Abstract
The optimal thermal environment for sick preterm infants is unknown. Incubator temperature can be regulated to an abdominal wall temperature of 36.5°C [neutral temperature (NT)] or to a minimal temperature difference (<2°C) between abdominal wall and extremities [comfort temperature (CT)]. This could affect the microcirculation, particularly in infants with impaired perfusion. We assessed the microvascular perfusion with near-infrared photoplethysmography (NIRP) at these two target temperatures between d 1 and 4 of life in preterm infants with normal (NL group) or impaired (RED group) microcirculation as determined by a clinical score. Signal strength variables such as area under the curve (AUC) and the first derivate of the amplitude (FLUX) were calculated. Starting temperature was randomized to NT or to CT and then followed by the other temperature. A significant increase of FLUX and AUC in the RED group was found with NT as starting temperature (FLUX: 282 ± 76 at NT versus 627 ± 211 at CT; p = 0.025; AUC: 73 ± 47 at NT versus 234 ± 112 at CT; p = 0.009), but not with CT. In NL infants, both parameters did not change significantly. Increasing the incubator temperature to CT changes thermoregulatory flow to the extremities in preterm infants with impaired microvascular perfusion and might improve tissue flow.
Similar content being viewed by others
Main
The optimal thermal environment for nursing sick preterm infants is not known. Neutral thermal zone is traditionally defined as a range of environmental temperatures within which the metabolic rate is minimal and thermoregulation is achieved by nonevaporative means (1,2). NT corresponds to a body surface temperature of about 37°C above the liver, in accordance with a core temperature of approximately 36.5°C regardless of the temperature of the extremities (3,4). CT is defined as a minimal temperature difference between the abdominal wall and the extremities (<2°C) (3). CT corresponds to an approximate core temperature of 37°C and higher and is close to an intrauterine temperature of 38°C (2,4).
Changes in body temperature of about 1°C are primarily associated with changes in total peripheral skin blood flow (5). In term infants with NL exposed to phototherapy an increase of 224% in skin blood flow has been observed using electrocapacitance plethysmography (6). The effect of incubator temperature on circulation and microcirculation of preterm infants with signs of an impaired peripheral microcirculation (e.g. mottled skin) has not yet been reported. In the present study, we compared the effect of different incubator temperatures (NT and CT) on indices of peripheral blood flow in preterm infants matched for gestational age either with infants showing clinical signs of an impaired microcirculation or those without such signs. Microcirculation was assessed noninvasively with NIRP (7).
Photoplethysmography was first described by Hertzman and Spealman in 1937 (8). It is a noninvasive technique consisting of a red/infrared light-emitting diode and a photosensitive detector (9). The emitted light is either absorbed in solid tissues or reflected by the erythrocytes and the vessel walls. Photoplethysmography can be used to monitor heart rate and oxygen saturation and to detect hemodynamic disturbances such as hypovolemia (10). The signal is related to blood flow and depends on the peripheral vasomotor tone, ambient temperature, hemoglobin (Hb) content, the response of the autonomic nervous system to stressful situations, and nociceptive stimulation (11–15).
We hypothesized that we would be able to observe and measure changes in microcirculation with incubator temperature changes and that these changes would be more pronounced in infants with clinically perturbed skin circulation.
PATIENTS AND METHODS
Patients.
After parental consent, all preterm infants with a gestational age >30 wk admitted to the intensive care unit were enrolled in the study during 1 y. All investigations were made between d 1 and 4. Gestational age in completed weeks was determined by maternal dates of the last menstrual period and by early ultrasound measurements and was confirmed by clinical examination. Infants with congenital malformations were excluded. The Institutional Review Board of the Medical Faculty of the Ludwig Maximilian University Munich approved the study protocol and the consent forms. The peripheral microcirculation of the infants was classified as NL or RED according to a clinical score (Table 1). NL was defined as a score <3, RED as a score ≥3. Scores were determined at the beginning of the study. Infants with disturbed microcirculation were matched to the next infant with the same gestational age (by week) and no clinical evidence of a microcirculatory disturbance.
Methods.
