Abstract
Lack of prolidase I (PD I) leads to prolidase deficiency, a disease characterized by intractable skin lesions, recurrent respiratory infections, and mental retardation. The present study was undertaken to characterize and determine the physiologic roles of different prolidase isoenzymes. Two isoforms of prolidase were isolated from rat kidney. PD I showed higher activity against seryl-proline and alanyl-proline, whereas PD II was active especially against methionyl-proline. PD I was highly concentrated in the small intestine and kidney, whereas PD II was shown not to vary in the organs examined. Expression of PD I and PD II in the small intestine were maximal within 1 wk of birth, and then rapidly declined. The changes of prolidase in the kidney and heart were found to differ slightly. N-benzyloxycarbonyl-l-proline and captopril inhibited PD I dose-dependently, but showed no inhibition of PD II at low concentrations. NiCl2 inhibited PD II much more effectively than PD I. Our findings suggest that PD I functions by way of an intestinal peptide carrier, which may also be regulated by the uptake of various iminodipeptides. Similarly, age-related alterations of prolidase isoenzymes suggest that intestinal PD II also participates in absorption of proline and other amino acids early in life.
Similar content being viewed by others
Main
Prolidase (E.C.3.4.13.9) is a manganese-requiring homodimeric iminodipeptidase, cleaving only iminodipeptides with carboxy-terminal proline or hydroxyproline. Its putative biologic roles include the deactivation of neuropeptides (1), the terminal degradation in the catabolic pathway of both exogenous and endogenous proteins, and providing large amounts of proline required for collagen synthesis. It also is thought to play an important role in proline nutrition and in the recycling of proline for protein synthesis and cell growth (2,3). Prolidase may also provide the substrate for proline oxidase, which generates reactive oxygen species during apoptosis (4,5).
Prolidase deficiency is a rare autosomal recessive disease characterized by chronic ulcerative dermatitis, mental retardation, frequent infections, and massive urinary excretion of iminodipeptides (6–8). The disease has been confirmed to be due to hereditary prolidase deficiency (9–11). It has been reported that the activity of the enzyme against Gly-Pro was almost totally missing in patients with prolidase deficiency, whereas the activity against other substrates remained close to normal (12,13).
Two forms of prolidase, PD I and PD II, were isolated from normal human erythrocytes, leukocytes, and cultured skin fibroblasts, and the characteristics of these enzymes have been investigated (14–16). We have also purified PD I and PD II from normal human erythrocytes, and found that the two isolated isoforms of prolidase differed in molecular mass (PD I, 56 kD, and PD II, 95 kD, in SDS-PAGE), response to manganese, substrate specificity, and heat stability (17,18). Moreover, prolidase activity is indeed present in patients with prolidase deficiency, and enzymatic properties of the patient's prolidase are essentially the same as those of PD II (19). Therefore, we attempted to give further insight into the understanding of the biosynthesis and physiologic roles of PD I and PD II isoenzymes.
In the present study, we investigated the characteristics of two isoforms of rat prolidase, including substrate specificity, organ distribution, developmental changes, and reaction to inhibitors. We also examined protein expression using polyclonal antibodies against human PD I and PD II.
METHODS
Chemicals.
Iminodipeptides including Gly-Pro, Ala-Pro, leucyl-proline (Leu-Pro), valyl-proline (Val-Pro), Met-Pro, Ser-Pro, prolyl-proline (Pro-Pro), and phenylalanyl-proline (Phe-Pro) were purchased from Bachem AG (Bubendorf, Switzerland); Hydroxyapatite Bio-Gel HTP gel and protein assay dye reagent concentrate were from Bio-Rad (Hercules, CA); horseradish peroxidase-linked anti-rabbit IgG and enhanced chemiluminescence (ECL) plus from Amersham Biosciences (Piscataway, NJ); and Cbz-Proline from Fluka Chemie AG (Buchs, Switzerland). All other reagents were of analytical grade and obtained from Wako Pure Chemical (Osaka, Japan).
