Abstract
Cardiac contractility depends on calcium sensitivity of the myofilaments and cytosolic free calcium concentration ([Ca2+]i) during activation. During development, the cardiac troponin T isoform cTnT1 is replaced by shorter cTnT isoforms, including cTnT4, and changes occur in other myofibrillar proteins and in calcium regulation. We expressed rabbit recombinant (r)cTnT1 and rcTnT4 in Spodoptera frugiperda cells and determined their effect on calcium binding to TnC in solution and on the calcium sensitivity of myofilaments in skinned rabbit ventricular fibers in vitro. We measured [Ca2+]i and L-type calcium current (ICa) in ventricular myocytes from 3-wk-old and adult rabbits. The dissociation constant (Kd) of Ca-TncTnT1 in solution was smaller than that of Ca-TncTnT4 (mean ± SE: 0.52 ± 0.08 μmol/L versus 0.83 ± 0.09 μmol/L). The Ca2+ sensitivity of force development was greater in fibers reconstituted with rcTnT1 (pCa50 6.07 ± 0.04) than those reconstituted with rcTnT4 (pCa50 5.75 ± 0.07). Systolic [Ca]i was lower in 3-wk-old than adult cells (443 ± 35 nmol/L versus 882 ± 88 nmol/L) as was ICa (5.8 ± 0.9 pA/pF versus 14.2 ± 1.6 pA/pF). The higher calcium sensitivity of Tn-Ca binding and of force development conferred by rcTnT1 suggest that higher neonatal cTnT1 expression may partially compensate for the lower systolic [Ca2+]i.
Similar content being viewed by others
Main
Myocardial contractility increases with development. Adult myocardium develops more force than fetal myocardium (1). Adult cardiac myocytes shorten further and faster than immature myocytes (2). Potential contributors to these differences include developmental increases in calcium ([Ca2+]i) transients and calcium sensitivity of myofilaments (1,3). Calcium transients increase postnatally in the rabbit (4). The calcium sensitivity of myofilaments, however, decreases with development in the rat, chick, and rabbit (5–7); this fall could result from a developmental switch in isoform expression of cTnT, a thin filament protein essential for Ca2+-regulated force development (8). Expression of cTnT1 and cTnT2 falls and that of cTnT3 and cTnT4 increases with development in the rabbit.
We expressed recombinant rabbit (r)cTnT1 and rcTnT4 in Sf9 cells and compared their effects on Ca2+-binding of isolated troponin in solution and in skinned muscle fibers in vitro. Further, we compared calcium transients and L-type calcium currents (ICa) in cardiac myocytes from 3-wk-old rabbits expressing cTnT1 and from adults that express predominantly cTnT4 and no cTnT1.
We found that (a) in solution, Tn comprising cTnT1 (TncTnT1) has higher calcium affinity than Tn comprising cTnT4 (TncTnT4), (b) fibers reconstituted with cTnT1 were more sensitive to calcium than those reconstituted with cTnT4, and (c) peak [Ca2+]i and ICa were significantly greater in adult than in 3-wk-old myocytes. Our data provide no evidence that incorporation of Tn into the myofilaments alters Tn-calcium binding affinity.
By imparting to the myofilaments a higher calcium sensitivity, the higher expression of cTnT1 and cTnT2 in immature myocytes would compensate partially for their smaller [Ca2+]i transients.
MATERIALS AND METHODS
Animals used in this study were handled according to the animal welfare regulations of Duke University (Durham, NC). The protocol was approved by the Institutional Animal Care and Use Committee. The rabbits were anesthetized (100 mg/kg ketamine and 10 mg/kg xylazine i.p.), and the heart was removed to obtain ventricular muscle fibers or isolated myocytes.
Preparation of cTnT.
cTnT isoforms were expressed in Sf9 cells using the baculovirus system (Invitrogen). The cDNA of rabbit cTnT1 (9) was cloned at the EcoR I restriction site into pVL1392 by releasing the cDNA from pBSII KS(+) with HindIII and KpnI and cloning it into pSP72 (Promega) where a second XbaI site was added. The cTnT1 cDNA was then excised with XbaI and cloned into pVL1392. Orientation of inserts and sequences encoding cTnT1 and cTnT4 were verified by polymerase chain reaction and DNA sequencing. SF9 cells were transfected with pVL1392-containing cTnT1 cDNA or pVL1393-containing cTnT4 cDNA and infected with wild-type AcMPEV (Invitrogen). Clones were identified using monoclonal antibody (MAb) 13-11, a cTnT-specific MAb, and Western blots (10).
