Abstract
We determined the contributions of IGF-I, IGFBP-3 and leptin to growth in -extremely premature infants over the first two years. Weight (Wt), crown-to heel length (CHL), plasma IGF-I, IGFBP-3 and leptin were measured in infants (gestation 24–33 wk) at birth (n = 54), expected date of delivery (EDD) and 6, 12 and 24 mo post-EDD (n = 29). Area under the curve (AUC) for hormone levels was calculated over 4 periods: birth–EDD, EDD–200 d, EDD–350 d and EDD–700 d. IGFBP-3, but not IGF-I or leptin, on day 1 correlated with birth Wt SD scores (SDS) (r = 0.46, p = 0.002) and CHL SDS (r = 0.41, p = 0.01). Wt SDS at EDD correlated with AUC IGF-I, IGFBP-3 and leptin (birth–EDD), but leptin was the best predictor in multiple regression(r = 0.65, p < 0.0001). Wt at EDD + 700 d correlated with AUC leptin (EDD–700 d) (r = 0.62, p = 0.002). CHL SDS at EDD correlated with AUC IGFBP-3 and leptin (birth–EDD), but IGFBP-3 was the best predictor (r = 0.55, p < 0.0001). CHL at EDD + 700 d correlated with AUC IGF-I and IGFBP-3 (EDD–700 d), but IGFBP-3 was the best predictor (r = 0.47, p = 0.01). Wt and CHL at birth were associated with IGFBP-3 levels in these infants. Wt at EDD and EDD + 700 d was predicted by concurrent leptin output while linear growth at EDD and EDD + 700 d was predicted by IGFBP-3 output.
Similar content being viewed by others
Main
Preterm birth interrupts the physiologic fetal phase of growth when growth velocity is highest. Infants born at gestational age (GA) <32 wk have a different pattern of postnatal growth than those born moderately premature (GA ≥32 wk) or at term (1,2) and often exhibit growth failure, particularly over the first months of life. Signs of catch-up growth in weight and length can be observed by expected date of delivery (EDD) in infants born at GA 29–31 wk but are not seen until after one year corrected age for more premature infants <29 wk gestation (2). Growth restriction, especially if associated with later catch-up, is of particular concern in such infants owing to the association with cardiovascular disease in adulthood (3). Life-long programming of the insulin like growth factor-I (IGF-I) system by intrauterine and postnatal hormonal and nutritional environment has been suggested to be a key mediator of this association (4,5).
Nutrition is a key regulator of pre- and early postnatal somatic growth. Other factors thought to be important include IGF-I, its principal binding protein insulin like growth factor binding protein-3 (IGFBP-3) and leptin. Leger et al. (6) examined growth hormone (GH), IGF-I and IGFBP-3 levels in relation to growth over the 1st 24 mo in 254 growth retarded children (birth weight <3rd percentile; 81 born at GA 29–37 wk) and 84 appropriate-for-GA (AGA) controls (40 born at GA 29–37 wk). The growth retarded infants had high GH but low IGF-I and IGFBP-3 levels at birth. However, these biologic parameters were not predictive of later growth or of short stature at 2 y of age, and levels over 24 mo did not correlate with weight or length gain. A number of studies have characterized longitudinal growth in very preterm children (7–9) and examined the influence of endocrine factors up to 120 d of age (10–12). However, none have studied the influence of the IGF-I system and leptin on the pattern of growth beyond the early postnatal period. We therefore undertook this study to determine the contributions of plasma IGF-I, IGFBP-3 and leptin on growth in extremely premature infants over the first two years of life.
METHODS
Study population.