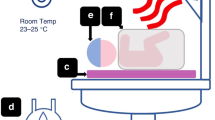
The infant's skin temperatures of the anterior abdominal wall above the liver and at the sole of one foot were measured during the entire study period using small skin thermometers (HP 21091; Hewlett Packard, Böblingen, Germany), which were fixed with a special foam pad. The temperature of the incubator was determined using a thermometer (HP 21078A) hanging freely 5 cm above the infant's abdomen. A standard thermometer in a water bath was used to calibrate the temperature probes to a precision of ±0.1°C (25 E; Thermo Schneider, Wertheim, Germany). NT was defined as the required incubator temperature to achieve a body surface temperature of 36.5°C at the anterior abdominal wall regardless of temperature of the extremities. CT was defined as the required incubator temperature to obtain a minimal temperature difference between abdominal wall and extremities (less than 2°C). Relative humidity in the incubators was kept at 80% to avoid generalized body cooling by heat loss from the skin surface in a low humidity environment.
Microvascular perfusion was assessed at the sole of the foot using NIRP. Microcirculatory parameters were determined with a combined red and near-infrared emission and detection system that has been described previously in detail (7,16). In brief, the volume pulse signal is obtained using a red and near-infrared light source emitting 840 nm and 640 nm light with a sample rate of 128 Hz. Pulsed light is used with a mean power of 1.25 mW and a maximum power of 5 mW. The signal is mainly reflected from the erythrocytes and originates from periodic changes in the Hb content of the tissue. The remitted light allows the detection of a volume pulse where only the pulsatile component is displayed. Two emitters and the sensor are embedded in a plastic disk, which is attached to the sole of the foot. To reduce light interference, the entire foot is then covered with black felt. The data were continuously recorded for 5–10 min before and after the intervention. The signals are analyzed offline and signal strength parameters as AUC and the maximum of the FLUX are calculated with a custom-built software (7,17). The time sequences are measured in each volume pulse for both the RED and NIRP signal. The data are calculated for each volume pulse and as a mean for the entire recording to reduce artifacts. Penetration of the signal has been shown to be approximately 3–5 mm (18). Blood pressure was measured at the beginning of the study at NT and at CT by using a HP Neonatal Blood Pressure Cuff (Hewlett-Packard Medical Products Group, Andover, MA). Heart rate and pulse oximeter saturation (Spo2) were calculated as the mean value obtained with a Hewlett-Packard cardiac and respiratory monitor over the 1 h of NIRP measurements.
Protocol.
Starting incubator temperature was randomized either to NT or CT (see above). Measurements were started approximately 10 min after the target temperature had been reached, and the infants were in quiet sleep in supine position in their incubators. Quiet sleep was assumed when no movements were observed apart from occasional startle reactions and when respiration was regular. After the first measurements, incubator temperature was adjusted in increments of 1.0–1.5°C to reach the second target body temperature. On average, it took 45 min to achieve the target temperature. The next measurement was obtained approximately 10 min after the target temperature had been reached, and the infants were in quiet sleep.
Muscular activity such as feeding or crying can be associated with a significant increase in total peripheral blood flow (5). The optimal time to obtain recordings for preterm infants is between 30 and 90 min postprandial (6). In this study, measurements were obtained nearly 45, 90, and 150 min after a feeding. Whenever activity was registered, the measurements were interrupted and were resumed when the infant had settled.
Statistical analysis.
The data were analyzed by using the SPSS statistical package (SPSS Inc., Chicago, IL). Statistical analyses were performed to test for significant changes in the difference of the increase (NT as starting temperature) or decrease (CT as starting temperature) of AUC and the FLUX between the NL and the RED groups. Data are presented as mean (standard deviation). Mann-Whitney U tests were used to indicate significance at an overall α of 0.05 (Table 1).
RESULTS
The clinical data and characteristics of the 28 infants are given in Table 2. The majority of infants in both groups were born prematurely because of premature rupture of membranes, but only two infants (in the NL groups) had elevated C-reactive protein levels indicating infection. Since the measurements were obtained during the first 2 d of life, nearly all infants still received antibiotics. Eight infants (four in each group) received volume boluses in the first hours of life for mean arterial blood pressures below 30 mm Hg and eight infants were ventilated, four in each group. None of the infants showed clinical signs of a patent ductus arteriosus.
The clinical score was 1.3 ± 1.0 in the NL group and 3.1 ± 0.9 in the RED group (p = 0.007) when the investigations were started with the NT range. After reaching CT, the clinical score was 0 ± 0 in the NL group and 0.3 ± 0.5 in the RED group (p = 0.141). This change in the clinical score was only observed when the CT was targeted after NT.