Sf9 cells (3–4 ×106 cells/mL) were infected with cTnT1 or cTnT4 recombinant baculovirus. cTnT was purified according to Potter (11), and frozen at −70°C in 6 mol/L urea.
Circular dichroism.
CD spectra for cTnT1 and cTnT4 were obtained at 25°C (0.5-nm steps with 1-nm bandwidth) in a buffer containing 0.5 mol/L KCl and 10 mmol/L potassium phosphate, and 3 mg/mL protein, pH 7.2.
Preparation of cTnC and cTnI.
Human cTnC was expressed in BL21 (DE3) Escherichia coli using an expression vector provided by Merck and Co. Inc. and purified (12). Recombinant mouse cTnI was expressed using a pET-3d vector and purified using established methods (13). Stock protein solution concentrations were measured using quantitative amino acid analysis.
2-[4′-(Iodoacetamido)aniline]-naphthalene-6-sulfonate (IAANS) labeling of cTnC.
Purified cTnC was dissolved in buffer A (0.2 mol/L KCl, 30 mmol/L 3-(N-morpholino)propane-sulfonate (MOPS), pH 7.0) to a final concentration of 50 μmol/L, and dialyzed serially against buffer A containing 1 mmol/L DTT and buffer A containing 6 mol/L urea without DTT (1,4-Dithiothreitol). cTnC was labeled using a 15-fold molar excess IAANS (Molecular Probes, Eugene, OR). The labeling stoichiometry was determined by 326-nm UV absorbance (IAANS molar extinction coefficient 26,000). Absorbance of cTnC at 280 nm was corrected for fluorophore absorbance (14). Labeling reactions yielded 2 mol bound IAANS/mol cTnC.
Preparation of soluble troponin complexes.
Binary and ternary troponin complexes were formed at 20°C in titration buffer (in mmol/L: KCl 400, ethyleneglycol-bis-(β-aminoethylether)-N,N,N′,N′ (EGTA) 1, MOPS 25, DTT 1.5, pH 7.2). Molar ratios of IAANS-cTnC:cTnI:cTnT were 1:2:2 to avoid dimer formation. Complexes of IAANS-cTnC:cTnI, IAANS-cTnC:cTnT1, and IAANS-cTnC:cTnT4 were prepared similarly.
Calculation of [Ca2+]free.
[Ca2+]free was calculated using CATCONC (15) software and the logarithmic association constants: H+:EGTA4− 9.47, H+:HEGTA3− 8.85, H+:H2EGTA2− 2.66, H+:H3EGTA− 2.0; Ca2+:EGTA4− 10.97, Ca2+:HEGTA3− 5.29, Mg2+:EGTA4− 5.21, Mg2+:HEGTA3− 3.43.
Ca2+-troponin affinity in solution.
Fluorescence measurements were made using a SLM 8100 spectrofluorometer (SLM/Aminco). Titration buffer (mmol/L): KCl 0.4, EGTA 1, and MOPS 25, pH 7.2, 20°C. Fluorescence emission spectra were obtained using 335-nm excitation (excitation and emission bandwidths: 4 and 32 nm, respectively). Uncorrected fluorescence intensities between 400 and 500 nm were integrated and the fraction of Ca2+-bound IAANS-cTnC (Fb) calculated: MATH

where Iobsv is the integrated fluorescence intensity observed after each addition of Ca2+, IEGTA is the integrated fluorescence in the absence of Ca2+, and Isat is the fluorescence under saturating Ca2+). Data were fit to a form of the Hill equation:

where nH is the Hill coefficient and Kd is the dissociation constant).
Force-pCa relationship.
Right ventricular muscle fibers from adult rabbit hearts (16) were skinned (0.5% Triton X-100) in relaxing solution. Compositions of relaxing (pCa 9) and pCa 4.5 solutions (6) and their preparation were as described previously (17), except pMg was 3.0, and all solutions contained 0.1 mg/L pepstatin A. Apparent Ca-EGTA stability constant used was 3.702 × 106 L/mol.