A longitudinal study with follow-up from birth (26 female, 28 male) until 24 mo post-EDD (29 infants) was undertaken in infants born September 1998 to May 1999 at GA 24–33 wk at two hospitals in Sheffield (Jessop Hospital for Women and Northern General Hospital). Infants were recruited prospectively within 24 h of birth (median birth weight and length 1260 g and 39.7 cm, respectively). Infants with severe intrauterine growth retardation (birth weight less than 3 SD scores (SDS)), congenital malformations, metabolic conditions, hydrops fetalis or maternal diabetes were excluded because these conditions could have altered growth rates and hormone levels. Infants transferred to a hospital in another region within 24 h of birth (4 infants) and those who did not survive the neonatal period (10 infants) were also excluded. GA was determined from date of mother's last menstrual period if estimates from maternal dates and fetal ultrasound scans performed at 12 postconceptual weeks agreed to within 14 d, and from fetal ultrasound if the difference was greater. Obstetric problems included prolonged rupture of membranes in 13, pre-eclampsia in 6, antepartum hemorrhage in 6 and fetal distress in 10 cases. Forty infants received antenatal glucocorticoids and 24 were delivered by Caesarean section. Median Apgar scores were 8 at 1 min and 9 at 5 min. Thirty-three infants received mechanical ventilation (median duration 10 h), 6 had chronic lung disease and 3 received postnatal glucocorticoids (total median dexamethasone dose 5.5 mg and duration 16 d). Postnatal complications included sepsis in 20, necrotising enterocolitis in 5 and intraventricular hemorrhage in 14 infants. The number of infants from whom follow-up data were obtained fell owing to parents declining further participation and unsuccessful venepuncture.
Ethical approval.
Ethical approval was granted by the North and South Sheffield Ethics Committees. Written informed consent was obtained from parents.
Growth measurements.
Weight (Wt), crown-to-heel length (CHL), knee-to-heel (lower leg) length (LLL) and head circumference (HC) were measured at birth, EDD and approximately 6, 12 and 24 mo post-EDD. Measurements were made by a single observer (EC) who had been trained by a skilled auxologist. Wt was measured using electronic scales after the nappy and all clothing were removed. CHL measurements were made using the Pedobaby® plastic ruler for infants in incubators and a Raven rollameter® for those in a cot. For the Pedobaby® the intraobserver coefficient of variation (CV) of means was 2.9% at a mean length of 42.3 cm. For the Raven rollameter® it was 2.4% at a mean length of 44.8 cm. LLL was measured with a neonatal knemometer using the technique described by Gibson and colleagues (13). Both legs were measured and the mean of the two was recorded as the LLL. Intra-observer CV was 1.5% at a mean measurement of 114.8 mm. HC was measured with a reusable Lasso® tape. The intraobserver CV was 1.1% at a mean measurement of 33.4 cm.
Blood samples and hormone assays.
Blood samples for IGF-I, IGFBP-3 and leptin were collected in lithium-heparin tubes at birth from the umbilical cord, within 24 h of birth, at the estimated EDD and close to 6, 12 and 24 mo post-EDD. After centrifugation, plasma samples were stored at –20°C until assayed. All samples from an individual were analysed in the same assay with appropriate sample dilution to fall within the detection range of each assay. Plasma IGF-I was measured using a previously reported in-house RIA (14). The detection range of the assay was 0.8–25 μg/L. The intra-assay CV was 4.0–5.7% and the inter-assay CV was 5.3–6.9%. The mean CV for all unknown samples analyzed was 4.5%. Plasma IGFBP-3 (IDS Ltd., UK) and leptin (Diagnostic Systems Ltd, Texas) were measured using commercially available RIA and ELISA kits. The detection range of the IGFBP-3 assay was 1–60 mg/L. The intra-assay CV was 2.6–6.0% while the inter-assay CV was 6.9% at 1.4 mg/L, 6.1% at 3.9 mg/L and 4.9% at 6.8 mg/L. The mean CV for plasma samples was 10.5%. The detection range of the leptin assay was 0.5–50 μg/L. The intra-assay CV was 1.5–6.2% while the inter-assay CV was 4.9% at 4.7 μg/L, 5.3% at 11.1 μg/L, 3.3% at 15.5 μg/L and 4.2% at 37.9 μg/L. The mean CV for plasma samples analysed was 7.7%.
Data analysis.