Preparation of anti-PD I and anti-PD II antiserum.
Rabbit antiserum was raised against PD I and II protein purified from normal human erythrocytes, as described previously (18). Purification of the protein with molecular mass of about 56 kD (PD I) and 95 kD (PD II) was monitored by SDS-PAGE stained with Coomassie. The purified PD I and PD II (0.9–1 mg in 0.75 mL) was emulsified with an equal volume of Freund's adjuvant, and the emulsion was injected subcutaneously at multiple sites along the back of a New Zealand White rabbit (SLC, Japan) every 2 wk for four times total. The animal was bled 7 d after the final injection, and serum fraction was prepared. The antiserum was titrated by Western blotting.
Preparation of enzymes from rat tissues.
Adult male Wistar rats (260–300 g weight, Charles River Japan, Yokohama, Japan) were deeply anesthetized with diethyl ether and killed by cervical dislocation. Various organs were dissected, cut into small pieces, and then homogenized to 10 times the volume (v/w) in 50 mM ice-cold Tris/HCl (pH 7.4) containing 0.32 M sucrose with a Polytron homogenizer. The homogenates were centrifuged at 800 × g for 15 min and the resultant supernatant was used for experiments. Similarly, the organs of fetus (embryonic d 19) and postnatal rats aged 1, 2, 4, 7, 14, 28, 35, 42, and 56 d were obtained. For the experiments shown in Figure 5, homogenates of rat kidney were subjected to sequential centrifugation at 1,000 × g for 10 min, at 22,000 × g for 30 min, and at 105,000 × g for 60 min. The pellets obtained by each centrifugation were resuspended in 50 mM Tris/HCl (pH 7.4). Protein concentration was determined according to the method of Bradford (20) using BSA as a standard. The animal experimental study was approved by Animal Experimental Committee of Kochi Medical School and followed the guidelines of the ethical committee on the care and use of laboratory animals at our university.
Developmental changes of PD I and PD II in rat small intestine, kidney, and heart. Homogenates (40 μg of protein) of the small intestine (A), kidney (B), and heart (C) from rats indicated ages were allowed to react with 5 mM Gly-Pro and Met-Pro in the presence of 1 mM MnCl2. Activity[Gly-Pro] (black circle) and Activity[Met-Pro]-[Gly-Pro] (white circle) were regarded as the special activity of PD I and PD II, respectively. The results are expressed as the means ± SD (n = 3). (D) Homogenates from rat small intestine were analyzed by Western blot with anti-PD I and anti-PD II antiserum.
Separation of PD I and PD II from rat kidney.
Rat kidney was homogenized in 10 times the volume (v/w) of 50 mM ice-cold Tris/HCl (pH 7.4) containing 10 mM mercaptoethanol (buffer A). The sample (5 mg protein) was loaded on a DEAE-cellulose column (0.5 × 10 cm) and pre-equilibrated with buffer A. After washing the column with 30 mL buffer A, the enzyme was eluted with stepwise addition of Tris/HCl buffer (pH 7.4) from 150 to 250 mM. Prolidase activity against Gly-Pro and Met-Pro in each chromatographic fraction was determined.
Enzyme assay.
Prolidase activity was assayed using various iminodipeptides as substrate. The reaction mixture, containing 10 μL of enzyme solution, 80 μL of 50 mM Tris/HCl (pH 7.8), and 10 μL of 20 mM MnCl2, was preincubated at 37°C for 10 min and then incubated with 100 μL of 10 mM substrate (pH 7.8) at 37°C for 30 min. The reaction was stopped by adding 200 μL of 10% TCA. The amount of proline liberated was determined by spectrophotometry using Chinard's method (21).
Western blotting.