Fibers were mounted on a force transducer (Güth OP1M (18)) at 22.0°C and sarcomere length of 2.3 μm (determined by light diffraction). Active force was determined versus pCa.
Dialysis of rcTnT.
rcTnT was dialyzed at 4°C for 24 h, first against 2 mol/L urea and 1 mol/L KCl, then sequentially against 1, 0.8, 0.6, and 0.4 mol/L KCl, all containing 20 mmol/L MOPS, 1 mmol/L DTT, and 0.1 mg/L pepstatin A, pH 7.0.
Solutions for skinned preparation experiments.
The washing solution used (in mmol/L) was KCl 100, MgCl2 5, MOPS 20, DTT 0.5, pepstatin A 0.1, pH 7.0. The extraction solution was KCl 250, EGTA 5, MgCl2 5, MOPS 20, DTT 0.5, pepstatin A 0.1 (pH 6.5), and 1–3 mg/mL rcTnT1 or rcTnT4. The reconstitution solution was 2.5 mg/mL cTnI-cTnC complex added to either (in mmol/L) KCl 100, EGTA 5, MgCl2 5, DTT 0.5, pepstatin A 0.1 mg/L, MOPS 20, or imidazole 120, KCl 29, MgCl2 16, EGTA 7, ATP 5, phosphocreatine 16 (pH 7).
Extraction-reconstitution assay.
We superfused the preparation in washing solution (10 min), then in extraction solution (70–90 min) to remove troponin (19). Loss of Ca2+-regulated force was verified using pCa 4.75. The fiber was then washed (10 min) and placed in reconstitution solution 75 min). In some preparations, extraction-replacement was performed in one step: preparations were exposed for 100 min to extraction solution containing TncTnT1 or TncTnT4 complex. The force-pCa relationship was measured, the fibers placed in sample buffer, and their proteins resolved using sodium dodecyl sulfate–polyacrylamide gel electrophoresis (SDS-PAGE) and silver stained. Western blots were probed with MAb 13-11 (10,20).
Cell isolation.
Myocytes were isolated following the procedure of Mitra and Morad (21). The digestion solution for 3-wk-old animals contained 0.5% collagenase (Worthington type II) and 0.006% protease (Sigma Chemical Co., type XIV) in 50 mL of physiologic solution (no added calcium); for adult animals, we used 1% collagenase and 0.006% protease.
Electrophysiology and calcium measurement.
Cells were studied under a Zeiss IM microscope using a Nikon 40x, 0.85-N.A. Fluor objective. Electrophysiologic data were acquired using Axopatch 200A, Digidata 1200, and pClamp 6.0 (Axon Instruments) (in early experiments, we used a Dagan Corporation Model 8900). FluoroPlex-III system (Tracor Northern) was used for fluorescence measurements (excitation wavelengths 350 and 380 nm, chopper rate, 60 s−1). Ventricular myocytes were whole-cell clamped (22) at room temperature (pipette resistance 1.8–2.5 MΩ). ICa was acquired at 10 or 20 kHz (2-kHz, four-pole, low-pass Bessel filter). Capacitance and 70–80% series-resistance compensation were applied. Holding potential (VH) was −80 mV; five conditioning 400-ms pulses to +10 mV at 4-s intervals standardized sarcoplasmic reticulum calcium load. VH from the end of the last conditioning pulse to the 400-ms test pulse was −40 mV (to inactivate sodium and T-type calcium channels). Resting potential (Em) was recorded immediately upon patch rupture and [Ca2+]i and ICa 5–10 min post-rupture.
Solutions for cardiac myocyte experiments.
The extracellular solution (in mmol/L) was NaCl 141.8, KCl 5.1, MgCl2 1.0, dextrose 12, MOPS (acid) 3.75, MOPS (Na salt) 6.25, and CaCl2 2.0, pH 7.4 and the pipette solution was KCl 143, Na2ATP 5.1, MgCl2 5.04, MOPS (acid) 10, EGTA 0.002, and K5fura-2 0.05 or 0.1, pH 7.2.
Calibration of fura-2 fluorescence.
We carried out in-cell calibration at room temperature as described by Li et al. (23).
Data analysis.
