Abstract
A delayed chylomicron (CM) clearance rate, a known risk factor for atherosclerosis, has been described in adults with diabetes type 1 (DM1). We determined the CM clearance rate in late teenagers with DM1, and the relationship between CM clearance rate and elevated plasma lipid concentrations in DM1 teenagers in poor metabolic control (as characterized by HbA1c percentage). Plasma lipids and CM clearance were determined in nine patients with DM1 (mean age ± SD: 17.5 ± 0.6 y) and four healthy controls (mean age ± SD: 20.1 ± 0.8 y), by measuring breath 13CO2, plasma triglyceride, retinyl palmitate, and 13C-labeled oleic acid concentrations, after oral administration of a fat-rich meal together with vitamin A and 13C-oleic acid. In patients with DM1, fasting triglyceride and cholesterol concentrations were positively correlated with HbA1c percentage (p < 0.05). Neither in DM1 patients, nor in controls, was an elevated triglyceride concentration (above 1.7 mmol/L) found. Yet, in 22% of DM1 patients, cholesterol concentration was above 5.2 mmol/L, but not in any of the controls. CM clearance rate in DM1 patients was similar to that in controls and did not significantly correlate with HbA1c percentage. Fasting lipid concentrations in DM1 patients were not significantly correlated with CM clearance rate. Present data indicate that elevated lipid concentrations in late teenagers with DM1 are not attributable to a delay in CM clearance rate. A delayed CM clearance rate at late teenager age is not a risk factor contributing to the increased risk for atherosclerosis in DM1.
Similar content being viewed by others
Main
Diabetes mellitus type 1 (DM1) is associated with a 2–4 fold increased risk for cardiovascular disease (1). Specific diabetes-related phenomena have been suggested to play a role in the observed association, such as glycosylation of proteins (2), presence of renal disease (3), elevated concentrations of plasma cholesterol, triglycerides, and low density lipoprotein (LDL) cholesterol (4), and altered composition of very low density lipoprotein (VLDL) and LDL (5, 6). In children and late teenagers, studies on risk factors associated with DM1 and atherosclerosis have mainly focused on cholesterol and triglyceride concentrations (7–9). Elevated concentrations of both cholesterol and triglycerides have been reported in children with DM1 in poor metabolic control, which tended to decrease to nondiabetic values upon improvement of metabolic control (7–9).
A delayed chylomicron (CM) clearance has been identified as a risk factor for atherosclerosis in patients with coronary heart disease, in patients with type 2 diabetes, and in adult patients with DM1 (10–12). Insulin enhances the metabolism of CM by stimulating the hydrolysis of CM triglycerides by lipoprotein lipase and the hepatic uptake of CM remnants (11). A relative deficiency of insulin, such as present in DM1, could therefore lead to a delay in CM clearance. A delay in CM clearance would imply a prolonged postprandial hyperlipidemia, which supposedly leads to increased deposition of CM contents into the arterial wall and to an unfavorable translocation of cholesteryl-esters from HDL to CM (13, 14). Delays in CM clearance are amenable to dietary intervention, by changing the intake of fish oil (long chain PUFA) (15), monounsaturated-fat (16), or carbohydrate (17). Yet, it is not known whether CM clearance is delayed in late teenagers with DM1. In nondiabetic individuals CM clearance rate appeared to be inversely correlated with age (i.e. a delayed clearance in older individuals) (18, 19). Specific dietary recommendations to late teenagers with DM1 are only warranted if CM clearance would be delayed at their age. In the present study, we investigated, whether a delayed clearance rate of chylomicrons (CM) can be a risk factor for atherosclerosis and may contribute to higher lipid concentrations, especially triglycerides, in late teenagers with DM1. We also determined whether CM clearance is correlated with the level of metabolic control, as characterized by HbA1c. CM clearance was investigated by determination of plasma concentrations of three CM constituents (triglycerides, vitamin A, and 13C-oleic acid) after their prior oral ingestion.
METHODS
Subjects.