Each sample (10 μg protein for PD I, 1 μg protein for PD II) was subjected to SDS-PAGE on a 10% gel. After separation by SDS-PAGE, proteins were transferred electrically to a hydrophobic PVDF membrane (Hybond P). The transferred proteins were probed with anti-PD I (1:1,000 dilution) or anti-PD II (1:10,000 dilution) antiserum followed by the incubation with the horseradish peroxidase-labeled secondary antibody (1:2,000 dilution). Finally, PD I and PD II were detected using ECL plus. The PD I and PD II (0.1 μg protein) purified from human erythrocytes were used as positive controls.
RESULTS
Separation of the two isoforms of kidney prolidase.
We confirmed that two isoforms of prolidase, PD I and PD II, exist in the rat kidney as well as in other organs examined (data not shown). Prolidase was separated from rat kidney using ion-exchange chromatography (DEAE-cellulose). Two main peaks (PD I and PD II) isolated from rat kidney were eluted from the column using a stepwise Tris/HCl buffer gradient ranging from 150 to 250 mM, as shown in Figure 1A. Using Gly-Pro as a substrate, only active PD I was detected. However, both enzymes showed activity when Met-Pro was used as the substrate. Similar results were seen in the isolation of PD I and PD II from human erythrocytes (17,18). As shown in Figure 1B, the antibodies raised against human prolidase reacted with rat PD I and PD II and were substantially identical with respect to enzyme activity.
Separation of the two isoforms of kidney prolidase. (A) Homogenates of rat kidney (5 mg of protein) were applied to a DEAE-cellulose column. Fractions (1 mL) were eluted from the column with stepwise addition of Tris/HCl buffer (pH 7.4) from 150 to 250 mM. Prolidase activity (10 μL of each fraction) was determined with Gly-Pro (black circles) and Met-Pro (white circles), respectively. (B) Five microliters of each fraction was analyzed by western blot with anti-PD I and anti-PD II antiserum, respectively. Human PD I or PD II was used as a positive control (lane H).
Substrate specificity of PD I and PD II.
We next examined substrate specificity of the partially purified PD I and PD II with various iminodipeptides (5 mM final concentration) in the presence of 1 mM MnCl2 (Fig. 2, A and B). PD I showed higher activities against Ser-Pro and Ala-Pro (260% and 225%, relative activity to Gly-Pro), and showed almost the same activity against Gly-Pro, Met-Pro, Val-Pro, and Leu-Pro. It was different from human PD I, which showed the highest activity against Gly-Pro (18). On the other hand, PD II showed significantly higher activity against Met-Pro, low activities against the other substrates (<20% of the activity against Met-Pro), and the activity was barely detectable against Gly-Pro.
Substrate specificity of partially purified PD I (A) and PD II (B). Separated PD I and PD II were allowed to react with various substrates (5 mM) in the presence of 1 mM MnCl2. (A) The activity of PD I against Gly-Pro (15.3 nmol/min per mg of protein) was expressed as 100%. (B) The activity of PD II against Met-Pro (8.3 nmol/min per mg of protein) was expressed as 100%. The results are expressed as the means ± SD (n = 3).
Organ distribution of rat PD I and PD II.
We prepared homogenates from nine organs (heart, liver, small intestine, stomach, brain, spleen, kidney, lung, and pancreas) of adult rats. In Figures 1 and 2, we found that the activity of partially purified PD I against Gly-Pro and Met-Pro were almost same, and the activity of partially purified PD II against Gly-Pro was very low. Thus, we regarded total activities of homogenates against Gly-Pro (Activity[Gly-Pro]) as the special activity of PD I, and the difference of total activity of homogenates against Met-Pro and Gly-Pro (Activity[Met-Pro]-[Gly-Pro]) as the special activity of PD II. Both of the enzymes were detected in all of the organs tested. PD I activity in small intestine and kidney was 10–15-fold higher than in other organs, whereas PD II activity was not significantly different amongst the other organs (Fig. 3, A and B). Protein expression of PD I and PD II were detected by Western blotting with anti-PD I and anti-PD II antisera. The distribution of protein levels was not completely identical but similar to that of the enzyme activity (Fig. 3C). We also found that PD I was predominantly concentrated in renal cortex, where its activity was 2-fold higher than in renal medulla. PD II activity was similar throughout the kidney (Fig. 4, A and B).