[Ca2+]i was calculated according to Grynkiewics et al. (24) using Ca-fura-2 Kd of 200 μmol/L. Background was subtracted before calculating fluorescence ratio. ICa was measured as negative peak minus steady-state current at 300 ms; this was equal, both in adult and 3-wk-old animals, to the (200 μmol/L) Cd2+-sensitive current. Cell capacitance was calculated from the time constant of response to small (±5 mV) test pulses from Vh = −80 mV sampled at 250 kHz (10-kHz filter) before compensation.
We used the notation pCa50 = −log10[Ca2+] for the pCa that elicited half-maximal tension, and pKd = −log10Kd for protein solutions.
Statistics.
Means or fitted values ± their standard error (SE) are given unless otherwise stated. We used an unpaired t test or t test for unequal variance. The paired t test was used to assess the difference in binding of Ca2+ to IAANS-TncTnT1 and IAANS-TncTnT4. ICa was corrected for cell capacitance by linear regression; ICa density was based on Cm of 80 pF. Statistical analysis was carried out using R (http://www.R-project.org)
RESULTS
Characterization of recombinant protein.
The purity and integrity of rcTnT1 and rcTnT4 were verified by comparison to native cTnT using SDS-PAGE and Western blots (Fig. 1A), and CD spectra, which exhibited two minima at 208 and 222 nm and were like the spectrum of native cTnT (25).
Recombinant and native cardiac troponin T. A, Silver stains comparing electrophoretic mobilities of purified recombinant (r)cTnT1 (a) and rcTnT4 (b). Western blots, probed with MAb 13-11, which recognizes an epitope specific to cTnT, compare electrophoretic mobilities of purified rcTnT1 (d) and rcTnT4 (e) to the four native isoforms (cTnT1–4) from 2-d-old rabbit myocardium (c). cTnT isoform expression in neonatal heart (c) differs from that of adult rabbit heart (C) in which cTnT1 is absent and TnT4 is the major isoform. B, Proteins from a control (I) and an experimental (II) fiber (8.5% acrylamide gel). Endogenous cTnT isoforms in II were replaced with rcTnT1. The third lane contains rcTnT1. C, Western blots (probed with MAb 13-11) of a control adult fiber (a) and two experimental fibers (b, c), reconstituted, with rcTnT1 (b) and rcTnT4 (c). In the experimental fibers, only rcTnT1 (b) and rcTnT4 (c) are evident.
Ca2+ concentration effects on fluorescence of IAANS-cTnC, IAANS-cTnC:cTnI, IAANS-cTnC:cTnT, and IAANS-cTnC:cTnI:cTnT containing rcTnT1 or rcTnT4.
We examined the effects of binding of Mg2+ or Ca2+ to cTnC high-affinity sites III and IV and of Ca2+ to the low-affinity site II on IAANS-cTnC fluorescence. In the absence of Mg2+, IAANS-cTnC fluorescence intensity decreased slightly between pCa 8.5 and 6.0, then increased as pCa was further reduced to 3.2, the increase being attributable to Ca2+ binding to site II (Fig. 2A). The apparent Kd of free IAANS-cTnC:Ca2+ was 0.104 μmol/L for the high-affinity sites and 9.7 μmol/L for the low-affinity site (data not shown).
The IAANS-cTnC:cTnI complex was titrated with Ca2+ in the presence of 4 mmol/L Mg2+ to saturate both cTnC high-affinity sites III and IV (Fig. 2B). Like Putkey et al. (26), we found a minimal decrease in fluorescence with increasing [Ca2+]. Fluorescence of IAANS-cTnC:cTnT1 and IAANS-cTnC:cTnT4 complexes did not change with [Ca2+] (data not shown).
The fluorescence intensity of IAANS-TncTnT1 and IAANS-TncTnT4 decreased with [Ca2+]: Kd for IAANS-TncTnT1:Ca2+ was 0.52 ± 0.08 μmol/L and for IAANS-TncTnT4:Ca2+ 0.83 ± 0.09 μmol/L (p = 0.001; paired t test, five determinations); nH were 1.0 ± 0.1 and 1.3 ± 0.2, respectively (Fig. 3).
Extraction-reconstitution experiments.
Mean pCa50 of the fibers with endogenous cTnT was 5.99 ± 0.07 and nH was 2.3 ± 0.5 (n = 10). SDS-PAGE (Fig. 1B) and Western blots (Fig. 1 C) demonstrated replacement of endogenous cTnT by rcTnT1 or rcTnT4. pCa50 postreconstitution was 6.07 ± 0.04 (n = 6) for rcTnT1, and 5.75 ± 0.07 (n = 4) for rcTnT4, p = 0.003 (Fig. 4) and nH was 2.4 ± 0.4 and 1.9 ± 0.1, respectively (p = 0.34).
