gene deletion in endothelial cells. Dev Dyn 235: 759–767
Petersen BE, Bowen WC, Patrene KD, Mars WM, Sullivan AK, Murase N, Boggs SS, Greenberger JS, Goff JP 1999 Bone marrow as a potential source of hepatic oval cells. Science 284: 1168–1170
Asahara T, Masuda H, Takahashi T, Kalka C, Pastore C, Silver M, Kearne M, Magner M, Isner JM 1999 Bone marrow origin of endothelial progenitor cells responsible for postnatal vasculogenesis in physiological and pathological neovascularization. Circ Res 85: 221–228
Spradling A, Drummond-Barbosa D, Kai T 2001 Stem cells find their niche. Nature 414: 98–104
Helisch A, Schaper W 2003 Arteriogenesis: the development and growth of collateral arteries. Microcirculation 10: 83–97
Scholz D, Ziegelhoeffer T, Helisch A, Wagner S, Friedrich C, Podzuweit T, Schaper W 2002 Contribution of arteriogenesis and angiogenesis to postocclusive hindlimb perfusion in mice. J Mol Cell Cardiol 34: 775–787
Fazel S, Cimini M, Chen L, Li S, Angoulvant D, Fedak P, Verma S, Weisel RD, Keating A, Li RK 2006 Cardioprotective c-kit+ cells are from the bone marrow and regulate the myocardial balance of angiogenic cytokines. J Clin Invest 116: 1865–1877
Heil M, Ziegelhoeffer T, Pipp F, Kostin S, Martin S, Clauss M, Schaper W 2002 Blood monocyte concentration is critical for enhancement of collateral artery growth. Am J Physiol Heart Circ Physiol 283: H2411–H2419
Alvarez-Dolado M, Pardal R, Garcia-Verdugo JM, Fike JR, Lee HO, Pfeffer K, Lois C, Morrison SJ, Alvarez-Buylla A 2003 Fusion of bone-marrow-derived cells with Purkinje neurons, cardiomyocytes and hepatocytes. Nature 425: 968–973
Terada N, Hamazaki T, Oka M, Hoki M, Mastalerz DM, Nakano Y, Meyer EM, Morel L, Petersen BE, Scott EW 2002 Bone marrow cells adopt the phenotype of other cells by spontaneous cell fusion. Nature 416: 542–545
Bailey AS, Willenbring H, Jiang S, Anderson DA, Schroeder DA, Wong MH, Grompe M, Fleming WH 2006 Myeloid lineage progenitors give rise to vascular endothelium. Proc Natl Acad Sci USA 103: 13156–13161
Wurmser AE, Nakashima K, Summers RG, Toni N, D'Amour KA, Lie DC, Gage FH 2004 Cell fusion-independent differentiation of neural stem cells to the endothelial lineage. Nature 430: 350–356
Acknowledgements
The authors thank Saul Karpen for sharing his liver expertise, the Baylor Comparative Pathology Laboratory for transaminase analysis, the Baylor Flow Cytometry Core, and Dorothy Burton for excellent animal care.
Author information
Authors and Affiliations
Corresponding author
Additional information
These studies were supported by National Institutes of Health grants EB005173 and HL06148 to KKH.
Rights and permissions
About this article
Cite this article
Kienstra, K., Jackson, K. & Hirschi, K. Injury Mechanism Dictates Contribution of Bone Marrow-Derived Cells to Murine Hepatic Vascular Regeneration. Pediatr Res 63, 131–136 (2008). https://doi.org/10.1203/PDR.0b013e31815b481c
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1203/PDR.0b013e31815b481c