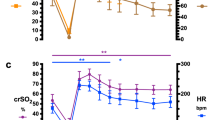
In RED infants, AUC (73 ± 47 versus 234 ± 112 p = 0.009) and FLUX (282 ± 76 versus 627 ± 211; p = 0.025) increased significantly when measurements were started at NT followed by CT (Figs. 1 and 2). However, there was no change in AUC and FLUX when measurements were first obtained at CT, followed by NT (AUC: 114 ± 42 versus 78 ± 28; FLUX: 364 ± 93 versus 272 ± 56). In infants without clinical signs of impaired microcirculation (NL), both AUC and FLUX did not change significantly (NT versus CT: AUC 93 ± 58 versus 140 ± 59; FLUX 310 ± 136 versus 446 ± 163; CT versus NT: AUC 145 ± 76 versus 77 ± 40; FLUX 462 ± 239 versus 293 ± 77). Incubator temperature had to be increased from 31°C to >38°C to reach the body CTs for all infants, RED and NL, and both groups required a similar increase in incubator temperature (Table 3).
Changes in FLUX. FLUX increased significantly in RED infants when incubator temperature was increased to CT. NT-CT: neutral to comfort temperature; CT-NT: comfort to neutral temperature. *p = 0.025, different from CT when started at NT, followed by CT. NL: n = 7 neutral to comfort, n = 7 comfort to neutral. RED: n = 7 neutral to comfort, n = 7 comfort to neutral.
Changes in AUC. AUC increased significantly for in RED infants when incubator temperature was increased to comfort temperature. NT-CT: neutral to comfort temperature; CT-NT: comfort to neutral temperature. *p = 0.009, different from CT when started at NT, followed by CT. NL: n = 7 neutral to comfort, n = 7 comfort to neutral. RED: n = 7 neutral to comfort, n = 7 comfort to neutral.
Despite these large temperature differences, clinical variables such as heart rate, blood pressure, and Spo2 did not differ between the two groups. Increasing incubator temperature to CT did not change any of these parameters significantly in any of the groups (Table 4). Overall there was a trend to lower blood pressure, and higher respiratory and heart rate at CT than at NT, which was more pronounced in the RED-group, but did not reach statistical significance.
DISCUSSION
We have shown that increasing incubator temperature to CT with a temperature difference of less than 2°C between abdominal wall and extremities significantly changes skin blood flow in the extremities of premature infants with clinical signs of compromised microcirculation but not in infants without such signs. Since NIRP primarily assesses the nutritive flow in the deeper layers of the skin, we assume that microvascular perfusion was impaired in these infants.
The prominent role of the microcirculation in disease and recovery has been the focus of many recent studies in adults and in animal models (19–21). In adults with septic shock, microcirculatory distress not corrected for 24 h was the single independent factor predicting patient outcome (21). In the newborn, cardiac output normalized to mass is much higher than in the adult, and skin surface area is significantly larger in relation to mass. The neonate is able to compensate for heat loss by maximal contraction of skin vessels immediately after birth (1). The compensatory mechanism of vasoconstriction mainly of skin arteriovenous shunts is particularly profound in neonates, whereas autoregulatory capability of the skin is poor. These characteristics make neonates notably susceptible to microcirculatory disturbances.
The optimal thermal environment for sick preterm infants is under debate since the invention of incubators, but so far the effect of incubator temperature on microvascular perfusion has not been studied. The current recommendations for body and ambient temperature are based on investigations carried out 20–40 y ago (1,4,22–24), but recently a Cochrane Review showed that maintaining body temperature still plays a role in reducing mortality (25). Just as low temperature is associated with increased mortality, overheating might stress infants and might also pose a risk. Increasing temperature to the recommended CT might increase blood flow to the skin, and in compromised infants, this may be to the expense of blood flow to vital organs.
Capillary refill time seems to be an unreliable indicator of microcirculatory impairment in term neonates shortly after birth (26). NIRP has been used in neonates to assess skin blood flow (27,28). In contrast to laser Doppler technology, which uses two laser beams to measure the velocity of erythrocytes in blood vessels, NIRP uses two wavelengths of light and is easier to apply (29). The major part of the photoplethysmographic signal represents blood flow through arteriovenous shunts from a greater depth of the skin and measures parameters representative of total blood flow (30–32). These shunts are innervated by sympathetic nerves and are part of the thermoregulatory system of the skin. In a comfortable ambient temperature, 80% of skin blood flow consists of blood flow through the arteriovenous shunts (32). Since NIRP is able to measure skin blood flow and its changes in different ambient environments, it might be a suitable method to evaluate the influence of incubator temperature on skin microcirculation. Taken together with macrocirculatory parameters, it might aid optimal temperature control in sick neonates.