Chylomicron clearance was studied in nine late teenagers with DM1 (four boys, five girls; mean age ± SD: 17.5 ± 0.6 y) and four healthy controls (two boys, two girls; mean age ± SD: 20.1 ± 0.8 y). Patients were studied at late teenager age to rule out the effect of puberty on lipid metabolism, as puberty is associated with a relative insulin resistance (20). DM1 patients were selected from our outpatient clinic population with fair or poor metabolic control (5 with HbA1c <8.7%, 4 with HbA1c >9.5%). Patients were selected on basis of their HbA1c (<8.7%, >9.5%) and differed only in metabolic control. HbA1c ± SD at the test day were 7.8 ± 0.5% and 10.9 ± 1.5%, respectively. Mean HbA1c in the year preceding the test were 7.6 ± 0.4% and 11.2 ± 1.9%, respectively. Patients with microvascular complications like microalbuminuria (defined as albumin excretion rate >30 mg/24 h in 24 h urine collection), or retinopathy (ophthalmoscopy through dilated pupils by ophthalmologist) were excluded from the study. Other exclusion criteria were severe insulin resistance (insulin dosages above 1.5 U/kg/d), with diabetes associated diseases like hypothyroidism and celiac disease, renal or liver disease, obesity (weight for height >90th percentile), anorexia nervosa (weight for height <10th percentile), first grade relatives with lipid disturbances, or cardiovascular disease under the age of 60. Patients, who had had diabetes for less than one year were also excluded. No medication known to affect lipoprotein metabolism was used by the subjects, except for oral contraceptives (3 of DM1, 2 of controls) and insulin. In control subjects, the absence of microalbuminuria was tested. They had none of the above mentioned diseases. At palpation control subjects did not have a goiter, and they had no known first grade relatives with lipid disturbances or cardiovascular disease under the age of 60. Mean insulin dosage was not significantly different between patients with HbA1c below 8.7% or above 9.5% (0.9 ± 0.2 U/kg/d in each group). Patients with DM1 did not have ketones in their urine nor any sign of illness during the three days preceding the test. Informed consent was obtained from all patients and controls over 18 y of age. Parental informed consent was obtained for minors. The study was approved by the medical ethical committee of our hospital.
Study design.
In DM1 patients and controls, CM metabolism was investigated after an overnight fast. CM metabolism was investigated by analyzing plasma disappearance of 3 CM constituents. First, the disappearance rate of the main CM core component, triglycerides, was measured after ingestion of a high-fat meal. Second, a classic test to quantitate uptake of CM remnants by the liver, the vitamin A test, was applied (21, 22). After oral ingestion, vitamin A is taken up by enterocytes and incorporated into the core of CM in the form of retinyl esters. Retinyl esters remain associated with the CM in the circulation until the remnant stage, after which they are taken up by the liver (21). Third, hydrolysis of CM triglycerides by lipoprotein lipase was investigated by ingestion of a stable isotopically labeled fatty acid (13C-oleic acid). Orally ingested 13C labeled fatty acid will be incorporated in CM triglycerides. After appearance in plasma, 13C-triglycerides will be hydrolyzed by lipoprotein lipase and the hydrolyzed 13C-fatty acid will be taken up into body cells and metabolized. The metabolism of 13C labeled CM fatty acids can be quantified by measuring the disappearance of 13C labeled fatty acid from plasma and, indirectly, by determining the appearance of 13CO2 in breath, reflecting oxidation of the parent compound. Subjects were instructed to avoid food naturally enriched in 13C, such as corn, pine apple, corn flour, cane sugar, and millet for three days before the test.
After an overnight fast, a standardized high-fat meal (composition: 1 g fat/kg body weight (BW), 60% (wt/wt) saturated fat; 1 g carbohydrate/kg BW, 0.5 g protein/kg BW), together with vitamin A (50.000 IU/m2) and [1- 13C]-oleic acid (5 mg/kg BW) was ingested between 8:30 and 9:00 AM (time 0). The high-fat meal consisted of a milkshake and wheat bread with butter and cheese. For 6 h after ingestion of the test-meal, no other food was ingested. DM1 patients administered their insulin in an adjusted dosage 30 min before the test-meal. The dosage of short acting insulin was adjusted for the carbohydrate composition in the test-meal (compared with their normal carbohydrate intake at breakfast and at 10:30 AM) and the glucose value at 8.00 AM. The dosage of long acting insulin was lowered in view of the fasting period of 6 h after the test-meal. Before, and for 6 h after ingestion of the test-meal, every half hour breath samples were collected to quantitate breath 13CO2, and every hour blood samples were collected to quantitate plasma concentrations of retinyl palmitate, triglycerides, cholesterol, 13C-oleic acid, glucose, and FFA. In the first blood sample taken, HbA1c concentration was determined.
Analytical methods.
Glucose levels were measured on a calibrated Companion 2 glucometer (Medisense, Abbott laboratories, Abbott Park, IL). Triglyceride and cholesterol concentrations were determined in plasma by enzymatic methods using commercial kits (Triglycerides/GB and Chol respectively, Boehringer, Mannheim, Germany), as were FFA in plasma (NEFA C, Wako, Neuss, Germany). Plasma retinyl palmitate concentrations were determined by high-pressure liquid chromatography (HPLC), according to Bui and to Zaman et al. (23, 24). HbA1c was determined by ion-exchange HPLC (VARIANTTM HbA1c Program with Bio-Rad VARIANT Hb Testing System, Bio-Rad, Hercules, CA). Normal range of HbA1c in nondiabetic individuals in our hospital is 4.6–6.1%.