Distribution of PD I and PD II in rat tissues. Homogenates of the indicated rat organs (20–50 μg of protein) were allowed to react with 5 mM Gly-Pro and Met-Pro, respectively, in the presence of 1 mM MnCl2. (A) Activity[Gly-Pro] (the total activity against Gly-Pro) was regarded as the special activity of PD I. (B) Activity[Met-Pro]-[Gly-Pro] (the deference of total activity against Met-Pro and Gly-Pro) was regarded as the special activity of PD II. Means ± SD are shown (n = 3). (C) The homogenates were analyzed by Western blot with anti-PD I and anti-PD II antiserum. Human PD I or PD II was used as a positive control (lane H).
Distribution of PD I (black columns) and PD II (white columns) in renal cortex and medulla. (A) Activity[Gly-Pro] and Activity[Met-Pro]-[Gly-Pro] were regarded as the special activity of PD I and PD II, respectively. Means ± SD are shown (n = 3). (B) The homogenates were analyzed by Western blot with anti-PD I and anti-PD II antiserum.
We also examined the total activity of homogenates against various substrates (Table 1) in the heart, liver, brain, small intestine, and kidney. We found that the total activity against Ser-Pro, Ala-Pro, and Met-Pro were higher than the other substrates in the detected tissue. Moreover, the total activities in the small intestine and kidney were markedly higher than in other organs.
Developmental changes of rat PD I and PD II.
The developmental changes of rat PD I and PD II were examined in various organs of fetal (19 d; E19) and 1–56 d-old rats. As shown in Figure 5A, a marked increase in PD I activity (Activity[Gly-Pro]) in the small intestine was observed from E19 to the neonatal period and remained steady during the first 7 d, then decreased between d 7 and d 28. After this period, the activity remained constantly low until d 56. A similar change was observed in PD II activity (Activity[Met-Pro]-[Gly-Pro]). Notably, the highest activity of PD I and PD II (d 2) was approximately five times higher than the lowest activity (E19). The changes of prolidase activity in kidney and heart were found to differ slightly. PD I activity in kidney increased from E19 to d 4 (2-fold), and remained at this level until d 56, but PD II activity did not markedly change over this time period (Fig. 5B). On the other hand, the activities of PD I and PD II in heart decreased from E19 to d 14 (2.5-fold decrease), and remained constant until d 56 (Fig. 5C). Western blot analysis revealed that protein expression of PD I and PD II in the small intestine changed with development, strongly correlating with the observed changes in activity (Fig. 5D).
Subcellular distribution of rat PD I and PD II.
Sequential centrifugation was performed to determine subcellular distribution of rat kidney PD I and PD II. After centrifugation, approximately 90% of the total activity of homogenates against Gly-Pro and Met-Pro remained in the 105,000 × g supernatant fluid (Fig. 6A). The distribution of PD I and PD II protein examined by Western blot analysis also showed a similar tendency (Fig. 6B). These results confirmed that PD I and PD II existed as a cytosolic protein in rat kidney.
Subcellular distribution of PD I and PD II in rat kidney. (A) Each fraction (25–40 μg of protein) obtained from rat kidney homogenates by sequential centrifugation (as described in Methods) was allowed to react with 5 mM Gly-Pro (black columns) and Met-Pro (white columns) in the presence of 1 mM MnCl2. Total prolidase activities are shown as means ± SD (n = 3). Lane 1, homogenate; lane 2, 1,000 × g pellet; lane 3, 22,000 × g pellet; lane 4, 105,000 × g pellet; lane 5, 105,000× g supernatant fluid. (B) The same samples (50 μg of protein) were analyzed by Western blot with anti-PD I and anti-PD II antiserum.