Changing core body temperature from low normal (36.1–36.5°C) to high normal values (37.7–37.9°C) leads to changes in spontaneous breathing pattern in ventilated preterm infants (24). In that publication, incubator temperature varied less, both in the low-normal (34.3 ± 0.9°C) and the high-normal (36.3 ± 1.0°C) groups, to reach the two target body temperature ranges than in our current study. In the above-mentioned study, infants who were ventilated were significantly less mature and had lower birth weights, but were studied at a slightly older postnatal age. Warmed humidified air from a ventilator is a substantial source of heat for small infants, which might explain why significantly higher body temperatures were achieved with smaller incubator temperature changes. In both studies, the larger gradient in core to peripheral body temperature, the colder environment likely represents an active thermoregulatory response with peripheral vasoconstriction.
In our trial, the changes in blood pressure and heart and respiratory rates did not reach significance, probably due to the small number. Group size was calculated from AUC or FLUX values based on previous studies (16) and might have been too small to detect changes in these clinical variables.
Our study shows that under the CT condition, preterm infants with signs of a compromised microcirculation have higher skin blood flow and therefore increased perfusion of the extremities than under NT. The impact that this observation has on the assessment of vital organ perfusion is not known. Intravital microscopy studies or positron emission tomography may help to confirm this correlation. This would make NIRP a valuable clinical patient monitor to assess the thermoregulatory response to postnatal adjustment after birth and to disease due to its easy use and the fact that it is ready available.
Abbreviations
- AUC:
-
area under the curve
- CT:
-
comfort temperature
- FLUX:
-
first derivate of the pulse wave amplitude
- NIRP:
-
near-infrared photoplethysmography
- NL:
-
normal microcirculation
- NT:
-
neutral temperature
- RED:
-
impaired microvascular perfusion
- Spo2:
-
pulse oximeter saturation
References
Brueck K, Parmelee AH Jr, Brueck M 1962 Neutral temperature range and range of thermal comfort in premature infants. Biol Neonate 4: 32–51
Thomas K 1994 Thermoregulation in neonates. Neonatal Netw 13: 15–22
Lemburg P 1963 Thermal monitoring of very preterm infants. Which temperature should be measured. In: Okken A, Koch J (eds) Thermoregulation of Sick and Low Birth Weight Neonates: Temperature Control, Temperature Monitoring, Thermal Environment. Springer, Berlin pp 63
Sauer PJ, Dane HJ, Visser HK 1984 New standards for neutral thermal environment of healthy very low birthweight infants in week one of life. Arch Dis Child 59: 18–22
Wu PY, Wong WH, Guerra G, Miranda R, Godoy RR, Preston B, Schoentgen S, Levan NE 1980 Peripheral blood flow in the neonate; 1. Changes in total, skin, and muscle blood flow with gestational and postnatal age. Pediatr Res 14: 1374–1378
Wu PY, Wong WH, Hodgman JE, Levan N 1974 Changes in blood flow in the skin and muscle with phototherapy. Pediatr Res 8: 257–262
Bauer A, Bruegger D, Christ F 2005 [Microcirculatory monitoring of sepsis]. Anaesthesist 54: 1163–1175
Hertzman AB, Spealman CR 1937 Observations on the finger volume pulse recorded photoelectrically. Am J Physiol 119: 334–335
Christ F, Nehring I, Abicht J, Baranov V, Kotov A, Gartside I, Gamble J, Messmer K 1998 Changes in the arteriolar volume pulse of the finger during various degrees of tilt using near infra-red and red photoplethysmography. Eur J Med Res 3: 249–255
Partridge BL 1987 Use of pulse oximetry as a noninvasive indicator of intravascular volume status. J Clin Monit 3: 263–268
Oishi M, Nishida H, Kabe K, Hoshi J 1993 Monitoring neonatal peripheral circulation by electrocardiogram-to-oximeter pulse velocity. Pediatr Res 33: 653–657
Thurston GB 1990 Light transmission through blood in oscillatory flow. Biorheology 27: 685–700
