Abstract
Previous studies have demonstrated increased oxidative damage to proteins and increased lipid peroxidation products in the plasma of hypoxic newborns at birth. We tested the hypothesis that hypoxic preterm newborns are at increased risk for oxidative stress in the first week of life. Heparinized blood samples of 34 hypoxic and 15 control preterm newborns were obtained at birth from the umbilical vein immediately after delivery and from a peripheral vein on postnatal d 7. Plasma levels of hypoxanthine, total hydroperoxide (TH), and advanced oxidation protein products (AOPP) were measured in cord blood and blood drawn on d 7. Hypoxanthine, TH, and AOPP levels were significantly higher in cord and d 7 blood samples of hypoxic newborn than control infants. Statistically significant correlations were observed between AOPP and hypoxanthine and between AOPP and TH plasma levels on d 7. AOPP and TH plasma levels significantly increased from cord to d 7 blood in neonates without hypoxia. These findings show that the oxidative stress observed in cord blood of hypoxic preterm newborns is still higher than control infants on d 7. The significant increase in TH and AOPP levels in nonhypoxic preterm newborns at the end of the first postnatal week indicates that damage caused by free radicals also occurs in nonhypoxic babies with normal clinical course. In summary, TH and AOPP production is prolonged for several days after birth in hypoxic preterm babies. The risk of free radical damage is lower but still exists in preterm neonates with normal clinical course.
Similar content being viewed by others
Main
Perinatal hypoxic-ischemic injury is an important cause of neonatal morbidity. Numerous experimental studies have demonstrated FR production and oxidative damage caused by hypoxia in fetal life (1–3). Although the clinical consequences of hypoxia are readily observed, the specific biochemical processes preceding the onset of FR damage are not well understood. Oxidative stress exists and tissue damage is possible when there are low levels of antioxidants or increased FR activity (4). Newborns and particularly preterm infants are at high risk for oxidative stress and are very susceptible to oxidative damage by FR (5). Indeed, there is evidence of an imbalance between antioxidant and oxidant-generating systems, which causes oxidative damage (6). Birth is an oxidative challenge for the newborn. The sharp postnatal transition from the relatively low oxygen intrauterine environment to the significantly higher oxygen extrauterine environment exposes the newborn to FR. The increase in FR generation is exacerbated by the low efficiency of natural antioxidant systems in the newborn, especially if preterm, predisposing it to oxidative damage (7). High FR generation and protein oxidation products have been detected in the plasma of preterm hypoxic newborns at birth (8). Sick preterm newborns may be particularly exposed to FR in the first postnatal week because of frequent intensive care, involving oxygen supply, assisted ventilation, surfactant administration, and total parenteral nutrition, which may enhance and prolong oxidative stress by increasing oxygen delivery to the tissues. The present paper tests the hypothesis that the high risk of FR injury of preterm newborns at birth persists during the first postnatal week.
METHODS
Patients.
Forty-nine preterm babies of gestational age 26–36 wk (for more details, see Table 1), admitted consecutively to the Neonatology Division, University Hospital of Siena, were enrolled in the study. All babies with congenital malformations, inborn errors of metabolism, blood group incompatibility, sepsis, diabetic mothers, or multiple gestation and those not born in the clinic were excluded. Thirty-four of the 49 newborns were regarded as hypoxic if they fulfilled at least two of the following criteria: pH ≤ 7.20 in umbilical vein, Apgar score ≤ 6 at 5 min, and fraction of inspired oxygen ≥ 0.4 needed to achieve oxygen saturation ≥ 86% at birth. These criteria, less severe than those established by the American College of Obstetricians and Gynecologists to define newborns with birth asphyxia (9), were chosen, as in previous papers of ours (8, 10, 11), to evaluate all hypoxic babies, even those with mild hypoxia. Ten of 34 hypoxic babies were reanalyzed separately to verify whether stricter criteria of hypoxia (pH < 7.15 in umbilical vein, Apgar score < 5 at 5 min) changed our results. Fifteen babies, without signs of perinatal hypoxia and with normal clinical course, were used as control subjects.