To remove variability in the time period between birth, EDD and age after birth and to standardize against time, 5 time points were chosen: birth, EDD, and 200, 350 and 700 d post-EDD (the latter 3 corresponding approximately to 6, 12 and 24 mo post-EDD). For each infant, growth data at 200, 350 and 700 d post-EDD was determined from the measurements obtained either side of these time points using regression analysis. Values for Wt, CHL and HC were expressed as SDS using the standards from Keen and Pearse (15) and Freeman et al. (16). For LLL the data from Gibson et al. (13) were used. To better represent the exposure to hormones over a prolonged period we calculated the area under the curve (AUC) between the 5 time points using the trapezium rule. Calculating the AUC per day allowed us to compare the overall levels of hormones over variable time periods. Thus AUC per day represented daily hormone output over 4 periods: birth to EDD, EDD to 200 d, EDD to 350 d and EDD to 700 d. Variables at different time points and periods were compared using paired t-test (growth SDS) and Wilcoxon signed rank test (hormone levels and output). Spearman's correlation, univariate and multiple linear regression were used to examine the relationships between growth parameters and hormone AUC/d. Statistical significance was defined as p ≤ 0.01.
RESULTS
Growth at birth and during the first 24 months.
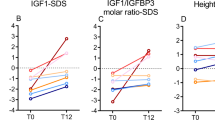
Anthropometric data at birth of the infants subdivided according to GA are shown in Fig. 1. Of the 54 infants, the number of infants with Wt, CHL and HC below –2 SDS were 5, 1 and 7 respectively. Subsequent measurements showed significant decline between birth and EDD in mean Wt (–0.5 to –1.1 SDS), CHL (–0.3 to –1.3 SDS) (Fig. 2) and LLL SDS (0.3 to –0.5 SDS) (p < 0.001). Thereafter catch-up in Wt to –0.5 SDS was seen by EDD + 200 d and in CHL to –0.2 SDS by EDD + 700 d. HC remained below zero SDS throughout the observation period (mean at birth –0.7 SDS and at EDD + 700 d –0.9 SDS). Postnatal changes in growth measurements did not differ between patients who had been exposed to antenatal or postnatal glucocorticoid treatment and those who had not received steroids (p > 0.1).
Hormone levels at birth and during the first 24 months.
Compared with cord concentrations, leptin levels on day 1 were lower (p < 0.0001; Table 1). Cord hormone concentrations did not correlate significantly with GA. However, plasma IGF-I (r = 0.37, p = 0.01) but not IGFBP-3 (r = 0.30, p = 0.05) and leptin (r = 0.13, p = 0.7) on day 1 correlated significantly with GA. IGF-I from cord blood and at EDD correlated with corresponding IGFBP-3 levels (cord r = 0.46, p = 0.002; EDD r = 0.44, p = 0.001) but not with leptin. No significant correlations were observed between the hormone concentrations on day 1. Between day 1 and EDD, IGFBP-3 (p < 0.0001) and leptin levels (p = 0.02) increased but IGF-I did not change significantly (p = 0.3; Table 1).
AUC/d (daily output) increased for all 3 hormones between the periods before and 200 d after EDD (Fig. 3; p < 0.001). While IGF-I and leptin output remained stable thereafter to 700 d post-EDD, IGFBP-3 output increased steadily (p ≤ 0.001). The relationships between IGF-I, IGFBP-3 and leptin output in the different time periods are shown in Fig. 4. Leptin output correlated significantly with IGFBP-3 output in the birth to EDD and EDD to 200 d periods, and with IGF-I output in the period EDD to 200 d (Fig. 4). The correlation between IGF-I output and IGFBP-3 output was significant in the period from birth to EDD (p = 0.01), with an increasing tendency to be related thereafter (p = 0.1–0.02).
Relationships between growth parameters and hormone levels at birth.
Cord IGF-I levels, but not IGFBP-3 or leptin, correlated with birth Wt SDS (r = 0.40, p < 0.01) and CHL SDS (r = 0.45, p < 0.01). IGFBP-3 levels on day 1, but not IGF-I or leptin, correlated with birth Wt SDS (r = 0.46, p = 0.002), CHL SDS (r = 0.41, p = 0.01), LLL SDS (r = 0.43, p = 0.006) and HC SDS (r = 0.44, p = 0.004).
Relationships between growth parameters and daily hormone output over the first 24 months. The biochemical parameters which correlated significantly in univariate analysis with growth measurements at different time points are shown in Table 2. Wt SDS at EDD, at EDD + 200 d and at EDD + 700 d was best predicted by leptin output over the corresponding periods. CHL SDS at EDD and EDD + 200 d was best predicted by IGFBP-3 output from birth to EDD, while at EDD + 700 d it was IGFBP-3 output over the corresponding period. HC SDS at EDD and at EDD + 200 d was best predicted by leptin output from birth to EDD, while at EDD + 700 d it was leptin output over the corresponding period (Table 2).