Effect of several inhibitors on PD I and PD II.
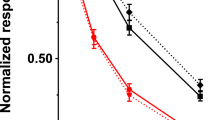
Several potential inhibitors of prolidase were tested to determine their effects on rat prolidase isoenzymes. We used partially purified PD I, PD II, and rat kidney homogenate as prolidase sources, and Gly-Pro and Met-Pro as substrates. The results indicated that Cbz-Proline and captopril inhibited PD I dose-dependently with IC50 values of 5 and 50 μM, respectively. However, up to 1000 μM Cbz-Proline and 100-μM captopril showed no effect on PD II (Fig. 7, A and B). On the other hand, NiCl2 markedly inhibited PD II (IC50 50 μM), while weakly inhibiting PD I (Fig. 7C). Furthermore, Cbz-Proline dose-dependently inhibited total prolidase activity in kidney homogenates against Gly-Pro and Met-Pro, with more efficient inhibition against Gly-Pro (Fig. 8A). We then examined the inhibitory effects of 0.5 mM Cbz-Proline, 0.5 mM captopril, and 1 mM NiCl2 on prolidase in kidney homogenate against various substrates. As shown in Figure 8B, the total activities of homogenates against various substrates were strongly inhibited, with the exception of Met-Pro (50% inhibition at 0.5 mM Cbz-Proline).
Inhibitory effects of Cbz-Proline, captopril, and NiCl2 on partially purified PD I and PD II. Separated PD I and PD II from rat kidney were allowed to react with 5 mM Gly-Pro (black circles) and Met-Pro (white circles), respectively, in the presence of 1 mM MnCl2 and the indicated concentrations of Cbz-Proline (A), captopril (B), and NiCl2 (C). The activity of separated PD I against Gly-Pro (14.5 nmol/min per mg of protein) and the activity of separated PD II against Met-Pro (8.7 nmol/min per mg of protein) in absence of the inhibitors was normalized to 100%. The results are expressed as the means ± SD (n = 3).
Inhibitory effects of Cbz-Proline, captopril, and NiCl2 on total prolidase activity from rat kidney homogenates. (A) Homogenates (40 μg of protein) from kidney was allowed react with 5 mM Gly-Pro (black circles) and Met-Pro (white circles), respectively, in the presence of 1 mM MnCl2 and the indicated concentrations of Cbz-Proline. The total activities of prolidase against Gly-Pro and Met-Pro (2.06 and 3.38 nmol/min per mg of protein) in absence of the inhibitors were normalized to 100%. (B) Homogenates (40 μg of protein) from kidney was allowed to react with 5 mM of various substrates, in the presence of 1 mM MnCl2. Cbz-Proline at 0.5 mM (white columns), captopril at 0.5 mM (striped columns), NiCl2 at 1 mM (shaded columns) or no inhibitors (black columns) was included in the reaction mixture. The results are expressed as the means ± SD (n = 3).
DISCUSSION
Prolidase is an important enzyme for protein metabolism, endogenous amino acid recycling, and proline-containing prodrug activation. Prolidase deficiency is caused by deficiency of PD I, which is composed of 492 amino acid residues with high homology between human and rat (86%). The gene is located on the short arm of chromosome 19 (22,23). Prolidase activity is present in patients with prolidase deficiency and enzymatic properties of the patient's prolidase are essentially the same as those of PD II (19). Therefore, this study was undertaken to characterize and determine physiologic roles of different prolidase isoenzymes and to further investigate the molecular basis of prolidase deficiency.