Heparinized blood samples were obtained from the umbilical vein after cord clamping immediately after delivery and from a peripheral vein on d 7. The study was approved by the Human Ethics Committee of the Medical Faculty, University of Siena. Informed written parental consent was obtained before enrollment of each infant.
Methods.
Blood was immediately centrifuged, and all analyses (Hx, AOPP, and TH) were performed in plasma within 2 h of blood sampling to avoid the effects of storage. After centrifuging, the plasma and buffy coat were removed. Hx levels were evaluated by HPLC, using a Varian Vista 5500 high-performance liquid chromatograph equipped with a variable-wavelength UV detector (model 4290, Varian, Palo Alto, CA, U.S.A.). A ready-to-use prepacked Supelcosil LC-18 column by Supelco (250 × 4.6 mm internal diameter, 5 μm), with precolumn (20 × 4.6 mm internal diameter) filled with the same packing (Supelguard, Supelco, St. Louis, MO, U.S.A.), completed the analytical system. The mobile phase gradient used was T 0′, (A = 100%; B = 0%); T10′, (A = 90%; B = 10%); T 20′, (A = 80%; B = 20%); and T 30′, (A = 100%; B = 0%), with A = 10−2 M potassium phosphate buffer at pH 5.5 and B = methanol. The next sample was injected 10 min later. The flow rate was 1 mL/min, and the wavelength 220 nm. AOPP were measured as described by Witko-Sarsat et al.(12) using spectrophotometry on a microplate reader. AOPP concentrations were expressed as micromolar chloramine-T equivalents. TH production was measured with a d-ROMs Kit by Diacron Srl, Grosseto, Italy, as previously described (8).
The data, expressed as mean ± SD and median, were analyzed for statistically significant differences by the nonparametric test for two independent or related samples (Mann-Whitney U and Wilcoxon tests) and by linear correlation, using SPSS release 6.0 statistical package (Dynamic Microsystems Inc., Silver Springs, MD, U.S.A.).
RESULTS
Hx, TH, and AOPP data at birth were similar to those previously reported (8). Hx, TH, and AOPP plasma levels were significantly higher in hypoxic newborns than in control infants at birth and on d 7 (Table 2). TH and AOPP also increased from birth to d 7 in neonates without perinatal hypoxia (Table 2).
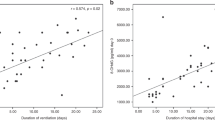
Similar results, but more statistically significant, were found in 10 of 34 hypoxic babies meeting stricter criteria of hypoxia with respect to nonhypoxic babies, both at birth [Hx, 7.50 ± 3.42 (median, 5.82; 95% CI: 5.38–10.68) versus 1.67 ± 1.42 (median, 1.22; 95% CI: 0.67–2.28) μg/mL, p < 0.0001; TH, 208.50 ± 114.33 (median, 187.50; 95% CI: 117.24–266.64) versus 45.72 ± 22.40 (median, 46; 95% CI: 27–59.25) U.Carr/L, p < 0.0001; AOPP, 182.38 ± 52.27 (median, 184.15; 95% CI: 132.67–247.88) versus 134.92 ± 60.53 (median, 107.5; 95% CI: 88.09–189.20) μmol/L, p = 0.02] and on d 7 of life [Hx, 4.94 ± 1.94 (median, 6; 95% CI: 4–7) versus 1.68 ± 0.94 (median, 1.81; 95% CI: 0.35–2.80) μg/mL, p = 0.0002; TH, 227.40 ± 125.13 (median, 172.5; 95% CI: 128–330) versus 84.61 ± 38.62 (median, 73; 95% CI: 47.86–127) U.Carr/L, p = 0.0002; AOPP, 558.77 ± 194.27 (median, 603; 95% CI: 348.12–737.54) versus 250.57 ± 128.90 (median, 222.3; 95% CI: 187.5–319.5) μmol/L, p = 0.001]. Statistically significant correlations were observed between AOPP and Hx (Fig. 1), TH and Hx (Fig. 2), and AOPP and TH (Fig. 3) on d 7.