DISCUSSION
Growth during the first two years.
In this study of extremely premature infants, poor growth in the immediate postnatal period was followed by catch-up in weight to –0.5 SDS by 6 mo and in length to –0.2 SDS by 2 y. Others have noted a deficit in weight compared with height even at 3 y corrected age in AGA preterm infants born at ≤30 wk GA (mean –0.34 versus 0.36 SDS) (17). The delayed catch-up in length could be considered an adaptive response to first restore fat mass and energy stores and to defer linear growth until a critical weight has been attained. We found lack of catch-up in HC throughout the 2 y study period and other observations at age 5 y indicate the lag in postnatal growth of the brain is prolonged (7). The implication of subnormal head size is neurodevelopmental deficit (18–20), and severe impairment has been reported in 38% of small-for-GA and 16% of AGA children born at GA <28 wk (7).
Hormone levels in the early postnatal period.
Although we did not have a control group of infants born at term, earlier studies have reported that IGF-I, IGFBP-3 and leptin levels at birth are lower in preterm infants compared with term infants and increase with GA (21–23). IGF-I sensitivity in utero is likely to vary according to insults which adversely influence growth and preterm birth (24), and may partly explain the wide individual variations in IGF-I levels we found from cord and day 1 samples and which others have observed in the fetus and in early life (6,25,26). The low leptin levels at birth in our extremely preterm infants and in other studies (27–29) were anticipated since it is synthesized and secreted exclusively by white adipose tissue, fat mass accumulates during the third trimester and levels in newborn infants correspond closely with fat stores (30,31). Ng (29) suggested the low leptin levels in extremely preterm infants may be a physiologic advantage as body energy utilization can be minimized and nutritional reserves conserved for subsequent growth and development.
While weight declined and linear growth was poor between birth and EDD, we observed an increase in leptin and IGFBP-3 levels but not in IGF-I. Findings from other studies vary, possibly due to demographic differences in populations, and time and frequency of blood sampling. While some reported an increase in leptin levels in the first few weeks in infants born <34 wk gestation and an inverse exponential relationship with postnatal weight gain by day 28 (32,33), others found leptin levels decreased and remained relatively low until critical adipose stores were reached and body mass index increased above 9 kg/m2 (11,12,29). Studies which used frequent blood sampling noted a decline in IGF-I and IGFBP-3 levels during the first week followed by a gradual rise concurrent with changes in clinical condition, nutrition and growth velocity in preterm infants (34–36).
Relationship between growth and hormone output during the first two years.
While a number of studies have described growth in extremely preterm infants and the potential influence of growth factors, observations are limited to the early postnatal period and thus cannot be compared with our findings. We found birth weight and length were associated with cord IGF-I and all growth measurements at birth were associated with IGFBP-3 levels on day 1. Postnatally, leptin output was the key correlate of weight and head circumference, and IGFBP-3 was the key correlate of length. It appears that when weight velocity normalizes, leptin levels stabilize rather than steadily increase, possibly as an adaptive response to sustain nutritional intake and allow for linear growth.
We found IGF-I output was higher over the 200-d-post-EDD than the interval up to EDD and then remained stable thereafter, but IGFBP-3 output increased steadily throughout the study period. The changes in IGF-I and IGFBP-3 output in our preterm infants correspond to circulating hormone levels seen in term AGA and intrauterine growth retarded infants (6,26). The levels of hormones in these preterm infants will be influenced on the one hand by nutrition and on the other by the sensitivity within the GH axis. A GH resistant state persisting outside the neonatal period is recognized when gestation is interrupted prematurely (10).