Force vs pCa. Normalized force vs pCa data for fibers in which the endogenous cTnT was replaced with either rcTnT1 (+, solid curve) or rcTnT4 (○, dashed curve) illustrates the greater calcium sensitivity of force development in fibers containing rcTnT1. The curves correspond to mean values for pCa50 and nH.
Comparison of Tn-Ca binding in solution and in myofilaments.
To assess whether incorporation of troponin into the myofilaments changed troponin:calcium affinity, we compared the differences (Δ) in Gibbs free energy (ΔG): ΔΔGsolution = ΔGTnT1 − ΔGTnT4 = 2.303RT·(pKd [TnT1] − pKd [TnT4]) for Tn in solution, and ΔΔGmuscle = 2.303RT·(pCa50[TnT1] − pCa50[TnT4]) in the myofilaments (R, universal gas constant; T, absolute temperature). This assumes that at pCa50, calcium binding sites of myofilament-bound cTnC are half-occupied. The two quantities were not significantly different: ΔΔGsolution was 0.29 ± 0.07 (95% confidence interval: 0.13–0.46) kcal/mol, and ΔΔGmuscle was 0.42 ± 0.10 (0.18–0.66) kcal/mol (Fig. 5).
Ventricular myocytes.
Body weights of 11 adult and 16 3-wk-old rabbits from which myocytes were obtained were 2840 (standard deviation [SD] = 300) g and 300 (SD = 100) g. Cells from 13 animals yielded [Ca]i and electrophysiologic data; cells from 13 animals yielded [Ca]i data.
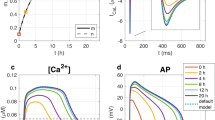
Peak [Ca2+]i in adult myocytes was twice that in 3-wk-old myocytes (Table 1, Fig. 6A). Diastolic [Ca2+]i and resting potentials in adult and 3-wk-old myocytes were not significantly different. Membrane capacitance (Cm) was significantly greater in adult myocytes due to their larger cell size (2). ICa and ICa density (Table 1, Fig. 6B, C) were both greater in adult myocytes. The peak of the ICa versus voltage curve in both age groups occurred at +10 mV (Fig. 6C).
Comparison of calcium transients and L-type calcium currents from 3-wk-old and adult rabbit ventricular myocytes. A, Calcium transients from the 3-wk-old (upper trace) show much smaller transients than the adult. Diastolic levels were 155 and 103 nmol/L, respectively. Black traces: filtered. B, L-type calcium currents from the 3-wk-old (black trace) show a significantly smaller current than the adult (gray trace) myocyte. C, ICa-voltage relations from a 3-wk-old (capacitance 61 pF, closed symbols) and an adult (capacitance 109 pF, open symbols) myocyte.
DISCUSSION
Myocardial contractility has been shown to increase with development.
The [Ca2+]i transient increases postnatally, but the calcium sensitivity of the myofilaments decreases. cTnT isoform expression also changes with development and is altered by heart disease (9,27,28). Although these changes, with opposite effects on contractility, appear to be at cross-purposes, the greater calcium sensitivity of tension would seem to partially compensate for the lower [Ca]i in the immature myocyte.
Four cTnT isoforms, cTnT1-4, expressed in the rabbit and human heart (9,27,29), are products of combinatorial alternative splicing of two exons (9). Expression of cTnT1 peaks in fetal human (36% of total cTnT) (27) and neonatal rabbit heart. In adult rabbit heart, cTnT4 is the dominantly expressed isoform, whereas in the normal adult human, only cTnT3 is expressed (9,27). These changes in isoform expression suggest that cTnT isoforms have functional consequence.
cTnT isoforms in solution.
We determined for the first time, in solution, that the affinity of TncTnT1 was greater than TncTnT4 and that this difference did not require incorporation of Tn into the myofilaments.
Calcium titration did not affect IAANS-cTnC:cTnT fluorescence, although it affected IAANS:Tn fluorescence, the effect depending on the presence of cTnT1 or cTnT4, suggesting that cTnT isoform amino-terminal regions, which are distant from known cTnT, cTnC, and cTnI interaction sites, affect the interactions among cTnC, cTnI, and cTnT, and this effect requires cTnI.
cTnT isoforms in the myofilament: the force-pCa relationship.