DISCUSSION
Birth is an oxidative challenge for the newborn. The fetal to neonatal transition exposes the newborn to a much more oxygen-rich world than the intrauterine environment (7). The relatively high oxygen concentrations after birth could be toxic to fetal tissues. A potential mechanism of toxicity and pathophysiologic cell alterations is believed to be mediated by increased production of FR (13).
FR may be generated by various mechanisms, such as hypoxia, ischemia-reperfusion, hyperoxia, neutrophil and macrophage activation, mitochondrial dysfunction, Fenton chemistry, endothelial cell damage, FFA, and prostaglandin metabolism (14–17). FR and their products, lipid and protein peroxides, are thought to be among the major causes of cell membrane destruction and cell damage. Cells normally respond to oxidative stress by up-regulating antioxidant defenses and other protective systems, but overproduction of FR damages proteins, lipids, and DNA and leads to cell transformation or cell death by apoptotic or necrotic mechanisms (18).
Newborns and particularly preterm infants are at high risk for FR damage. In these subjects, there is evidence of an imbalance between antioxidant and oxidant-generating systems, which causes oxidative damage (6). Neonatal plasma has an antioxidant profile with low glutathione peroxidase, superoxide dismutase, β-carotene, riboflavin, α-proteinase, vitamin E, selenium, copper, zinc, ceruloplasmin, transferrin, and other plasma factors (19, 20).
Increased production of FR is a feature of most, if not all, neonatal disease (21). Hypoxia is one of the main factors inducing FR generation in the perinatal period. During hypoxia the cutback in oxidative phosphorylation rapidly diminishes reserves of high-energy phosphates, and high levels of adenosine and hypoxanthine accumulate in a few minutes (14, 22). ATP degradation products are the substrate for the xanthine oxidase reaction, leading to enhanced FR production in the reperfusion phase (23). Other biochemical events capable of inducing FR during hypoxia are reduction of electron transport chain components that undergo autooxidation, increased release of free iron from ferritin under conditions of decreased cellular high-energy compounds, and excessive accumulation of intracellular Ca2+(18, 24, 25).
We previously found higher Hx, xanthine, and uric acid in hypoxic newborn infants than in control infants at birth (8). Increased production of ATP degradation products was associated with higher TH and AOPP levels. The more severe the hypoxia, the greater the lipid and protein damage by FR. Additional pathways can contribute to expose preterm newborns to FR damage in the postnatal period. In the first week of life, preterm babies are at high risk for oxidative stress because of postnatal environmental oxygen concentrations, which are higher than those of the intrauterine environment, therapeutic use of oxygen, and elevated non–protein-bound iron concentrations in red blood cells and plasma (10, 26, 27). Activation of polymorphonuclear leukocytes, often associated with preterm labor and delivery, can be harmful because activated neutrophils produce a flood of FR (28). FR of any origin cause oxidative stress, which can be detected in plasma in postnatal life. The present study shows higher cord blood Hx, TH, and AOPP concentrations in hypoxic than in nonhypoxic preterm newborns as we previously observed (8). Higher Hx, TH, and AOPP plasma levels were also found on d 7 in the hypoxic group than in nonhypoxic control infants. These data strongly suggest that the oxidative stress observed in cord blood of hypoxic preterm newborns remains higher than in control infants at least until d 7. TH and AOPP are also increased significantly in the first week of life in nonhypoxic preterm newborns. This increased generation in protein and lipid peroxidation products strongly suggests that oxidative stress also occurs in nonhypoxic babies with normal clinical course. The higher Hx levels in hypoxic than nonhypoxic control infants on d 7 may indicate an increase in ATP degradation products as a consequence of the low ATP levels after birth, particularly in hypoxic preterm babies with low caloric intake in the first days of life.