Thus the different time periods over the 1st 24 mo in very preterm infants could represent changes in the evolution of mechanisms that control the GH–IGF-I axis. The period between birth and EDD is the phase of GH insensitivity when levels of IGF-I, IGFBP-3 and leptin are low. Over this period, the stronger relationship between leptin and IGFBP-3 than between IGF-I and IGFBP-3 indicates that nutrition is the major initial driver for the generation of IGFBP-3. Over the 6 mo post-EDD, both IGFBP-3 and IGF-I correlate significantly with leptin, indicating that nutrition remains important, and that IGF-I has now become more sensitive to nutritional state. Thereafter between 6–12 mo post-EDD, the relationship between IGFBP-3 and IGF-I becomes stronger and could herald a shift to GH sensitivity. The period between 12 to 24 mo when IGF-I and IGFBP-3 output correlate strongly with each other but not with leptin could represent a phase when GH-dependence is now fully established. The importance of maintaining a pool of IGFBP-3 is reflected by the steady increase in output up to 24 mo post-EDD. However IGFBP-3 is the most significant factor correlating with linear growth, although IGF-I output does correlate in univariate analysis. As IGFBP-3 modulates IGF-I bioavailability and IGF-I output remains steady from EDD onwards, then this period may also be characterized by increasing tissue sensitivity to IGF-I, with rising IGFBP-3 levels acting to increase circulating pool of IGF-I. As there is evidence from animal models and cell lines that IGFBP-3 can inhibit growth independent of IGF-I, then we might expect rising IGFBP-3 levels to inhibit statural growth (37). However, in preterm infants IGFBP-3 appears to be predominantly associated with promoting growth.
Proteolysis of IGFBP-3 can give apparently high concentrations of IGFBP-3 because the assay identifies intact protein as well as fragments, and may also increase the bioavailability of IGF-I by reducing its affinity for IGFBP-3 (38). If this were to explain the pattern of change in IGFBP-3 in our preterm infants, increased proteolytic activity would be expected over the first 24 mo. The presence of circulating IGFBP-3 fragments indicative of proteolytic degradation has been identified in serum from healthy and growth retarded term infants at birth and at 2 mo (39) but has not been investigated in older infants or in preterm infants.
The consequences of suboptimal growth in infants born prematurely include abnormal body composition, short adult stature, poor neurodevelopmental outcome and life-long programming for cardiovascular disease in later life. Our study highlights the contribution of IGF-I, IGFBP-3 and leptin to the complex mechanisms which orchestrate growth in extremely preterm infants and the importance of optimizing circulating levels early. A rising IGFBP-3 level is a good marker of appropriate nutrition management and strategies to enhance it should improve catch-up growth in early life and reduce the risk of complications seen in adulthood.
Abbreviations
- AGA:
-
appropriate for gestational age
- AUC:
-
area under the curve
- CHL:
-
crown-to-heel length
- CV:
-
coefficient of variation
- EDD:
-
expected date of delivery
- HC:
-
head circumference
- IGFBP-3:
-
insulin-like growth factor binding protein-3
- LLL:
-
lower leg length
- SDS:
-
standard deviation scores
- Wt:
-
weight
References
Casey PH, Kraemer HC, Bernbaum J, Yogman MW, Sells JC 1991 Growth status and growth rates of a varied sample of low birth weight, preterm infants: a longitudinal cohort from birth to three years of age. J Pediatr 119: 599–605
Gibson AT, Carney S, Cavazzoni E, Wales J 2000 Neonatal and postnatal growth. Horm Res 53: 42–49
Barker DJ 1999 Fetal origins of cardiovascular disease. Ann Med 31: 3–6
Holt RI 2002 Fetal programming of the growth hormone-insulin-like growth factor axis. Trends Endocrinol Metab 13: 392–397