PD I showed high activity against various iminodipeptides and was concentrated in the small intestine and kidney. Total prolidase activity from the small intestine and kidney were markedly higher than in other organs examined. It suggested that the principal function of PD I is the absorption and reabsorption of proline and other amino acids. The distribution pattern and developmental expression of PD I are consistent with the expression pattern of PEPT 1, which is a peptide transporter existing in intestine and kidney as well as mediates the absorption of small peptides (24). Hu et al. (23) also found that the distribution pattern of PD I activity in the small intestine correlates with peptide absorption. In another study, the uptake of Gly-Pro gradually increased in the small intestine with a striking perinatal peak, and then declined continually after the first 6 d of postnatal life. Moreover, the preferential uptake of small peptides over their free amino acids was also found in newborns and infants (25). In the present study, the activities and protein levels of intestinal PD I showed a similar pattern in age-dependent expression, suggesting the possibility that PD I functions by way of an intestinal peptide carrier, which may also be regulated by the uptake of various iminodipeptides.
PD II showed a wide distribution and significantly higher activity against Met-Pro. Compared with the small intestine and kidney, total prolidase activities in various tissues tested were low, but more pronounced against Met-Pro. These results suggested that PD II is responsible for providing high cleaving activity against Met-Pro in variety of tissues rather than for absorption and reabsorption of proline and other amino acids. Interestingly, significant activity and protein expression of intestinal PD II were observed at postnatal d 1–7, followed by a rapid decrease to fetal levels at d 21, about the time of weaning. This suggested that PD II participates in the absorption of proline and other amino acids early in life. This age-related alteration of PD II may be particularly advantageous in newborns because of high requirement of free amino acids for growth and metabolic function during this period.
Lack of PD I leads to abnormalities in absorbing proline arising from digestion of dietary proteins in the small intestine, as well as in reabsorbing proline generated from endogenous protein in the kidney. The latter results in massive urinary excretion of iminodipeptides, a typical symptom of prolidase deficiency. We considered the significant hindrance in intestinal absorption of proline during the neonatal period, is an important contributor to the pathogenesis of prolidase deficiency. Proline is found as an essential dietary amino acid for neonatal pigs, but not for post weaning pigs, because arginine- and glutamine-dependent proline synthesis are not detectable in enterocytes of newborn or sucking pigs (26). Wu et al. (27) also demonstrated the presence of high activity of mitochondrial proline oxidase in the small intestine, and that the intestinal mucosa plays a major role in initiating proline degradation in the body. Thus, the decrease of intestinal absorption of proline decreased directly the amount of proline available to extraintestinal tissues in the first pass by portal-drained viscera. Only weak prolidase activity exists in extraintestinal and extrarenal tissues, which do not have the ability to provide essential proline. In addition, relatively large amounts of arginine and its immediate precursors (ornithine, citrulline) are synthesized from proline by pig enterocytes in a concentration-dependent manner, and account for 80–90% of metabolized proline carbons (27). Wu (27) also found that proline oxidase activity and rates of synthesis of citrulline and arginine from proline are high in enterocytes of newborn pigs and markedly decreased in 7-d-old pigs. Arginine is a nutritionally essential amino acid for neonates and remarkably deficient in the milk of many species including humans, pigs, and rats (28). Therefore, the endogenous synthesis of arginine form proline plays a crucial role in maintaining arginine homeostasis in neonates. The decrease of intestinal absorption of proline might indirectly decrease the amount of endogenous synthesis of arginine for neonates. Arginine deficiency can cause growth retardation, impaired immune and neurologic development, which are also typical symptoms of prolidase deficiency. Thus, we propose that dietary supplementation of free proline plus arginine for neonates; it might be helpful for treating patients with prolidase deficiency.
Cbz-Proline, captopril and Ni2+ inhibition of prolidase were described in previous studies (29–31), but as these studies used Gly-Pro as substrate, it would seem that the inhibitory effect of these compounds would contribute to PD I function only. In the present study, we found that these three compounds inhibited PD I in agreement with the above papers, but Cbz-Proline and captopril showed no effect on PD II at low concentrations and NiCl2 inhibited PD II more strongly than PD I. Cbz-Proline, which strongly inhibited PD I (200 μM, 90% inhibition), but showed no effect on PD II, might be useful in developing an experimental animal model for prolidase deficiency.