Positive statistically significant correlations were found between Hx and TH, Hx and AOPP, and TH and AOPP on d 7 as previously found in cord blood (8). This finding suggests that protein oxidative damage and lipid peroxidation are interrelated and linked to breakdown of ATP.
In conclusion, although the present study was conducted with a small number of subjects, it is a first step in the evaluation of postnatal oxidative stress. In particular, it is interesting to note that the evidence of oxidative stress on d 7 of life exists not only in hypoxic but also in nonhypoxic preterm babies. This suggests that all preterm infants are at high risk for oxidative stress at birth because the extrauterine environment (Po2 100 mm Hg) is richer in oxygen than the intrauterine environment (20–25 mm Hg). This 4- to 5-fold increase is exacerbated by the low efficiency of natural antioxidant systems in the newborn, especially the preterm newborn. The evidence of persistent oxidative stress in postnatal life both in hypoxic and nonhypoxic preterm babies suggests that it may be a physiologic event. Therefore, resuscitation routines should be assessed more carefully than is done at present to reduce postnatal oxidative stress, using room air without adding oxygen (29), avoiding neonatal acidosis, and limiting sepsis. These strategies aimed at reducing FR generation and oxidative stress are advisable until new reliable antioxidant therapies are found.
Abbreviations
- Hx:
-
hypoxanthine
- AOPP:
-
advanced oxidation protein products
- TH:
-
total hydroperoxide
- FR:
-
free radical
- CI:
-
confidence interval
References
Maulik D, Numagami Y, Ohnishi ST, Mishra OP, Delivoria-Papadopoulos M 1998 Direct detection of oxygen free radical generation during in utero hypoxia in the fetal guinea pig brain. Brain Res 798: 166–172
Mishra OP, Delivoria-Papadopoulos M 1989 Lipid peroxidation in developing fetal guinea pig brain during normoxia hypoxia. Dev Brain Res 45: 129–135
Hasegawa K, Yoshioka H, Sawada T, Nishikawa H 1991 Lipid peroxidation in neonatal mouse brain subjected to two different types of hypoxia. Brain Dev 13: 101–103
Halliwell B 1994 Free radicals, antioxidants human disease: curiosity, cause, or consequence?. Lancet 344: 721–724
Saugstad OD 1996 Mechanisms of tissue injury by oxygen radicals: implications for neonatal disease. Acta Paediatr 85: 1–4
Jacob RA 1995 The integrated antioxidant system. Nutr Res 15: 755–766
Frank L, Sosenko RS 1987 Development of lung anti-oxidant enzyme system in late gestation: possible implications for the prematurely born infant. J Paediatr 110: 9–14
Buonocore G, Perrone S, Longini M, Terzuoli L, Bracci R 2000 Total hydroperoxide advanced oxidation protein products in preterm hypoxic babies. Pediatr Res 47: 221–224
American College of Obstetricians and Gynecologists 1994 Fetal Distress and Birth Asphyxia. Washington, DC: American College of Obstetricians and Gynecologists. ACOG Committee Opinion 137
Buonocore G, Zani S, Perrone S, Caciotti B, Bracci B 1998 Intraerythrocyte nonprotein-bound iron plasma malondialdehyde in the hypoxic newborn. Free Radic Biol Med 25: 766–770
Buonocore G, Perrone S, Gioia D, Gatti MG, Massafra C, Agosta R, Bracci R 1999 Nucleated red blood cell count at birth as an index of perinatal brain damage. Am J Obstet Gynecol 181: 1500–1505
Witko-Sarsat V, Friedlander M, Capeillere-Blandin C, Nguyen-Khoa T, Thu Nguyen A, Zingraff J, Jungers P, Descamps-Latsca B 1996 Advanced oxidation protein products as a novel marker of oxidative stress in uremia. Kidney Int 49: 1304–1313