13C-oleic acid content was measured in total plasma lipid. To determine 13C-oleic acid, plasma lipids were methylated and extracted according to Lepage and Roy (25). Resulting fatty acid methyl esters were analyzed by gas chromatography (GC) to quantitate oleic acid concentration (26) and by GC combustion isotope ratio mass spectrometry (GC-C-IRMS) to determine 13C-enrichment of oleic acid. Fatty acids were quantified using heptadecanoic acid (17:0) as internal standard. 13C-enrichment of the oleic acid methyl esters was determined by using a Finnigan MAT Delta S isotope ratio mass spectrometer interfaced to a Varian 3400 gas chromatograph via a capillary oxidation furnace (Finnigan MAT, Bremen, Germany). Separation of the methyl esters and determination of [13C] abundance was performed according to the method described by us previously (27). The concentration of 13C-oleic acid in plasma is expressed as the percentage of the dose administered per liter plasma (% Dose/L).
13C-enrichment in the breath samples was determined by means of continuous flow isotope ratio mass spectrometry (Finnigan Breath MAT, Finnigan MAT Gmb, Bremen, Germany). The 13C abundance of breath CO2 was expressed as the difference (%o) compared with the reference standard Pee Dee Belemnite limestone (δ 13Cpdb,%o). The proportion of 13C-label excreted in breath CO2 was expressed as the percentage of administered 13C-label recovered per hour (%13C Dose/h), and was corrected for individual CO2 production as determined every hour for 5 min by indirect calorimetry (Oxycon, model ox-4, Dräger, Breda, The Netherlands).
Statistical analysis.
Results are expressed as means ± SD. Group means were compared by Student-t test, or, if SDs were significantly different, by Welch's alternate-t test. The postprandial clearance rates of retinyl palmitate and triglycerides were calculated in individual subjects by calculating the slope of the linear regression line, using the peak concentration and plasma concentrations up to 3 h after peak concentration as dependent variables and corresponding time points as independent variables in each individual. The slopes in DM1 and control late teenagers (means ± SD) were compared by Student-t test. The postprandial clearance of retinyl palmitate was also investigated by calculating the area under the retinyl palmitate curves of each individual and comparing the two group means and SDs by Student-t test.
RESULTS
Baseline characteristics of DM1 patients and controls.
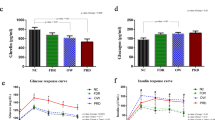
Table 1 shows the clinical data and fasting cholesterol and triglyceride concentrations in DM1 patients and controls. For triglyceride concentrations in children and teenagers, a cut-off concentration of 1.7 mmol/L is used (as defined by the European Non-Insulin-Dependent Diabetes Policy Group) (7). In none of the DM1 patients or controls were fasting triglyceride concentrations higher than 1.7 mmol/L. According to the National Cholesterol Education Program (NCEP), fasting cholesterol concentrations in healthy children and teenagers are classified as “borderline increased” when between 4.4 and 5.2 mmol/L and as “high” when exceeding 5.2 mmol/L (28). Fasting cholesterol concentrations were higher than 5.2 mmol/L in 22% (2 of 9) of the DM1 patients and none of the controls. Borderline increased cholesterol concentrations were found in 1 of 9 DM1 patients and 1 of 4 controls. DM1 patients and controls had similar body mass indices. As expected, HbA1c percentage was significantly higher in patients with DM1 compared with controls (p < 0.01). The controls were slightly older than the patients (p < 0.01). In DM1 patients, fasting triglyceride and cholesterol concentrations were positively correlated to HbA1c percentage (p < 0.05). Fasting plasma triglyceride concentration was correlated with HbA1c, with the following equation: [fasting plasma triglyceride concentration] = 0.164[HbA1c]-0.794 (r = 0.80, p < 0.01). Fasting plasma cholesterol concentration was correlated with HbA1c with the following equation: [fasting plasma cholesterol concentration] = 0.45[HbA1c]-0.147 (r = 0.76, p < 0.05).
Postprandial response: glucose, FFA, and triglycerides.
Fasting and postprandial glucose concentrations were significantly higher in patients with diabetes compared with controls (p < 0.01, Fig. 1 A). In patients with diabetes, maximal glucose concentrations were found at 2 h after ingestion of the high-fat meal. FFA concentrations were significantly higher in patients with diabetes compared with controls, before (time point 0) and at 1 and 2 h after administration of the test-meal (p < 0.01), but were similar thereafter (Fig. 1 B).