We found that the calcium sensitivity of active tension was higher in rabbit fibers reconstituted with rabbit rcTnT1 than with rabbit rcTnT4, in agreement with Gomes et al. (30), who examined the effects of human (h)cTnT isoforms on the force-pCa relationship of porcine fibers. There was an apparent rightward shift of the force-pCa relationship post-replacement. This may be due to our using mouse cTnI, which is 92% homologous with rabbit cTnI (we also used human cTnC, but that has 100% homology with rabbit cTnC). Also, extraction can affect other myofilament proteins, e.g. myosin binding protein C (31). Whatever the reason, both experimental groups would be affected similarly because the only difference between them was the cTnT isoform used. These results suggest that the developmental decrease in expression of cTnT1 and cTnT2 and increase in cTnT3 and cTnT4 contribute to the decrease in calcium sensitivity in rabbit and rat myocardium (7,32).
Developmental changes and species differences in isoform expression of slow skeletal (ss)TnI, cTnI, tropomyosin and myosin heavy chain (MHC) may contribute to differences in calcium sensitivity (3,33,34). The neonatal switch in expression from ssTnI to cTnI could lower calcium sensitivity (3,35,36) and modify cTnT isoform effects on tension (30). However, we found, in neonatal rabbit, a positive correlation between the sensitivity of myofilaments to calcium and cTnT2 expression but no evidence of a difference in relative cTnI to ssTnI expression (16).
Comparison of calcium binding of CTnT isoforms in solution and the myofilaments.
In the thin filament, the cTnT amino-terminus overlaps the head-to-tail binding of adjoining tropomyosin molecules, suggesting a structure-function relationship. We hypothesized that incorporation of troponin into the myofilaments would alter the calcium affinity induced by the troponin isoforms in solution. If so, ΔΔG between calcium binding to TncTnT1 and TncTnT4 in solution should differ from that in myofilaments: we found, however, that the difference in ΔΔG between fibers containing rcTnT1 and rcTnT4 resulted mainly from the intrinsic differences in TncTnT1 and TncTnT4 and not from incorporation of troponin into myofilaments (Fig. 5).
Systolic [Ca2+]i, Cm, and ICa density were larger in adult than in 3-wk-old rabbit ventricular myocytes, in agreement with previous reports (37–39). In an earlier study (2), we found larger and faster sarcomere shortening in the adult myocyte, consistent with its higher systolic [Ca2+]i. We found no developmental difference in diastolic [Ca2+]i, consistent with our previous finding of no difference in resting sarcomere length (2).
SUMMARY
In solution and in myofilaments, the affinity of TncTnT1 for calcium was higher than that of TncTnT4; our results provide no evidence that these affinities are modified upon incorporation of Tn into myofilaments. The higher calcium sensitivity of myofilaments from immature myocardium, due at least in part to developmental changes in cTnT isoform expression, may compensate for its smaller [Ca2+]i transient: for the same [Ca2+]i, a small shift of the force-pCa curve can result in a dramatic difference in generated force, given the steepness of the curve in the activation region (Fig. 4).
