Abstract
Previous studies of the maturation of periodic breathing cycle duration (PCD) with postnatal age in infants have yielded conflicting results. PCD is reported to fall in term infants over the first 6 mo postnatally, whereas in preterm infants PCD is reported either not to change or to fall. Contrary to measured values, use of a theoretical respiratory control model predicts PCD should increase with postnatal age. We re-examined this issue in a longitudinal study of 17 term and 22 preterm infants. PCD decreased exponentially from birth in both groups, reaching a plateau between 4 and 6 mo of age. In preterm infants, PCD fell from a mean of 18.3 s to 9.8 s [95% confidence interval (CI) is ± 3.2 s]. In term infants, PCD fell from 15.4 s to 10.1 s (95% CI is ± 3.1 s). The higher PCD at birth in preterm infants, and the similar PCD value at 6 mo in the two groups, suggest a more rapid maturation of PCD in preterm infants. This study confirms that PCD declines after birth. The disagreement between our data and theoretical predictions of PCD may point to important differences between the respiratory controller of the infant and adult.
Similar content being viewed by others
Main
Experimental evidence is accumulating that PB in the infant, as in the adult, is caused by instability in the respiratory control system, possibly mediated by increased hypoxic stimulation of the peripheral chemoreflex feedback loop during the early postnatal period (1,2). Furthermore, math modeling studies of respiratory control based on a downscaling of adult cardiorespiratory time constants to the infant (3,4) support such a conclusion. Of interest, the theoretical model of Khoo et al. (3) predicts that the PCD should increase progressively with age from approximately 18 s at birth to an adult value in excess of 30 s.
Surprisingly, most infant data contradict this model prediction, with three longitudinal studies in term infants (5–7) and one small study in preterm infants (1) reporting that PCD decreases significantly from birth to 6 mo postnatally. However, another cross-sectional study of selected long epochs of PB in a larger group of preterm infants studied just before discharge from the nursery reported that PCD showed no trend or a weakly increasing trend over the first 3 mo postnatally (8); while the results of this study could be interpreted as conforming with the predictions of the Khoo model scaled to the infant, we have argued previously that the study design may have biased the outcome (1).
To resolve the issue as to whether PCD decreases or increases with postnatal age, further longitudinal studies were warranted in a larger population of term and preterm infants. Based on our earlier studies, and consistent with recent findings that the ventilatory response to CO2 becomes brisker with age (9), we hypothesized that the reduction in PCD commencing at birth and completed by 6 mo postnatally in both term and preterm infants represents a similar adaptation to air breathing and is mediated principally by changes in the response time of the peripheral chemoreceptors. Accordingly, we measured PCD in term and preterm infants over this period. Our study design also allowed us to ascertain whether sleep state (SS) and sleep position (SP) impact on PCD.
METHODS
Subjects.
Twenty-four term infants (15 F/9 M) and 27 preterm infants (16 F/11 M) were recruited from the maternity wards at Monash Medical Centre, Melbourne, Australia. Before the study, written informed consent was obtained from the parents of all subjects. The Monash Medical Centre Human Research and Ethics Committee granted ethical approval for this project.
Term infants were born between 38 and 42 wk gestation, with normal birth weights (mean ± SE, 3578 ± 87 g; range, 3015–4190 g). Apgar scores were 5–10 (median, 9) at 1 min and 6–10 (median, 9) at 5 min. Preterm infants were born between 30 and 35 wk gestational age (GA), with a birth weight of 1806 ± 88 g (range, 920–2408 g). Apgar scores were 3–9 (median, 7) at 1 min and 7–10 (median, 9) at 5 min.