In summary, our studies suggest that the principal function of PD I is absorption and reabsorption of proline and other amino acids. PD I functions by way of an intestinal peptide carrier, which may also be regulated by the uptake of various iminodipeptide. The main function of PD II is to provide high cleaving activity against Met-Pro in various tissues. Similarly, age-related alterations of prolidase isoenzymes suggest that intestinal PD II also participates in absorption of proline and other amino acids in early life.
Abbreviations
- Ala-Pro:
-
alanyl-proline
- Cbz-Proline:
-
N-benzyloxycarbonyl-l-proline
- Gly-Pro:
-
glycyl-proline
- Met-Pro:
-
methionyl-proline
- PD I:
-
prolidase I
- PD II:
-
prolidase II
- PEPT 1:
-
intestinal oligopeptide transporter
- Ser-Pro:
-
seryl-proline
References
Hui KS, Lajtha A 1980 Activation and inhibition of cerebral prolidase. J Neurochem 35: 489–494
Jackson SH, Heininger JA 1975 Proline recycling during collagen metabolism as determined by concurrent 18O2-and 3H-labeling. Biochim Biophys Acta 381: 359–367
Myara I, Charpentier C, Lemonnier A 1984 Prolidase and prolidase deficiency. Life Sci 34: 1985–1998
Donald SP, Sun XY, Hu CA, Yu J, Mei JM, Valle D, Phang JM 2001 Proline oxidase, encoded by p53-induced gene-6, catalyzes the generation of proline-dependent reactive oxygen species. Cancer Res 61: 1810–1815
Maxwell SA, Rivera A 2003 Proline oxidase induces apoptosis in tumor cells, and its expression is frequently absent or reduced in renal carcinomas. J Biol Chem 278: 9784–9789
Powell GF, Rasco MA, Maniscalco RM 1974 A prolidase deficiency in man with iminopeptiduria. Metabolism 23: 505–513
Kodama H, Umemura S, Shimomura M, Mizuhara S, Arata J, Yamamoto Y, Yasutake A, Izumiya N 1976 Studies on a patient with iminopeptiduria. I. Identification of urinary iminopeptides. Physiol Chem Phys 8: 463–473
Der Kaloustian VM, Freij BJ, Kurban AK 1982 Prolidase deficiency: an inborn error of metabolism with major dermatological manifestations. Dermatologica 164: 293–304
Jackson SH, Dennis AW, Greenberg M 1975 Iminodipeptiduria: a genetic defect in recycling collagen; a method for determining prolidase in erythrocytes. Can Med Assoc J 113: 759–763
Powell GF, Kurosky A, Maniscalco RM 1977 Prolidase deficiency: report of a second case with quantitation of the excessively excreted amino acids. J Pediatr 91: 242–246
Sheffield LJ, Schlesinger P, Faull K, Halpern BJ, Schier GM, Cotton RG, Hammond J, Danks DM 1977 Iminopeptiduria, skin ulcerations, and edema in a boy with prolidase deficiency. J Pediatr 91: 578–583
Butterworth J, Priestman D 1984 Substrate specificity of manganese-activated prolidase in control and prolidase-deficient cultured skin fibroblasts. J Inherit Metab Dis 7: 32–34
Priestman DA, Butterworth J 1984 Prolidase deficiency: characteristics of human skin fibroblast prolidase using colorimetric and fluorimetric assays. Clin Chim Acta 142: 263–271
Kodama H, Ohhashi T, Ohba C, Ohno T, Arata J, Kubonishi I, Miyoshi I 1989 Characteristics and partial purification of prolidase and prolinase from leukocytes of a normal human and a patient with prolidase deficiency. Clin Chim Acta 180: 65–72
Myara I 1987 Effect of long preincubation on the two forms of human erythrocyte prolidase. Clin Chim Acta 170: 263–270