Fasting and postprandial plasma glucose concentrations (A) and FFA concentrations (B) in late teenagers with diabetes mellitus type 1 (n = 9, closed symbols), and nondiabetic controls (n = 4, open symbols), before and for 6 h after ingestion of a fat-rich meal at time point 0. Insulin dosage was adjusted for the carbohydrate composition of the test-meal, the glucose concentration at the beginning of the test, and the fasting period of 6 h after the test-meal. *p < 0.05, reflects a significant difference between the two groups.
Postprandial triglyceride concentrations showed maximal values at 2 h (DM1) or 3 h (controls) after ingestion of the test-meal (Fig. 2 A). Fasting and postprandial triglyceride concentrations were not significantly different between the two groups at any time point. Also, the rate of decrease in triglyceride concentration, after reaching its maximum, was not significantly different between the two groups, as calculated from individual decay regression lines (p > 0.05; slope of regression line in DM1, and controls −0.32 ± 0.19, and −0.14 ± 0.08 mmol L−1 h−1, respectively). If postprandial triglyceride concentrations were compared between DM1 patients with high and moderate HbA1c (Fig. 2 B), the patients with high HbA1c (>9.5%) had significantly higher plasma triglyceride concentrations at time point 2 and 6 h compared with the patients with moderate HbA1c (<8.7%)(p < 0.05). Yet, these significant differences disappeared when the percentage increase in triglyceride concentration from time point 0 was calculated; indicating that higher postprandial triglyceride concentrations in DM1 patients with HbA1c > 9.5% were due to higher fasting triglyceride concentrations.
Fasting and postprandial plasma triglyceride concentrations in late teenagers with diabetes mellitus type 1 (n = 9, closed symbols), in nondiabetic controls (n = 4, open symbols) (A), and in DM1 patients with HbA1c > 9.5% (n = 4, closed symbols) and HbA1c < 8.7% (n = 5, open symbols) (B). A fat-rich meal was ingested at time point 0. Afterward no food was taken for 6 h. Results between the total diabetes and control group were not significantly different at any time point (A). In DM1 patients with HbA1c > 9.5% plasma triglyceride concentrations were significantly higher at time point 2 and 6 h compared with DM1 patients with HbA1c < 8.7% (*p < 0.05) (B).
Vitamin A test.
Figure 3 shows that in diabetic and in control late teenagers, mean concentrations of retinyl palmitate were maximal at 2 h after the ingestion of the vitamin A-containing high-fat meal. Except for the 6-h time point, no significant differences were observed between the two groups in plasma concentrations of retinyl palmitate. The areas under the curve were 7.0 ± 3.1 and 8.9 ± 2.9 in DM1 patients and controls, respectively (p > 0.05). Based on individual measurements, the rates of decrease in retinyl palmitate concentrations, after reaching its maximum, were not significantly different between the 2 groups (p > 0.05; slope of regression line in DM1, and controls, −0.67 ± 0.45, and −0.75 ± 0.37 μmol L−1 h−1, respectively).
Plasma retinyl palmitate concentrations before and for 6 h after ingestion of a fat-rich meal and retinyl palmitate in late teenagers with diabetes mellitus type 1 (n = 9, closed symbols) and nondiabetic controls (n = 4, open symbols). *p < 0.05 reflects a significant difference between the two groups.
13C-oleic acid test.
Whereas the vitamin A test mainly reflects the uptake of CM remnants in the liver, the 13C-oleic acid test provides information, at least theoretically, on the lipolysis of CM-triglycerides. In case of a decreased activity of lipoprotein lipase during insulin deficiency, one would expect increased plasma 13C-oleic acid concentrations, a delayed disappearance of 13C-oleic acid from plasma, and a delayed appearance of 13CO2 in breath. However, plasma 13C-oleic acid concentrations were not significantly different between DM1 and control late teenagers after ingestion of 13C-oleic acid together with the high-fat meal (p > 0.05, Fig. 4 A). Unlike retinyl palmitate concentrations (Fig. 3), plasma 13C-oleic acid concentrations did not reach maximal values at time point 2 h, but rather continuously increased throughout the 6 h study period.
(A) Plasma 13C-oleic acid concentrations before and for 6 h after ingestion of 13C-oleic acid and a fat-rich meal in late teenagers with diabetes mellitus type 1 (n = 9, closed symbols) and nondiabetic controls (n = 4, open symbols). Results between the two groups were not significantly different at any time point. (B) Breath 13CO2 concentration before and for 6 h after ingestion of 13C-oleic acid and a fat-rich meal in late teenagers with diabetes mellitus type 1 (n = 9, closed symbols) and nondiabetic controls (n = 4, open symbols). Results between the two groups were not significantly different at any time point.