Term infants were studied longitudinally at 2–3 wk after birth, with a post-conceptional age (PCA) of 291 ± 9 d (range, 278–315 d), at 2–3 mo post-term (PCA of 349 ± 11 d; range, 336–365 d) and at 5–6 mo postnatal age (mean PCA of 461 ± 13 d; range, 445–475 d). The preterm infants were studied four times, the first study being at 36–38 wk GA (PCA of 257 ± 5 d; range, 249–267 d), the other three at ages that were age-matched postconceptionally to the term infants at 2–3 wk (PCA of 287 ± 6 d; range, 267–302 d), 2–3 mo (PCA of 352 ± 9 d; range, 329–367 d), and 5–6 mo (PCA of 443 ± 3 d; range, 442–447 d).
Recording methods.
A 16-channel Grass Polygraph (Model 78A, Grass Instrument Company, Quincy, MA) was used to record EEG, electrooculogram, submental electromyogram, ECG, instantaneous heart rate, thoracic and abdominal breathing movements (Resp-ez Piezo-electric sensor, EPM Systems, Midlothian, VA), expired CO2 (CO2/O2 Analyser, Engstrom Eliza MC, Bromma, Sweden), blood oxygen saturation (Biox 3700e Pulse Oximeter, GE Healthcare, Piscataway, NJ), and abdominal skin and rectal temperature (YSI 400 series thermistor NTC probes, YSI Inc., Yellow Springs, OH). SS was assessed as quiet sleep (QS), active sleep (AS), or indeterminant sleep using EEG, behavioral, heart rate, and breathing pattern criteria (10).
Protocol.
All polysomnography recordings were made between 1000 h and 1600 h. Electrodes were attached to the baby during a feed. When drowsy, infants were placed in a bassinet, or in a cot for infants at age 5–6 mo. The room was dimly lit and temperature was kept constant (22–23°C). Infants generally had a morning and afternoon sleep interrupted by a midday feed, when SP was changed. The initial SP (prone or supine) was randomized on the morning of the first study; the opposite position was used for the morning of the second study and the order of SP was again reversed for the final study.
Polysomnographic recordings began when the infant was in a stable SS. As part of a study of arousal, an air-jet was used at intervals during the sleep period to deliver 5-s pulses of pressurized air to the infant's nostrils (11). This involved presenting a variable intensity stimulus alternately to the left and right nostrils to determine the pressure that induced arousal. On occasions, the air-jet stimulus induced a sigh that was often followed by an epoch of PB.
Data analysis.
Analysis was performed on babies exhibiting at least one epoch of PB, defined using a modified Kelly criterion (12) as two or more sequential apneas lasting more than 3 s, separated by periods of ventilation with a duration of <20 s (1,5). Of 51 enrolled infants, this criterion was fulfilled by 17 term (11 F/6 M) and 22 preterm infants (12 F/10 M). PCD was measured as the time interval between the initial breath of one breathing cluster and the initial breath of the next cluster. Only epochs of PB preceded by a sigh were analyzed. For each epoch of PB we recorded the average PCD, SP, SS, number of cycles comprising an epoch, duration of the epoch, and the preceding stimulus type (ST). The stimulus was designated as “spontaneous” if the sigh occurred in isolation with no apparent preceding event, or as “stimulated” if the sigh was preceded within a 3-s interval by an air-jet stimulus. This definition was not intended to imply causality; it merely allowed us to determine whether the presence of the air-jet arousal stimulus before the sigh affects PCD.
The primary goals of the analysis were to characterize the postnatal maturation of PCD in term and preterm infants and to determine whether SP, SS or ST influences PCD. A regression analysis was performed with PCD as the dependent variable and baby ID, preterm versus term, PNA, SS, SP, and ST as independent variables. This analysis catered for the full range of models of the form:

where PCDi is the PCD for baby i; Bi is a constant term for baby i; and B0x kx are constants, each taking one of two values depending on whether x = P (preterm) or T (term); B1, B2, and B3 are further constants; and SS, SP, and ST are binary (0,1) variables.