Abbreviations
- IAANS:
-
2-[4′-(iodoacetamido)aniline]-naphthalene-6-sulfonate
- MOPS:
-
3-(N-morpholino)propane-sulfonate
References
Friedman WF 1972 The intrinsic physiologic properties of the developing heart. In: Friedman WF, Lesch M, Sonnenblick EH (eds) Progress in Cardiovascular Diseases: Neonatal Heart Disease. Grune & Stratton, New York, pp 87–111
Nassar R, Reedy MC, Anderson PA 1987 Developmental changes in the ultrastructure and sarcomere shortening of the isolated rabbit ventricular myocyte. Circ Res 61: 465–483
Siedner S, Kruger M, Schroeter M, Metzler D, Roell W, Fleischmann BK, Hescheler J, Pfitzer G, Stehle R 2003 Developmental changes in contractility and sarcomeric proteins from the early embryonic to the adult stage in the mouse heart. J Physiol 548: 493–505
Nakanishi T, Seguchi M, Tsuchiya T, Yasukouchi S, Takao A 1990 Effect of acidosis on intracellular pH and calcium concentration in the newborn and adult rabbit myocardium. Circ Res 67: 111–123
Fabiato A 1982 Calcium release in skinned cardiac cells: variations with species, tissues, and development. Fed Proc 41: 2238–2244
Fabiato A, Fabiato F 1979 Calculator programs for computing the composition of the solutions containing multiple metals and ligands used for experiments in skinned muscle cells. J Physiol (Paris) 75: 463–505
McAuliffe JJ, Gao LZ, Solaro RJ 1990 Changes in myofibrillar activation and troponin C Ca2+ binding associated with troponin T isoform switching in developing rabbit heart. Circ Res 66: 1204–1216
Potter JD, Sheng Z, Pan BS, Zhao J 1995 A direct regulatory role for troponin T and a dual role for troponin C in the Ca2+ regulation of muscle contraction. J Biol Chem 270: 2557–2562
Greig A, Hirschberg Y, Anderson PA, Hainsworth C, Malouf NN, Oakeley AE, Kay BK 1994 Molecular basis of cardiac troponin T isoform heterogeneity in rabbit heart. Circ Res 74: 41–47
Malouf NN, McMahon D, Oakeley AE, Anderson PA 1992 A cardiac troponin T epitope conserved across phyla. J Biol Chem 267: 9269–9274
Potter JD 1982 Preparation of troponin and its subunits. Methods Enzymol 85: 241–263
Pan BS, Johnson RG Jr 1996 Interaction of cardiotonic thiadiazinone derivatives with cardiac troponin C. J Biol Chem 271: 817–823
Guo X, Wattanapermpool J, Palmiter KA, Murphy AM, Solaro RJ 1994 Mutagenesis of cardiac troponin I. Role of the unique NH2-terminal peptide in myofilament activation. J Biol Chem 269: 15210–15216
Liao R, Wang CK, Cheung HC 1994 Coupling of calcium to the interaction of troponin I with troponin C from cardiac muscle. Biochemistry 33: 12729–12734
Taylor RB, Trimble C, Valdes JJ, Wayner MJ, Chambers JP 1992 Determination of free calcium. Brain Res Bull 29: 499–501
Nassar R, Malouf NN, Kelly MB, Oakeley AE, Anderson PA 1991 Force-pCa relation and troponin T isoforms of rabbit myocardium. Circ Res 69: 1470–1475
Nassar R, Malouf NN, Mao L, Rockman HA, Oakeley AE, Frye JR, Herlong JR, Sanders SP, Anderson PA 2005 cTnT1, a cardiac troponin T isoform, decreases myofilament tension and affects the left ventricular pressure waveform. Am J Physiol Heart Circ Physiol 288: H1147–H1156
Guth K, Potter JD 1987 Effect of rigor and cycling cross-bridges on the structure of troponin C and on the Ca2+ affinity of the Ca2+-specific regulatory sites in skinned rabbit psoas fibers. J Biol Chem 262: 13627–13635
Hatakenaka M, Ohtsuki I 1991 Replacement of three troponin components with cardiac troponin components within single glycerinated skeletal muscle fibers. Biochem Biophys Res Commun 181: 1022–1027
Anderson PA, Greig A, Mark TM, Malouf NN, Oakeley AE, Ungerleider RM, Allen PD, Kay BK 1995 Molecular basis of human cardiac troponin T isoforms expressed in the developing, adult, and failing heart. Circ Res 76: 681–686
Mitra R, Morad M 1985 A uniform enzymatic method for dissociation of myocytes from hearts and stomachs of vertebrates. Am J Physiol 249: H1056–H1060
Hamill OP, Marty A, Neher E, Sakmann B, Sigworth FJ 1981 Improved patch-clamp techniques for high-resolution current recording from cells and cell-free membrane patches. Pflugers Arch 391: 85–100