Detection of 13CO2 in breath samples implies oxidation of the administered 13C-oleic acid, indicating lipolysis of CM-triglycerides and cellular uptake of 13C-FFA. Increase in breath 13CO2 concentrations was not delayed in DM1 patients, and breath 13CO2 concentrations were not significantly different between the 2 groups at any time point (Fig. 4 B). Breath 13CO2- and plasma 13C-oleic acid concentrations appeared significantly correlated (r = 0.93, p < 0.01; data not shown).
Chylomicron clearance rate related to HbA1c, gender, and fasting triglycerides.
CM clearance in DM1 patients in poor metabolic control (arbitrarily chosen at HbA1c > 9.5%) was not delayed in any of the three tests (triglycerides, vitamin A, or 13C-oleic acid), compared either with DM1 patients with moderate good metabolic control (HbA1c < 8.7%) or with nondiabetic controls (data not shown). When the slope of the regression lines of triglyceride clearance and of retinyl palmitate clearance were plotted against HbA1c, no significant correlation was found (p > 0.05; r = 0.10, r = 0.12, respectively). Also, the area under the retinyl palmitate curves were not significantly correlated with HbA1c (r = 0.22, p > 0.05). There was no significant difference between boys or girls with DM1 in CM clearance rates for any of the three tests applied and no significant correlation was found between CM clearance rates and fasting triglyceride concentrations.
DISCUSSION
In the present study we aimed to identify if a delayed CM clearance rate, as a known risk factor for atherosclerosis, is present in late teenagers with DM1 and if a delayed CM clearance could contribute to the higher plasma lipid concentrations in DM1 teenagers in poor control. In DM1 patients, fasting triglyceride and cholesterol concentrations were positively correlated with the level of metabolic control, as characterized by HbA1c. Our results show that the clearance rate of CM was not delayed in late teenagers with DM1 compared with controls. CM clearance rate was not related with either metabolic control (HbA1c), gender, fasting cholesterol, or triglyceride concentrations in late teenagers with DM1, indicating that relatively higher triglyceride and cholesterol concentrations in late teenagers with DM1 cannot be attributed to a delayed clearance of chylomicrons.
The observed correlation between HbA1c and high plasma triglyceride and cholesterol concentrations is in agreement with other studies in children and late teenagers with DM1 (7–9, 29). The pathophysiological mechanisms of elevated lipid concentrations in diabetes type 1 are not fully understood. It has been hypothesized that lipid absorption from the intestine is increased in DM1. Studies in streptozotocin-induced diabetic rats showed an increased intestinal triglyceride production (30) and cholesterol synthesis (31) and an increased intestinal absorption of cholesterol (32). Another possible mechanism is an increased hepatic VLDL production, as a consequence of increased free-fatty acid release from adipose tissue and as a consequence of inefficient suppression of hepatic VLDL release by insulin. In poorly controlled adult patients with DM1 the antilipolytic effect of insulin is diminished, leading to a higher free-fatty acid flux from adipose tissue (33). Hepatic VLDL release in patients with type 2 diabetes was less inhibited by insulin compared with control subjects (34). Finally, a delayed metabolism and clearance of lipoproteins could lead to hypertriglyceridemia and hypercholesterolemia. A delayed chylomicron and VLDL clearance has been identified in patients with type 2 diabetes and in adult patients with DM1 (11, 12) and this could play a role in hypertriglyceridemia. Increased production of LDL from elevated VLDL and delayed clearance of (glycosylated) LDL could lead to hypercholesterolemia (35, 36). A reduced hepatic uptake of LDL particles from patients with type 2 diabetes was associated with an altered lipid composition of the LDL particle and glycosylation of LDL protein (36). Insulin stimulates the hydrolysis of CM and VLDL triglycerides by lipoprotein lipase (37) and the hepatic uptake of chylomicron-, VLDL-remnants, and LDL in the liver, probably by stimulating the LDL receptor (38, 39).
In the present study we investigated the possibility that a delayed CM clearance rate is a possible contributor to the higher lipid values in diabetes in poor control. CM metabolism was studied by analyzing plasma disappearance of three different CM constituents. None of the three tests applied indicated significant differences in CM clearance between late teenagers with diabetes and controls, nor between DM1 boys or girls, nor between fairly or poorly controlled DM1 patients. Present data indicates that in patients with DM1, at this late teenager age, a delayed CM clearance rate is not an important atherosclerotic risk factor and does not contribute to the relatively higher triglyceride concentrations in late teenagers with DM1 in poor metabolic control. It therefore remains to be determined whether the observed correlation between metabolic control and triglyceride concentrations in late teenagers with diabetes is caused by one of the other hypothesized mechanisms, like increased hepatic VLDL production.