Significance for this regression analysis was assessed using Fisher's F tests in an associated ANOVA (Table 1). Where the assessment involved an invariant characteristic of a baby (i.e. preterm versus term), the denominator was the residual mean square deviation between babies. Where it involved a characteristic that varied both over time and between babies, the denominator was the residual mean square deviation. However, there was considerable replication in the data in that several PCD observations (average = 6) were sometimes available for a baby at one PNA. Under these circumstances, there is a potential bias in using F tests based on residual mean square deviation. To ensure that the analysis was free of bias, F tests based on residual “babies × times” deviation were also used (last column in Table 1).
RESULTS
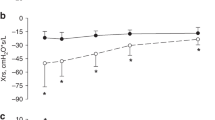
The PB pattern of a typical infant from an age of 28 d postnatally to 220 d shows a clear decline in PCD with PNA (Fig. 1). Figure 2, a and b, shows PCD plotted against PNA for all infants in the study. Also plotted in Figure 2, c and d, are published PCD data from previous studies. Note in Figure 2, c that the data of Glotzbach et al. (8) show a rising trend with PNA, whereas our previous (1) and current data demonstrate a progressive fall in PCD with maturation; the single point is from Rigatto et al. (13). All three existing studies in term infants (5–7) show a fall in PCD with PNA and are in good agreement with the regression curve derived from our current data (Fig. 2d).
Typical set of respi-band traces from a preterm infant (gestational age, 33 wk; birth weight, 2337 g) showing typical sigh-induced epochs of PB and progressive reduction in PCD with advancing PNA. Note increased damping of the sigh-induced respiratory oscillations, evident as a shortening of the PB epoch length with increasing PNA.
Postnatal trend in PCD. Each point in (a) and (b) represents a single estimate of PCD from this study. The solid line shows the regression equation for preterm (Eq. 2) and term infants (Eq. 3), with dotted lines representing ± 95% confidence intervals. In (c) and (d), data from previous studies are plotted with the regression lines and confidence intervals from (a) and (b).
Choice of regression model.
Many variants of Eq. 1 were considered, and five of these are included in the ANOVA set out in Table 1. The simplest (Model 1), which comprised only the Bi term (i.e. constant variability between babies but no dependence on PNA), accounted for 40% of the total variability in PCD. This compares with only 0.3% for the difference between the overall average PCD for preterm and term infants. Thus, without taking PNA into account, there was no significant difference between preterm and term infants (Table 1).
Model 2 incorporates the effect of PNA. It is the best fit obtained using a single exponential decay curve. The equation is
Eq. 4

The fit achieved with this model represents a significant improvement over Model 1 (p < 0.001), accounting for 72.5% of the total variability in PCD.
Model 3 comprises two exponential decay curves (one for preterm infants and one for term infants) with identical exponent k values. The equations are
Eq. 5

if preterm, and
Eq. 6

if term.
This model was significantly better than Model 2 (p < 0.001), accounting for 73.5% of the variability in PCD.
Model 4 comprised two exponential decay curves with different exponent values. This model, which accounted for 73.6% of the variability in PCD, was discarded because it provided no significant improvement over Model 3.
Model 5, which is our preferred model, is similar to Model 3, containing two exponential decay curves plus an additional term (–0.36 SS), which incorporates the significant effect of AS rather than QS in lowering PCD by a mean ± SE of 0.4 ± 0.1 s. Model 5 accounted for 73.9% of the total variation in PCD. The equation for the preterm infants is

For the term infants it is

Neither SP (p = 0.78) nor ST (p = 0.83) had a significant influence on PCD.
Limiting values of PCD.
Model 5 PCD maturation curves for preterm and term infants are illustrated in Figure 2. Use of a regression equation allowed determination of the limiting values of PCD at PNA = 0 (i.e. birth) and as PNA → ∞ (i.e. at the nadir of the falling phase of PCD during the first 4–6 mo postnatally). From the Model 5 equations, given QS, mean PCD values at PNA = 0 were 9.8 + 8.5 = 18.3 s for preterm infants and 10.1 + 5.3 = 15.4 s for term infants. The same equations, given QS, show that PCD values approach Bi as PNA → ∞. For preterm infants, this gave a mean ± SE for Bi of 9.8 ± 0.2 s and for term infants 10.1 ± 0.3 s; these two mean values were not significantly different (p = 0.31, t test). Model 5 is therefore consistent with there being no difference between the PCD in preterm and term infants after a sufficient interval of time.