Kumazawa T, Kobayashi M, Tagaki K 1964 A plethysmographic study of the human skin under various environmental conditions. Jpn J Physiol 14: 354–364
Johnstone M 1974 Digital vasodilation: a sign of anaesthesia. Br J Anaesth 46: 414–419
Young IM 1962 Vasomotor tone in the skin blood vessels of the newborn infant. Clin Sci 22: 325–332
Seidl T, Genzel-Boroviczeny O, Abicht JM, Christ F 2004 Does red blood cell transfusion change the near infra red photoplethysmography signal in infants?. Intensive Care Med 30: 1602–1606
Christ F 1996 [Methods of microcirculatory monitoring (laser Doppler flowmetry, photoplethysmography and computer-assisted venous occlusion plethysmography)]. Anasthesiol Intensivmed Notfallmed Schmerzther 31: S37–S43
Niklas M, Moser U, Buehrer A, Valentin R, Abicht J, Baschnegger H, Christ F 1998 Attenuation of the near-infrared and red photoplethysmographic signal by different depth of tissues. Eur J Med Res 3: 241–248
Trzeciak S, Rivers E 2005 Clinical manifestations of disordered microcirculatory perfusion in severe sepsis. Crit Care 9: S20–S26
Ellis CG, Jagger J, Sharpe M 2005 The microcirculation as a functional system. Crit Care Med 9: S3–S8
Sakr Y, Dubois MJ, De Backer D, Creteur J, Vincent JL 2004 Persistent microcirculatory alterations are associated with organ failure and death in patients with septic shock. Crit Care Med 32: 1825–1831
Sinclair JC 1972 Thermal control in premature infants. Annu Rev Med 23: 129–148
Glass L, Silverman WA, Sinclair JC 1968 Effect of the thermal environment on cold resistance and growth of small infants after the first week of life. Pediatrics 41: 1033–1046
Rieger-Fackeldey E, Schaller-Bals S, Schulze A 2003 Effect of body temperature on the pattern of spontaneous breathing in extremely low birth weight infants supported by proportional assist ventilation. Pediatr Res 54: 332–336
Sinclair JC 2000 Servo-control for maintaining abdominal skin temperature at 36C in low birth weight infants. Cochrane Database Syst Rev 2: CD001074.
LeFlore JL, Engle WD 2005 Capillary refill time is an unreliable indicator of cardiovascular status in term neonates. Adv Neonatal Care 5: 147–154
Babchenko A, Khanokh B, Nitzan M, Arad I 1999 Low frequency spontaneous fluctuations in tissue blood volume in neonates. J Basic Clin Physiol Pharmacol 10: 259–272
Oishi M, Nishida H, Hoshi J 1996 Evaluation of the peripheral circulatory status of the neonate during homeothermal adjustment by plethysmo-time-interval. Acta Paediatr Jpn 38: 147–150
Kvandal P, Landsverk SA, Bernjak A, Stefanovska A, Kvernmo HD, Kirkeboen KA 2006 Low-frequency oscillations of the laser Doppler perfusion signal in human skin. Microvasc Res 72: 120–127
Hales JR, Roberts RG, Westerman RA, Stephens FR, Fawcett AA 1993 Evidence for skin microvascular compartmentalization by laser-Doppler and photoplethysmographic techniques. Int J Microcirc Clin Exp 12: 99–104
Tur E, Guy RH, Tur M, Maibach HI 1983 Noninvasive assessment of local nicotinate pharmacodynamics by photoplethysmography. J Invest Dermatol 80: 499–503
Toms SL, Cooke ED 1995 A comparison of the functioning of arteriovenous anastomoses in secondary Raynaud's phenomenon and control subjects in response to local hand warming. Int Angiol 14: 74–79
Acknowledgements
The authors gratefully acknowledge the support from the staff of the NICU Grosshadern.
Author information
Authors and Affiliations
Corresponding author
Additional information
These data are a part of the dissertation of Tamara Seidl.
Rights and permissions
About this article
Cite this article
Genzel-Boroviczény, O., Seidl, T., Rieger-Fackeldey, E. et al. Impaired Microvascular Perfusion Improves With Increased Incubator Temperature in Preterm Infants. Pediatr Res 61, 239–242 (2007). https://doi.org/10.1203/pdr.0b013e31802d77a2
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1203/pdr.0b013e31802d77a2
This article is cited by
-
Definition of hypotension and assessment of hemodynamics in the preterm neonate
Journal of Perinatology (2009)