Kajantie E, Fall CH, Seppälä M, Koistinen R, Dunkel L, Ylihärsilä H, Osmond C, Andersson S, Barker DJ, Forsén T, Holt RI, Phillips DI, Eriksson J 2003 Serum insulin-like growth factor (IGF)-I and IGF-binding protein-1 in elderly people: relationships with cardiovascular risk factors, body composition, size at birth, and childhood growth. J Clin Endocrinol Metab 88: 1059–1065
Leger J, Noel M, Limal JM, Czernichow P 1996 Growth factors and intrauterine growth retardation. II. Serum growth hormone, insulin-like growth factor (IGF) I, and IGF-binding protein-3 levels in children with intrauterine growth retardation compared with normal control subjects: prospective study from birth to two years of age. Study Group of IUGR. Pediatr Res 40: 101–107
Bardin C, Piuze G, Papageorgiou A 2004 Outcome at 5 years of age of SGA and AGA infants born less than 28 weeks of gestation. Semin Perinatol 28: 288–294
Hack M, Schluchter M, Cartar L, Rahman M, Cuttler L, Borawski E 2003 Growth of very low birth weight infants to age 20 years. Pediatrics 112: E30–E38
Niklasson A, Engstrom E, Hard AL, Wikland KA, Hellstrom A 2003 Growth in very preterm children: a longitudinal study. Pediatr Res 54: 899–905
Engström E, Niklasson A, Albertsson Wikland K, Ewald U, Hellström A 2005 The role of maternal factors, postnatal nutrition, weight gain, and gender in regulation of serum IGF-I among preterm infants. Pediatr Res 57: 605–610
Ertl T, Funke S, Sarkany I, Szabo I, Rascher W, Blum WF, Sulyok E 1999 Postnatal changes of leptin levels in full-term and preterm neonates: their relation to intrauterine growth, gender and testosterone. Biol Neonate 75: 167–176
Spear ML, Hassink SG, Leef K, O'Connor DM, Kirwin SM, Locke R, Gorman R, Funanage VL 2001 Immaturity or starvation? Longitudinal study of leptin levels in premature infants. Biol Neonate 80: 35–40
Gibson AT, Pearse RG, Wales JK 1993 Knemometry and the assessment of growth in premature babies. Arch Dis Child 69: 498–504
Gill MS, Whatmore AJ, Tillmann V, White A, Addison GM, Price DA, Clayton PE 1997 Urinary IGF and IGF binding protein-3 in children with disordered growth. Clin Endocrinol (Oxf) 46: 483–492
Keen DV, Pearse RG 1988 Weight, length and head circumference curves for boys and girls of between 20 and 42 weeks' gestation. Arch Dis Child 63: 1170–1172
Freeman JV, Cole TJ, Chinn S, Jones PR, White EM, Preece MA 1995 Cross sectional stature and weight reference curves for the UK, 1990. Arch Dis Child 73: 17–24
Strauss RS, Dietz WH 1997 Effects of intrauterine growth retardation in premature infants on early childhood growth. J Pediatr 130: 95–102
Hack M, Breslau N, Fanaroff AA 1989 Differential effects of intrauterine and postnatal brain growth failure in infants of very low birth weight. Am J Dis Child 143: 63–68
Caravale B, Tozzi C, Albino G, Vicari S 2005 Cognitive development in low risk preterm infants at 3-4 years of life. Arch Dis Child Fetal Neonatal Ed 90: F474–F479
Sung IK, Vohr B, Oh W 1993 Growth and neurodevelopmental outcome of very low birthweight infants with intrauterine growth retardation: Comparison with control subjects matched by birthweight and gestational age. J Pediatr 123: 618–624
Giudice LC, de Zegher F, Gargosky SE, Dsupin BA, des Las Fuentes L, Crystal RA, Hintz RL, Rosenfeld RG 1995 Insulin-like growth factors and their binding proteins in the term and preterm human fetus and neonate with normal and extremes of intrauterine growth. J Clin Endocrinol Metab 80: 1548–1555
Lassarre C, Hardouin S, Daffos F, Forestier F, Frankenne F, Binoux M 1991 Serum insulin-like growth factors and insulin growth factor binding proteins in the human fetus. Relationships with growth in normal subjects and in subjects with intrauterine growth retardation. Pediatr Res 29: 219–225
Verhaeghe J, Van Herck E, Billen J, Moerman P, Van Assche FA, Giudice LC 2003 Regulation of insulin-like growth factor-I and insulin-like growth factor binding protein-1 concentrations in preterm fetuses. Am J Obstet Gynecol 188: 485–491
Jensen EC, Harding JE, Bauer MK, Gluckman PD 1999 Metabolic effects of IGF-I in the growth retarded fetal sheep. J Endocrinol 161: 485–494