Finally, since the exponent derived from the regression analysis was identical for preterm and term infants, a time constant of 1/k = 39.21 d characterized both infant groups. Thus, 95% of the total maturational change in PCD from birth to its nadir at 4–6 mo postnatally was completed within three time constants (117.6 d), or approximately 4 mo. In our previous studies of preterm and term infants we used a bilinear regression instead of an exponential regression to determine the PNA at which no further change in PCD occurs. This PCD plateau value (Fig. 3) started at a PNA of 110 d in preterm infants and at 90 d in term infants (1,5). Thus, the results from our current and previous studies are in reasonable agreement despite use of different methods to determine the start of the PCD plateau. Similarly, the asymptotic values of PCD at PNA = 0 are similar for the two methods: the current study yielded 18.3 s versus 17.1 s in our previous study for preterm infants (1), whereas in term infants PCD at PNA = 0 was 15.4 s in the current study versus 15.6 s in our previous study (5).
Postnatal trend in PCD from infant to adult showing the initial fall in PCD up to approximately 4 mo PNA followed by a plateau and a subsequent rise to adult values. Infant data are from our term group, whereas the PCD estimates marked with an asterisk are clinical data acquired from infants and children admitted to our sleep laboratory with suspected OSA in whom no evidence was found (unpublished data).
PCD variability.
A 95% confidence interval for the regression line of Model 5 was determined by estimating a total variance comprising two components. The first source of variability is the between-babies component. This was calculated as the variance of Bi for each infant group. For the preterm group it was 0.92 s2 and for the term group it was 0.95 s2. The second component is the residual mean square variability from the regression given in Table 1 as 1.56 s2. A pooled estimate of (the total) variance was calculated by adding these components giving a 95% confidence interval (i.e. ±2 SD) of ±3.1 s for the term group and ±3.2 s for the preterm group. These intervals are indicated as dotted curves in Figure 2.
Effect of postconceptional age on PCD.
Although the primary focus of our study was to determine the maturational change in PCD with PNA, the PCD data are plotted against PCA in Figure 4. At 257 d PCA, PCD for the preterm group was 14.1 ± 1.9 s (mean ± SD). At 290 d PCA, PCD in the term group was 13.9 ± 1.3 s, significantly greater than in the preterm group in which mean PCD was 11.2 ± 1.7 s (p < 0.001). At 350 d PCA, mean PCD in the term group (10.9 ± 0.9 s) was significantly greater than in the preterm group (10.2 ± 1.4 s; p < 0.01). At 450 d PCA, there was no significant difference (p = 0.75) between the two groups and the aggregate PCD was 9.4 ± 1.3 s.
DISCUSSION
Our study confirms and extends previous reports that PCD falls with advancing PNA (1,5–7) in both preterm and term infants. While the initial value of PCD in preterm infants of 18.3 ± 3.2 s (mean ± 2 SD) is significantly higher than that in term infants (15.4 ± 3.1 s), 95% of the exponential decrease in PCD that occurs between birth and 6 mo is completed by 117.6 d PNA in both infant groups. Furthermore, the PCD reached at around 6 mo is approximately 10 s in both cases. Our study therefore suggests a more rapid maturation of PCD with PNA in preterm compared with term infants. Interestingly, a significant but small decrease in PCD was found during AS but SP did not significantly influence PCD.
Term infants.
Our data are in accord with a study by Fleming et al. (6) in 15 normal full-term infants carried out from shortly after birth to 7 mo of age. From their Figure 8 (6), PCD can be calculated as PCD = 2π/ωn, where ωn is the natural radian frequency of the ventilatory oscillation. These calculated values, plotted in Figure 2 d, are in excellent agreement with our data. The high PCD and rapid PCD development reported by Fleming et al. during this period of adaptation to air breathing are consistent with the suggestion that PCD decreases with age as a result of reduced chemoreception delays and/or increased lung washout efficiency (1,5,7).