Myara I, Cosson C, Moatti N, Lemonnier A 1994 Human kidney prolidase-purification, preincubation properties and immunological reactivity. Int J Biochem 26: 207–214
Nakayama K, Awata S, Zhang J, Kaba H, Manabe M, Kodama H 2003 Characteristics of prolidase from the erythrocytes of normal humans and patients with prolidase deficiency and their mother. Clin Chem Lab Med 41: 1323–1328
Ohhashi T, Ohno T, Arata J, Sugahara K, Kodama H 1990 Characterization of prolidase I and II from erythrocytes of a control, a patient with prolidase deficiency and her mother. Clin Chim Acta 187: 1–10
Liu G, Nakayama K, Sagara Y, Awata S, Yamashita K, Manabe M, Kodama H 2005 Characterization of prolidase activity in erythrocytes from a patient with prolidase deficiency: comparison with prolidase I and II purified from normal human erythrocytes. Clin Biochem 38: 625–631
Bradford MM 1976 A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 72: 248–254
Chinard FP 1952 Photometric estimation of proline and ornithine. J Biol Chem 199: 91–95
Endo F, Tanoue A, Nakai H, Hata A, Indo Y, Titani K, Matsuda I 1989 Primary structure and gene localization of human prolidase. J Biol Chem 264: 4476–4481
Hu M, Cheng Z, Zheng L 2003 Functional and molecular characterization of rat intestinal prolidase. Pediatr Res 53: 905–914
Shen H, Smith DE, Brosius FC 3rd 2001 Developmental expression of PEPT1 and PEPT2 in rat small intestine, colon, and kidney. Pediatr Res 49: 789–795
Guandalini S, Rubino A 1982 Development of dipeptide transport in the intestinal mucosa of rabbits. Pediatr Res 16: 99–103
Wu G, Morris SM Jr 1998 Arginine metabolism: nitric oxide and beyond. Biochem J 336: 1–17
Wu G 1997 Synthesis of citrulline and arginine from proline in enterocytes of postnatal pigs. Am J Physiol 272: G1382–G1390
Davis TA, Nguyen HV, Garcia-Bravo R, Fiorotto ML, Jackson EM, Lewis DS, Lee DR, Reeds PJ 1994 Amino acid composition of human milk is not unique. J Nutr 124: 1126–1132
Lupi A, Rossi A, Vaghi P, Gallanti A, Cetta G, Forlino A 2005 N-benzyloxycarbonyl-L-proline: an in vitro and in vivo inhibitor of prolidase. Biochim Biophys Acta 1744: 157–163
Ganapathy V, Pashley SJ, Roesel RA, Pashley DH, Leibach FH 1985 Inhibition of rat and human prolidases by captopril. Biochem Pharmacol 34: 1287–1291
Miltyk W, Surazynski A, Kasprzak KS, Fivash MJ Jr, Buzard GS, Phang JM 2005 Inhibition of prolidase activity by nickel causes decreased growth of proline auxotrophic CHO cells. J Cell Biochem 94: 1210–1217
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Liu, G., Nakayama, K., Awata, S. et al. Prolidase Isoenzymes in the Rat: Their Organ Distribution, Developmental Change and Specific Inhibitors. Pediatr Res 62, 54–59 (2007). https://doi.org/10.1203/PDR.0b013e3180676d05
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1203/PDR.0b013e3180676d05
This article is cited by
-
The Prolidase Activity, Oxidative Stress, and Nitric Oxide Levels of Bladder Tissues with or Without Tumor in Patients with Bladder Cancer
The Journal of Membrane Biology (2017)
-
Serum prolidase activity is associated with non-diabetic metabolic syndrome
Diabetology & Metabolic Syndrome (2014)
-
Serum prolidase activity, oxidative stress, and nitric oxide levels in patients with bladder cancer
Journal of Cancer Research and Clinical Oncology (2012)