Buonocore G, Perrone S, Bracci R 2001 Free radicals brain damage in the newborn. Biol Neonate 79: 180–186
McCord JM 1985 Oxygen-derived free radicals in postischemic tissue injury. N Engl J Med 312: 159–163
Sutton HC, Winterbourn CC 1989 On the participation of higher oxidative states of iron copper in Fenton reactions. Free Radic Biol Med 6: 53–60
Kukreja RC, Kontos HA, Hess ML, Ellis EF 1986 PGH synthase lipoxygenase generate superoxide in the presence of NADH or NADPH. Circ Res 59: 612–619
Turrens JF 1997 Superoxide production by the mitochondrial respiratory chain. Biosci Rep 17: 3–8
Mishra OP, Delivoria-Papadopoulos M 1999 Cellular mechanisms of hypoxic injury in the developing brain. Brain Res Bull 48: 233–238
Bracci R, Buonocore G 1998 The antioxidant status of erythrocytes in preterm term infants. Semin Neonatol 3: 191–197
Gophinathan V, Miller NJ, Milner AD, Rice-Evans CA 1994 Bilirubin ascorbate: antioxidant activity in neonatal plasma. FEBS Lett 349: 197–200
Saugstad OD 2001 Update on oxygen radical disease in neonatology. Curr Opin Obstet Gynecol 13: 147–153
Saugstad OD 1988 Hypoxanthine as an indicator of hypoxia: its role in health disease through free radical production. Pediatr Res 23: 143–150
Saugstad OD 1996 Role of xanthine oxidase its inhibitor in hypoxia: reoxygenation injury. Pediatrics 98: 103–107
Turrens JG, Alexandre A, Lehninger AL 1985 Ubisemiquinone is the electron donor for superoxide formation by complex III of heart mitochondria. Arch Biochem Biophys 237: 271–278
Ying W, Han S-K, Miller JW, Swanson RA 1999 Acidosis potentiates oxidative neuronal death by multiple mechanisms. J Neurochem 73: 1549–1556
Buonocore G, Zani S, Sargentini I, Gioia D, Signorini C, Bracci R 1998 Hypoxia-induced free iron released in the red cells of newborn infants. Acta Paediatr 87: 77–81
Evans PJ, Evans R, Kovar IZ, Holton AF, Halliwell B 1992 Bleomycin-detectable iron in the plasma of premature full-term neonates. FEBS Lett 303: 210–212
Buonocore G, Gioia D, De Filippo M, Picciolini E, Bracci R 1994 Superoxide anion release by polymorphonuclear leukocytes in whole blood of newborns mothers during the peripartal period. Pediatr Res 36: 619–622
Saugstad OD, Rootwelt T, Aalen O 1998 Resuscitation of asphyxiated newborn infants with room air or oxygen: an international controlled trial: the Resair 2 study. Pediatrics 102: e1
Author information
Authors and Affiliations
Corresponding author
Additional information
Supported by the Italian Ministry for the University and Scientific-Technological Research (COFIN 99 Funds).
Rights and permissions
About this article
Cite this article
Buonocore, G., Perrone, S., Longini, M. et al. Oxidative Stress in Preterm Neonates at Birth and on the Seventh Day of Life. Pediatr Res 52, 46–49 (2002). https://doi.org/10.1203/00006450-200207000-00010
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1203/00006450-200207000-00010
This article is cited by
-
Association between adherence to a low carbohydrate dietary (LCD) pattern with breast milk characteristics and oxidative markers in infants’ urine: a cross-sectional study
Journal of Health, Population and Nutrition (2023)
-
Association between thiol-disulfide hemostasis and transient tachypnea of the newborn in late-preterm and term infants
BMC Pediatrics (2023)
-
Evaluation of pro-oxidant antioxidant balance in retinopathy of prematurity
Eye (2022)
-
Elucidating the involvement of apoptosis in postmortem proteolysis in porcine muscles from two production cycles using metabolomics approach
Scientific Reports (2021)
-
Short-term perinatal oxygen exposure may impair lung development in adult mice
Biological Research (2020)