Other data from term infants show a fall in PCD with PNA. For example, as is clear from Figure 2 d, the data of Barrington et al. (7), acquired from 10 full-term infants followed longitudinally out to 12 wk postnatally, lie well within the 95% confidence intervals of our data, as do previous term infant data on PCD collected during longitudinal studies in our laboratory (5).
Preterm infants.
In contrast to the situation in term infants, data on PCD maturation in preterm infants are contradictory. The current study and our earlier work (1) demonstrate PCD falls with PNA, whereas a study by Glotzbach et al. (8) reported no change in PCD with PNA. The possible reasons for this discrepancy have been discussed in some detail previously (1). Briefly, their study was cross-sectional, with infants randomly selected for a 24-h study just before discharge home. Crucially, 75% of their data were clustered within the first 6 postnatal weeks rather than being spread over 30 wk, as in our study. Since the infants were not followed longitudinally, the ability to detect a reduction in PCD over time was weakened, especially given the large between-baby variability demonstrated in this current study by the wide 95% confidence intervals for PCD in Figure 2.
A further issue in the study of Glotzbach et al. is that they selected only long epochs of PB for analysis in each infant. If a correlation exists between PCD and epoch length, the use of long epochs could lead to a selection bias favoring larger PCD values and, as we argue later, a different pattern of maturation. Such a correlation might be expected on theoretical grounds, inasmuch as our calculations using a math model of the infant control system (3,4) assuming mild hypoxia associated with intrapulmonary shunting in the early postnatal period (14) show that, if we assume changes in PCD are mediated through changes in circulatory delay (Fig. 5), then state-related changes in cardiac output would be expected to strongly mediate such changes. Accordingly, if for example cardiac output falls, the lung-chemoreceptor circulatory delay would increase and loop gain (LG) could exceed 1, spontaneously generating a long epoch of PB with a long PCD. Conversely, if cardiac output rises, the circulatory delay and LG would decrease producing a short epoch of PB with a shorter PCD.
Predicted effect of increasing circulatory delay (with respect to the nominal value of 3.5 s) between the lung and peripheral chemoreceptors on PCD and LG. As circulatory delay increases, both LG and PCD increase, destabilizing respiratory control; sustained PB is predicted when LG ≥1. The mildly hypoxic conditions assumed in this model simulation result in LG >1 even when circulatory delay is nominal and, accordingly, sustained PB with a PCD of 14 s is predicted.
A final mechanism by which choosing long epochs of PB for analysis may distort the postnatal trend in PCD is if there are two temporal components in the peripheral chemoreceptor feedback loop and each contributes to the PCD. If one of these falls with maturation (e.g. speed of chemoreceptor response) while the other rises (e.g. circulatory delay from lung to peripheral chemoreceptor), then whether PCD increases or decreases with maturation will depend on which of these components dominates. If long epochs of PB have longer PCD caused by increased circulatory delay, as argued above, then in these epochs the increase in circulatory delay with maturation would tend to dominate, giving PCD a rising trend (or perhaps no trend if the two trends are equal and opposite) with maturation. Conversely, shorter epochs with shorter circulatory delay would be dominated by the speed of response of the peripheral chemoreceptor, and in this case PCD would fall. Unfortunately, our data do not permit us to assess whether PCD maturation is different when long epochs are selected for analysis because potentially long epochs of PB were often terminated prematurely by the air jet stimulus used for arousal testing.
Sleep state and sleep position.
In our previous studies (1,5) and those of others (7), SS did not influence PCD. Interestingly, in the current study using a more sensitive multiple regression analysis, a small decrement in PCD (0.36 s) was identified in AS. We are unsure why this small decrement occurs, but it may mirror the effect of an increased cerebral blood velocity during AS (15,16) leading to a small reduction in lung-chemoreceptor delay and PCD. In addition, we found that SP had no effect on PCD maturation, suggesting that the respiratory control system and circulation delay are not influenced by whether the infant is prone or supine; this finding argues against a role for vestibular processes in the declining trend in PCD.