Several confounders could have influenced the outcome of the study. The relatively small study group could have confounded the results, however, because none of the three tests showed a trend toward delayed CM metabolism in DM1 patients, this possibility seems less likely. Due to the relatively small numbers of patients studied, we cannot exclude the possibility that a small difference in CM clearance exists, yet the clinical consequence of such a finding would then be questionable. Remarkably, studies performed in adult patients with type 1 or type 2 diabetes, who did show a difference in CM clearance rates, were based on patients studied comparable to the present study (12, 40).
It can be excluded that an acutely deteriorated metabolic control during the test confounded the results. The patients with diabetes did express a relative insulin deficiency compared with the nondiabetic controls, because fasting and postprandial glucose concentrations were increased in the former (Fig. 1 A). Postprandial FFA concentrations decreased in both groups and were not significantly different between both groups from time point 3 h after ingestion of the fat-rich meal, indicating that during the test-meal, insulin levels were sufficient to inhibit lipolysis. It could be possible that the metabolic control of the DM1 patients was better during the test day compared with their daily practice, because of regular glucose control and adapted insulin concentrations. However, insulin concentrations were calculated according to the dosages the patients normally injected themselves, and this adaptation of insulin dosage (based on the normally injected insulin dosage) was comparable with other studies (12, 41). It is therefore unlikely that “over-regulation” has confounded our present study. Finally, postprandial lipoprotein metabolism is highly dependent on fasting plasma triglyceride concentration (42), and in the presented study group none of the subjects had fasting triglyceride concentrations > 1.7 mmol/L. However, studies in normotriglyceridemic patients with type 2 diabetes and adults with DM1 did show a delayed postprandial chylomicron clearance (12, 43).
The presented results indicate that lipoprotein lipase activity and uptake of CM remnants in the liver are sufficiently stimulated in late teenagers in the diabetic state. The observed difference between the present results in late teenagers with DM1 (no delayed CM clearance rate) and previous data in adults with DM1 (delayed CM clearance rate) could be due to a worse metabolic regulation. Georgopoulos and Phair studied only adults with DM1 in poor control (HbA1 12.8 ± 0.6%; normal range: 3.9–7.7%) (12), whereas in our study DM1 patients were in fair or poor control (mean HbA1c 9.2 ± 1.9%; normal range: 4.6–6.1%). Comparison of metabolic regulation (HbA1c) between the two studies is difficult however, because in the first study HbA1 percentages were presented instead of HbA1c, and HbA1 was measured by agarose gel electrophoresis rather than by HPLC (44). Another explanation for the difference in CM clearance rate could be the age dependency of CM clearance. Studies in healthy individuals using the vitamin A test have shown that CM clearance rate delays with increasing age (18). It is tempting to speculate that DM1 patients do have an age dependency in CM clearance, similarly to subjects without DM1. However, the age-dependent delay in CM clearance rate is more pronounced in the former.
In conclusion, the present study showed that higher lipid concentrations in late teenagers with DM1 in poor control were not caused by a delay in CM clearance rate. Present data indicate that a delayed CM clearance rate at late teenager age is not a risk factor contributing to the increased risk for atherosclerosis in DM1.
Abbreviations
- DM1:
-
diabetes mellitus type 1
- LDL:
-
low density lipoprotein
- VLDL:
-
very low density lipoprotein
- CM:
-
chylomicron
- HDL:
-
high density lipoprotein
- BW:
-
body weight
- HPLC:
-
high pressure liquid chromatography
- GC:
-
gas chromatography
- GC-C-IRMS:
-
gas chromatography-combustion isotope ratio mass spectrometry
References
Kannel WB, McGee DL 1979 Diabetes and cardiovascular disease. The Framingham Study. JAMA 241: 2035–2038
Brownlee M, Cerami A, Vlassara H 1988 Advanced glycosylation end products in tissue and the biochemical basis of diabetic complications. N Engl J Med 318: 1315–1321