Li Q, Altschuld RA, Stokes BT 1987 Quantitation of intracellular free calcium in single adult cardiomyocytes by fura-2 fluorescence microscopy: calibration of fura-2 ratios. Biochem Biophys Res Commun 147: 120–126
Grynkiewicz G, Poenie M, Tsien RY 1985 A new generation of Ca2+ indicators with greatly improved fluorescence properties. J Biol Chem 260: 3440–3450
Burtnick LD, McCubbin WD, Kay CM 1976 The isolation and characterization of the tropomyosin binding component (TN-T) of bovine cardiac troponin. Can J Biochem 54: 546–552
Putkey JA, Liu W, Lin X, Ahmed S, Zhang M, Potter JD, Kerrick WG 1997 Fluorescent probes attached to Cys 35 or Cys 84 in cardiac troponin C are differentially sensitive to Ca(2+)-dependent events in vitro and in situ. Biochemistry 36: 970–978
Anderson PA, Malouf NN, Oakeley AE, Pagani ED, Allen PD 1991 Troponin T isoform expression in humans. A comparison among normal and failing adult heart, fetal heart, and adult and fetal skeletal muscle. Circ Res 69: 1226–1233
Saba Z, Nassar R, Ungerleider RM, Oakeley AE, Anderson PA 1996 Cardiac troponin T isoform expression correlates with pathophysiological descriptors in patients who underwent corrective surgery for congenital heart disease. Circulation 94: 472–476
Townsend PJ, Barton PJ, Yacoub MH, Farza H 1995 Molecular cloning of human cardiac troponin T isoforms: expression in developing and failing heart. J Mol Cell Cardiol 27: 2223–2236
Gomes AV, Venkatraman G, Davis JP, Tikunova SB, Engel P, Solaro RJ, Potter JD 2004 Cardiac troponin T isoforms affect the Ca(2+) sensitivity of force development in the presence of slow skeletal troponin I: insights into the role of troponin T isoforms in the fetal heart. J Biol Chem 279: 49579–49587
Wattanapermpool J, Guo X, Solaro RJ 1995 The unique amino-terminal peptide of cardiac troponin I regulates myofibrillar activity only when it is phosphorylated. J Mol Cell Cardiol 27: 1383–1391
Reiser PJ, Westfall MV, Schiaffino S, Solaro RJ 1994 Tension production and thin-filament protein isoforms in developing rat myocardium. Am J Physiol 267: H1589–H1596
Metzger JM, Wahr PA, Michele DE, Albayya F, Westfall MV 1999 Effects of myosin heavy chain isoform switching on Ca2+-activated tension development in single adult cardiac myocytes. Circ Res 84: 1310–1317
Muthuchamy M, Grupp IL, Grupp G, O'Toole BA, Kier AB, Boivin GP, Neumann J, Wieczorek DF 1995 Molecular and physiological effects of overexpressing striated muscle beta-tropomyosin in the adult murine heart. J Biol Chem 270: 30593–30603
Arteaga GM, Palmiter KA, Leiden JM, Solaro RJ 2000 Attenuation of length dependence of calcium activation in myofilaments of transgenic mouse hearts expressing slow skeletal troponin I. J Physiol 526: 541–549
Godt RE, Fogaca RT, Nosek TM 1991 Changes in force and calcium sensitivity in the developing avian heart. Can J Physiol Pharmacol 69: 1692–1697
Huynh TV, Chen F, Wetzel GT, Friedman WF, Klitzner TS 1992 Developmental changes in membrane Ca2+ and K+ currents in fetal, neonatal, and adult rabbit ventricular myocytes. Circ Res 70: 508–515
Osaka T, Joyner RW 1991 Developmental changes in calcium currents of rabbit ventricular cells. Circ Res 68: 788–796
Wetzel GT, Chen F, Klitzner TS 1993 Ca2+ channel kinetics in acutely isolated fetal, neonatal, and adult rabbit cardiac myocytes. Circ Res 72: 1065–1074
Author information
Authors and Affiliations
Corresponding author
Additional information
This work was supported in part by NIH RO1 HL20749, RO1 HL42250, RO1 GM42501, and RO1 HL22231 and by grants from the Gustavius and Louise Pfeiffer Memorial Fund and the Children's Miracle Network.
Rights and permissions
About this article
Cite this article
McCall, S., Nassar, R., Malouf, N. et al. Development and Cardiac Contractility: Cardiac Troponin T Isoforms and Cytosolic Calcium in Rabbit. Pediatr Res 60, 276–281 (2006). https://doi.org/10.1203/01.pdr.0000233004.95404.1f
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1203/01.pdr.0000233004.95404.1f
This article is cited by
-
Reduced troponin I phosphorylation and increased Ca2+-dependent ATP-consumption in triton X-skinned fiber preparations from Gαq overexpressor mice
Molecular and Cellular Biochemistry (2008)