Maturation of respiratory control.
Our findings provide insight into the maturation of respiratory control in infants. First, the theoretical prediction that PCD should rise with PNA was not observed, even though increasing body size and the dramatic fall in cardiac output/kg in the immediate postnatal period in infants (17) would be expected to increase the circulatory delay between the lung and peripheral chemoreceptors. This observation suggests that other factors must cause PCD to decline. By contrast, data from older infants, from adults with idiopathic central sleep apnea (18), and from young high altitude climbers (19) are consistent with theoretical model predictions, suggesting that increasing circulation delay assumes a greater importance some time after the first 6 mo of life (Fig. 3). Second, the temporally matched fall in PCD with PNA in the preterm and term infants suggests that the respiratory control system undergoes an adaptation to air breathing starting soon after birth, and that this adaptation is completed by 4 mo postnatally, regardless of whether birth is preterm or term. Third, since a number of studies suggest a dominant role for the peripheral chemoreceptors in the genesis of respiratory instability (3,4,6,14,20,21), the fall in PCD with PNA specifically points to a shortening of the time delays in the peripheral chemoreflex loop as causative. Interestingly, recent evidence suggests that delays in the response to a hypercapnic stimulus are significantly reduced in human infants over the first weeks of life while the peripheral O2 chemoreflex shows little change (9). This decrease in response time to a hypercapnic stimulus may be mediated through maturational changes in the peripheral chemoreceptors themselves, or it is possible that developmental changes associated with increased myelination of nerve fibers in the CNS, or changes in the central integration of sinus nerve traffic in the brain stem, play a role.
In conclusion, our study shows, contrary to theoretical prediction, that PCD falls exponentially from birth until 4 mo postnatally and thereafter is unchanged until at least 6 mo PNA, confirming our previous studies in smaller numbers of infants. In addition, a small but significant decrease in PCD in AS compared with QS was identified, whereas SP had no effect on PCD. As has been suggested (6), the observed changes in PCD and in the length of PB epochs with postnatal age may signify a switch from an immature unstable infant respiratory control system to a more mature and stable adult system. The results of our current study support the view that the mechanism responsible for the maturation of PCD is a decline in the response time of the peripheral chemoreceptor in the first 6 mo of life.
Abbreviations
- AS:
-
active sleep
- GA:
-
gestational age
- LG:
-
loop gain
- PB:
-
periodic breathing
- PCA:
-
post-conceptional age
- PCD:
-
periodic breathing cycle duration
- PNA:
-
postnatal age
- QS:
-
quiet sleep
- SP:
-
sleep position
- SS:
-
sleep state
- ST:
-
stimulus type
References
Wilkinson MH, Cranage S, Berger PJ, Blanch N, Adamson TM 1995 Changes in the temporal structure of periodic breathing with postnatal development in preterm infants. Pediatr Res 38: 533–538
Rigatto H 2003 Periodic breathing. In: Mathew OP (ed) Respiratory Control and Disorders in the Newborn. Marcel Dekker Inc, New York, pp 237–272
Khoo MC, Kronauer RE, Strohl KP, Slutsky AS 1982 Factors inducing periodic breathing in humans: a general model. J Appl Physiol 53: 644–659
Nugent ST, Finley JP 1987 Periodic breathing in infants: a model study. IEEE Trans Biomed Eng 34: 482–485
Wilkinson MH, Cranage S, Adamson TM, Wilson FE, Strangward A, Walker AM 1986 Incidence and temporal characteristics of oscillatory breathing patterns in full term neonates. IEEE/Eighth Annual Conf Eng Med. Biol Soc 2: 1257–1262