Borch-Johnsen K, Kreiner S 1987 Proteinuria: value as predictor of cardiovascular mortality in insulin dependent diabetes mellitus. BMJ Clin Res Ed 294: 1651–1654
Pietri A, Dunn FL, Raskin P 1980 The effect of improved diabetic control on plasma lipid and lipoprotein levels: a comparison of conventional therapy and continuous subcutaneous insulin infusion. Diabetes 29: 1001–1005
Patti L, Romano G, Di Marino L, Annuzzi G, Mancini M, Riccardi G, Rivellese AA 1993 Abnormal distribution of VLDL subfractions in type 1 (insulin-dependent) diabetic patients: could plasma lipase activities play a role?. Diabetologia 36: 155–160
Winocour PH, Durrington PN, Ishola M, Anderson DC 1986 Lipoprotein abnormalities in insulin-dependent diabetes mellitus. Lancet 1: 1176–1178
Azad K, Parkin JM, Court S, Laker MF, Alberti KG 1994 Circulating lipids and glycaemic control in insulin dependent diabetic children. Arch Dis Child 71: 108–113
Virtanen SM, Rasanen L, Virtanen M, Sippola H, Rilva A, Kaprio EA, Maenpaa J, Akerblom HK 1993 Associations of serum lipids with metabolic control and diet in young subjects with insulin-dependent diabetes mellitus in Finland. Eur J Clin Nutr 47: 141–149
Abraha A, Schultz C, Konopelska-Bahu T, James T, Watts A, Stratton IM, Matthews DR, Dunger DB 1999 Glycaemic control and familial factors determine hyperlipidaemia in early childhood diabetes. Oxford Regional Prospective Study of Childhood Diabetes. Diabet Med 16: 598–604
Groot PH, van Stiphout WA, Krauss XH, Jansen H, van Tol A, van Ramshorst E, Chin-On S, Hofman A, Cresswell SR, Havekes L 1991 Postprandial lipoprotein metabolism in normolipidemic men with and without coronary artery disease. Arterioscler Thromb 11: 653–662
De Man FH, Cabezas MC, Van Barlingen HH, Erkelens DW, de Bruin TW 1996 Triglyceride-rich lipoproteins in non-insulin-dependent diabetes mellitus: post-prandial metabolism and relation to premature atherosclerosis. Eur J Clin Invest 26: 89–108
Georgopoulos A, Phair RD 1991 Abnormal clearance of postprandial Sf 100–400 plasma lipoproteins in insulin-dependent diabetes mellitus. J Lipid Res 32: 1133–1141
Zilversmit DB 1979 Atherogenesis: a postprandial phenomenon. Circulation 60: 473–485
Patsch JR, Miesenbock G, Hopferwieser T, Muhlberger V, Knapp E, Dunn JK, Gotto AM, Patsch W 1992 Relation of triglyceride metabolism and coronary artery disease. Studies in the postprandial state. Arterioscler Thromb 12: 1336–1345
Williams CM 1998 Dietary interventions affecting chylomicron and chylomicron remnant clearance. Atherosclerosis 141: S87–92
Garg A 1998 High-monounsaturated-fat diets for patients with diabetes mellitus: a meta-analysis. Am J Clin Nutr 67: 577S–582S
Georgopoulos A, Bantle JP, Noutsou M, Swaim WR, Parker SJ 1998 Differences in the metabolism of postprandial lipoproteins after a high- monounsaturated-fat versus a high-carbohydrate diet in patients with type 1 diabetes mellitus. Arterioscler Thromb Vasc Biol 18: 773–782
Krasinski SD, Cohn JS, Schaefer EJ, Russell RM 1990 Postprandial plasma retinyl ester response is greater in older subjects compared with younger subjects. Evidence for delayed plasma clearance of intestinal lipoproteins. J Clin Invest 85: 883–892
Cohn JS, McNamara JR, Cohn SD, Ordovas JM, Schaefer EJ 1988 Postprandial plasma lipoprotein changes in human subjects of different ages. J Lipid Res 29: 469–479
Arslanian SA, Kalhan SC 1994 Correlations between fatty acid and glucose metabolism. Potential explanation of insulin resistance of puberty. Diabetes 43: 908–914
Berr F, Kern F 1984 Plasma clearance of chylomicrons labeled with retinyl palmitate in healthy human subjects. J Lipid Res 25: 805–812
Wilson DE, Chan IF, Ball M 1983 Plasma lipoprotein retinoids after vitamin A feeding in normal man: minimal appearance of retinyl esters among low-density lipoproteins. Metabolism 32: 514–517
Bui MH 1994 Simple determination of retinol, alpha-tocopherol and carotenoids (lutein, all-trans-lycopene, alpha- and beta-carotenes) in human plasma by isocratic liquid chromatography. J Chromatogr B Biomed Appl 654: 129–133
Zaman Z, Fielden P, Frost PG 1993 Simultaneous determination of vitamins A and E and carotenoids in plasma by reversed-phase HPLC in elderly and younger subjects. Clin Chem 39: 2229–2234