Leger J, Noel M, Benali SB, Blot P, Czernichow P 1996 Growth factors and intrauterine growth retardation. I. Serum growth hormone, insulin-like growth factor (IGF)-I, IGF-II, and IGF binding protein 3 levels in normally grown and growth-retarded human fetuses during the second half of gestation. Pediatr Res 40: 94–100
Low LC, Tam SY, Kwan EY, Tsang AM, Karlberg J 2001 Onset of significant GH dependence of serum IGF-I and IGF-binding protein 3 concentrations in early life. Pediatr Res 50: 737–742
Gomez L, Carrascosa A, Yeste D, Potau N, Rique S, Ruiz-Cuevas P, Almar J 1999 Leptin values in placental cord blood of human newborns with normal intrauterine growth after 30–42 weeks of gestation. Horm Res 51: 10–14
Harigaya A, Nagashima K, Nako Y, Morikawa A 1997 Relationship between concentration of serum leptin and fetal growth. J Clin Endocrinol Metab 82: 3281–3284
Ng PC, Lam CW, Lee CH, Wong GW, Fok TF, Chan HI, Ma KC, Wong E 2000 Leptin and metabolic hormones in preterm newborns. Arch Dis Child Fetal Neonatal Ed 83: F198–F202
Symonds ME, Mostyn A, Pearce S, Budge H, Stephenson T 2003 Endocrine and nutritional regulation of fetal adipose tissue development. J Endocrinol 179: 293–299
Vatten LJ, Ødegård RA, Nilsen ST, Salvesen KÅ, Austgulen R 2002 Relationship of insulin-like growth factor-I and insulin-like growth factor binding proteins in umbilical cord plasma to preeclampsia and infant birth weight. Obstet Gynecol 99: 85–90
Shekhawat PS, Garland JS, Alex C, Sasidharan P, Mick G, McCormick KL 2000 Cord blood and postnatal serum leptin and its relationship to steroid use and growth in sick preterm infants. J Pediatr Endocrinol Metab 13: 1571–1576
Ong KK, Ahmed ML, Sherriff A, Woods KA, Watts A, Golding J, Dunger DB 1999 Cord blood leptin is associated with size at birth and predicts infancy weight gain in humans. J Clin Endocrinol Metab 84: 1145–1148
Kajantie E, Dunkel L, Rutanen EM, Seppälä M, Koistinen R, Sarnesto A, Andersson S 2002 IGF-I, IGF binding protein (IGFBP)-3, phosphoisoforms of IGFBP-1, and postnatal growth in very low birth weight infants. J Clin Endocrinol Metab 87: 2171–2179
Smith WJ, Underwood LE, Keyes L, Clemmons DR 1997 Use of insulin-like growth factor I (IGF-I) and IGF-binding protein measurements to monitor feeding of premature infants. J Clin Endocrinol Metab 82: 3982–3988
Rajaram S, Carlson SE, Koo WW, Rangachari A, Kelly DP 1995 Insulin-like growth factor (IGF)-I and IGF-binding protein 3 during the first year in term and preterm infants. Pediatr Res 37: 581–585
Rechler MM 1997 Editorial: Growth inhibition by insulin-like growth factor (IGF) binding protein-3–what's IGF got to do with it?. Endocrinology 138: 2645–2647
Diamandi A, Mistry J, Krishna RG, Khosravi J 2000 Immunoassay of insulin-like growth factor-binding protein-3 (IGFBP-3): new means to quantifying IGFBP-3 proteolysis. J Clin Endocrinol Metab 85: 2327–2333
Cianfarani S, Germani D, Rossi P, Rossi L, Germani A, Ossicini C, Zuppa A, Argiro G, Holly JM, Branca F 1998 Intrauterine growth retardation: evidence for the activation of the insulin-like growth factor (IGF)-related growth-promoting machinery and the presence of a cation-independent IGF binding protein-3 proteolytic activity by two months of life. Pediatr Res 44: 374–380
Author information
Authors and Affiliations
Corresponding author
Additional information
Supported by Jessop Baby Fund and Serono Pharmaceuticals (UK), Ltd.
Rights and permissions
About this article
Cite this article
Patel, L., Cavazzoni, E., Whatmore, A. et al. The Contributions of Plasma IGF-I, IGFBP-3 and Leptin to Growth in Extremely Premature Infants During the First Two Years. Pediatr Res 61, 99–104 (2007). https://doi.org/10.1203/01.pdr.0000250036.34522.f1
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1203/01.pdr.0000250036.34522.f1
This article is cited by
-
An exploratory study of clinical factors associated with IGF-1 and IGFBP-3 in preterm infants
Pediatric Research (2024)
-
Growth factors, adiponectin, leptin and body mass index in pre-pubertal children born large for gestational age
Journal of Endocrinological Investigation (2011)