Fleming PJ, Goncalves AL, Levine MR, Woollard S 1984 The development of stability of respiration in human infants: changes in ventilatory responses to spontaneous sighs. J Physiol 347: 1–16
Barrington KJ, Finer NN, Wilkinson MH 1987 Progressive shortening of the periodic breathing cycle duration in normal infants. Pediatr Res 21: 247–251
Glotzbach SF, Tansey PA, Baldwin RB, Ariagno RL 1989 Periodic breathing cycle duration in preterm infants. Pediatr Res 25: 258–261
Sovik S, Lossius K 2004 Development of ventilatory response to transient hypercapnia and hypercapnic hypoxia in term infants. Pediatr Res 55: 302–309
Anders T, Emde R, Parmelee A 1971 A manual of standardized terminology, techniques and criteria for scoring of states of sleep and wakefulness in newborn infants. UCLA Brain Information Services/BRI Publications Office, Los Angeles
Horne RS, Sly DJ, Cranage SM, Chau B, Adamson TM 2000 Effects of prematurity on arousal from sleep in the newborn infant. Pediatr Res 47: 468–474
Kelly DH, Shannon DC 1979 Periodic breathing in infants with near-miss sudden infant death syndrome. Pediatrics 63: 355–360
Rigatto H, Brady JP 1972 Periodic breathing and apnea in preterm infants. I. Evidence for hypoventilation possibly due to central respiratory depression. Pediatrics 50: 202–218
Al-Matary A, Kutbi I, Qurashi M, Khalil M, Alvaro R, Kwiatkowski K, Cates D, Rigatto H 2004 Increased peripheral chemoreceptor activity may be critical in destabilizing breathing in neonates. Semin Perinatol 28: 264–272
Hajak G, Klingelhofer J, Schulz-Varszegi M, Matzander G, Sander D, Conrad B, Ruther E 1994 Relationship between cerebral blood flow velocities and cerebral electrical activity in sleep. Sleep 17: 11–19
Kuboyama T, Hori A, Sato T, Mikami T, Yamaki T, Ueda S 1997 Changes in cerebral blood flow velocity in healthy young men during overnight sleep and while awake. Electroencephalogr Clin Neurophysiol 102: 125–131
Walker AM 1993 Circulatory transitions at birth and the control of the neonatal circulation. Fetus and Neonate. Physiology and Clinical Applications. Cambridge University Press, New York, pp 160–196
Hall MJ, Xie A, Rutherford R, Ando S-I, Floras JS, Bradley TD 1996 Cycle length of periodic breathing in patients with and without heart failure. Am J Respir Crit Care Med 154: 376–381
West JB, Peters RM, Aksnes G, Maret KH, Milledge JS, Schoene RB 1986 Nocturnal periodic breathing at altitudes of 6300 and 8050m. J Appl Physiol 61: 280–287
Wilkinson MH, Berger PJ, Blanch N, Brodecky V, Jones C 1996 Source of respiratory drive during periodic breathing in lambs. Respir Physiol 104: 115–126
Wilkinson MH, Sia KL, Skuza EM, Brodecky V, Berger PJ 2005 Impact of changes in inspired oxygen and carbon dioxide on respiratory instability in the lamb. J Appl Physiol 98: 437–446
Author information
Authors and Affiliations
Corresponding author
Additional information
Supported by grants from the National Health and Medical Research Council of Australia, Grant Number 34836 and 194403, and the National SIDS Council of Australia, Grant G94-13/161.
Rights and permissions
About this article
Cite this article
Wilkinson, M., Skuza, E., Rennie, G. et al. Postnatal Development of Periodic Breathing Cycle Duration in Term and Preterm Infants. Pediatr Res 62, 331–336 (2007). https://doi.org/10.1203/PDR.0b013e3180db29e5
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1203/PDR.0b013e3180db29e5
This article is cited by
-
Ventilatory control instability as a predictor of persistent periodic breathing in preterm infants
Pediatric Research (2022)
-
Clinical associations with immature breathing in preterm infants: part 2—periodic breathing
Pediatric Research (2016)
-
Altitude, oxygen and the definition of bronchopulmonary dysplasia
Journal of Perinatology (2012)