Lepage G, Roy CC 1986 Direct transesterification of all classes of lipids in a one-step reaction. J Lipid Res 27: 114–120
Muskiet FA, van Doormaal JJ, Martini IA, Wolthers BG, van der Slik W 1983 Capillary gas chromatographic profiling of total long-chain fatty acids and cholesterol in biological materials. J Chromatogr 278: 231–244
Minich DM, Kalivianakis M, Havinga R, Van Goor H, Stellaard F, Vonk RJ, Kuipers F, Verkade HJ 1999 Bile diversion in rats leads to a decreased plasma concentration of linoleic acid which is not due to decreased net intestinal absorption of dietary linoleic acid. Biochim Biophys Acta 1438: 111–119
Anonymous 1992 National Cholesterol Education Program (NCEP): highlights of the report of the Expert Panel on Blood Cholesterol Levels in Children and Adolescents. Pediatrics 89: 495–501
Lopes-Virella MF, Wohltmann HJ, Loadholt CB, Buse MG 1981 Plasma lipids and lipoproteins in young insulin-dependent diabetic patients: relationship with control. Diabetologia 21: 216–223
Popper DA, Shiau YF, Reed M 1985 Role of small intestine in pathogenesis of hyperlipidemia in diabetic rats. Am J Physiol 249: G161–167
Feingold KR, Wilson DE, Wood LC, Kwong LK, Moser AH, Grunfeld C 1994 Diabetes increases hepatic hydroxymethyl glutaryl coenzyme A reductase protein and mRNA levels in the small intestine. Metabolism 43: 450–454
Young NL, McNamara DJ, Saudek CD, Krasovsky J, Lopez DR, Levy G 1983 Hyperphagia alters cholesterol dynamics in diabetic rats. Diabetes 32: 811–819
Jensen MD, Caruso M, Heiling V, Miles JM 1989 Insulin regulation of lipolysis in nondiabetic and IDDM subjects. Diabetes 38: 1595–1601
Malmstrom R, Packard CJ, Caslake M, Bedford D, Stewart P, Yki-Jarvinen H, Shepherd J, Taskinen MR 1997 Defective regulation of triglyceride metabolism by insulin in the liver in NIDDM. Diabetologia 40: 454–462
Howard BV 1987 Lipoprotein metabolism in diabetes mellitus. J Lipid Res 28: 613–628
Kramer-Guth A, Quaschning T, Galle J, Baumstark MW, Koniger M, Nauck M, Schollmeyer P, Marz W, Wanner C 1997 Structural and compositional modifications of diabetic low-density lipoproteins influence their receptor-mediated uptake by hepatocytes. Eur J Clin Invest 27: 460–468
Taskinen MR 1992 Quantitative and qualitative lipoprotein abnormalities in diabetes mellitus. Diabetes 41: 12–7
Cooper AD 1997 Hepatic uptake of chylomicron remnants. J Lipid Res 38: 2173–2192
Wade DP, Knight BL, Soutar AK 1988 Hormonal regulation of low-density lipoprotein (LDL) receptor activity in human hepatoma Hep G2 cells. Insulin increases LDL receptor activity and diminishes its suppression by exogenous LDL. Eur J Biochem 174: 213–218
Syvanne M, Hilden H, Taskinen MR 1994 Abnormal metabolism of postprandial lipoproteins in patients with non-insulin-dependent diabetes mellitus is not related to coronary artery disease. J Lipid Res 35: 15–26
Frayn KN, Coppack SW, Humphreys SM, Clark ML, Evans RD 1993 Periprandial regulation of lipid metabolism in insulin-treated diabetes mellitus. Metabolism 42: 504–510
Lewis GF, O'Meara NM, Soltys PA, Blackman JD, Iverius PH, Pugh WL, Getz GS, Polonsky KS 1991 Fasting hypertriglyceridemia in noninsulin-dependent diabetes mellitus is an important predictor of postprandial lipid and lipoprotein abnormalities. J Clin Endocrinol Metab 72: 934–944
Mero N, Syvanne M, Taskinen MR 1998 Postprandial lipid metabolism in diabetes. Atherosclerosis 141: S53–5
Weykamp CW, Penders TJ, Muskiet FA, van der Slik W 1993 Glycohaemoglobin: comparison of 12 analytical methods, applied to lyophilized haemolysates by 101 laboratories in an external quality assurance programme. Ann Clin Biochem 30: 169–174
Acknowledgements
The authors thank Henk Elzinga for his skillful contributions to GC-C-IRMS measurements.
Author information
Authors and Affiliations
Corresponding author
Additional information
Supported by an unrestricted grant from the Suikerstichting Nederland and by a Starter's Grant from the Department of Pediatrics, University Hospital Groningen. HJ Verkade is a Fellow of the Royal Netherlands Academy for Arts and Sciences.
Rights and permissions
About this article
Cite this article
van Waarde, W., Odink, R., Rouwé, C. et al. Postprandial Chylomicron Clearance Rate in Late Teenagers with Diabetes Mellitus Type 1. Pediatr Res 50, 611–617 (2001). https://doi.org/10.1203/00006450-200111000-00013
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1203/00006450-200111000-00013