Abstract
Toluene inhalant abuse during pregnancy may result in growth-retarded microcephalic newborns who subsequently demonstrate developmental impairment. By using a rat model of toluene-abuse embryopathy, we studied the effects of prenatal toluene exposure on the generation and migration of cortical neurons. Dams were exposed by gavage to either corn oil or toluene diluted in corn oil on d 6–21 of gestation. The time of origin of cortical neurons was determined in the mature pups of dams injected with the thymidine analogue 5′-bromodeoxyuridine on 1 d during the period from d 13–21 of gestation. 5′-Bromodeoxyuridine–labeled neurons were identified by immunohistochemistry in a 400-μm-wide column of somatosensory cortex. The brains of the toluene-exposed pups had a significant reduction in the number of neurons within each cortical layer (p < 0.001). Depending on the cortical layer, the generation of neurons in the toluene-exposed pups was delayed by 1 or 2 d. In addition, the brains of the toluene-exposed pups also showed evidence of abnormal neuronal migration. However, there were no differences in either brain weight or body weight between the control and toluene-exposed pups. These observations suggest that although prenatal toluene exposure results in abnormal neuronal proliferation and migration, brain weight in the toluene-exposed pups may be preserved by enhanced development of glia or the neuropil.
Similar content being viewed by others
Main
The intentional inhalation of organic solvents (inhalant abuse) is a common form of substance abuse, and a large number of abusers are adolescent and young adult women (1, 2). The aromatic hydrocarbon toluene is a component of commonly abused commercial products, and some abusers prefer to expose themselves to pure toluene (3). The fetus of a pregnant toluene abuser is also exposed to this substance, and several cases of clinical toluene-abuse embryopathy have been reported (4–10). These growth-retarded microcephalic children have a variety of nonspecific dysmorphic features as well as developmental delay, cerebellar dysfunction, language impairment, and hyperactivity. To study the neurologic and systemic teratologic effects of this solvent, our laboratory has developed a rat model of toluene-abuse embryopathy (11–13). Pregnant dams were exposed to daily gavage doses of toluene; their fetuses, examined on E19, demonstrated generalized growth retardation, and biochemical indices of brain development indicated that the forebrain contained fewer nuclei (lower DNA content). However, these changes were not found to be permanent. Prenatally toluene-exposed pups examined at P21 did not demonstrate differences in systemic growth or in forebrain DNA content when compared with control animals, suggesting that catch-up growth occurred.
Although the rat brain undergoes significant development during the first 3 wk of postnatal life, neurogenesis within the forebrain is essentially complete before birth with only the dentate granule cells originating after birth, primarily during the first postnatal week (14). Therefore, although the reduced number of forebrain nuclei secondary to prenatal toluene exposure resolved by P21, it is possible that prenatal toluene exposure results in a permanent reduction in the number of forebrain neurons and that the catch-up growth was due to enhanced glial development. To help answer this question, the present study was designed to evaluate the effects of prenatal toluene exposure on development of the cytoarchitecture of the rat cerebral cortex. For this study, neurogenesis was evaluated by labeling dividing neuroblasts on a specific day of gestation with BrdU (15, 16). In this manner, the effects of prenatal toluene exposure on neuronal proliferation and subsequent migration through the developing cortex could be assessed.
METHODS
Breeding of animals.
The procedures used in this study were approved by the Animal Use and Care Administrative Advisory Committee of the University of California, Davis. Animal care and breeding procedures were identical to those used in our previous studies (11–13). Specifically, female Sprague-Dawley rats (160–180 g) were purchased from Simonsen Laboratories, Inc. (Gilroy, CA) and housed in wire mesh cages in a temperature-controlled room at 22°C and under a 12-h light/dark cycle. The animals were provided with water and a diet consisting of dextrose, 62%; casein, 24%; corn oil, 8%; mineral mix, 6%; and vitamin mix ad libitum (17, 18). For breeding, male rats obtained from the same vendor were placed with the females overnight. The presence of copulation plugs the following morning was taken as evidence of successful mating, and that day was designated as E0.
Exposure to toluene.
Pregnant dams in the treatment group were exposed to toluene by gavage administration. From our previous work, we demonstrated that a specific oral dose of toluene will simulate a prolonged inhalation exposure (19). For these studies, a 650 mg/kg body weight gavage dose of toluene, which simulates a 4162-ppm 3-h inhalation exposure, was used. Analytical grade toluene was dissolved in corn oil to give a final concentration of 650 mg/mL, and the animals in the treatment group received 1 mL/kg body weight of dosing solution per day on E6–21. Control dams received daily gavage doses of corn oil, 1 mL/kg body weight.
Treatment with BrdU.
During gestation, each dam received a single intraperitoneal injection of BrdU (Sigma Chemical Co.) in normal saline, 50 μg/g body weight. Four dams, two control and two toluene-treated, received a BrdU injection on one of the 9 d of gestation between E13 and E21. Therefore, a total of 36 dams were used for the study (18 control and 18 toluene-treated). Developing neurons that are undergoing mitosis at the time of the BrdU injection will incorporate BrdU into their DNA. Cells that continue to divide will have the BrdU label within their nucleus diluted to background levels. Therefore, neurons that were generated on the day of gestation when the dam received BrdU will be labeled with BrdU (15, 16).
Delivery, postnatal care, and preparation of histologicspecimens.
The dams were allowed to deliver their pups, and the day of delivery was denoted as P0. The pups were weaned on P21 at which time they were weighed and then killed by CO2 vapor inhalation. The brains were removed and weighed, and then the brains from two male and two female pups per litter were submitted for histologic evaluation. The tissue was fixed in methanol:chloroform:glacial acetic acid (6:3:1) for 24 h. After fixation, the brains were embedded in paraffin. Serial coronal sections 10-μm thick were cut, and sections corresponding to level 8.8 of the atlas of Pellegrino et al. (20) were affixed to Superfrost/Plus glass slides (Fisher, Pittsburgh, PA). To be able to easily identify both BrdU-positive and BrdU-negative neurons within these sections, a double labeling immunohistochemical technique was developed (21). Sections were prepared to demonstrate immunoreactivity for both BrdU and NeuN (22). Briefly, the sections were treated with anti-BrdU monoclonal mouse antibody (Dako, Carpenteria, CA); anti-NeuN monoclonal mouse antibody (Dr. R.J. Mullen, Salt Lake City, UT); biotinylated anti-mouse IgG (Vector, Burlingame, CA); avidin-biotin-peroxidase complex (Vector) and 3–3′diaminobenzidine (DAB) substrate for peroxidase with nickel chloride (Vector) for BrdU staining; and avidin-biotin-alkaline phosphatase (Vector) and alkaline phosphatase substrate Vector-Red (Vector) for NeuN staining. BrdU-positive neurons demonstrated dark brown to black immunoreactivity within the nucleus together with some red immunoreactivity (NeuN) within the cytoplasm of the soma. BrdU-negative neurons demonstrated red immunoreactivity within the nucleus and the cytoplasm of the soma. Glia and vascular elements were unstained.
Microscopic analysis of somatosensory cortex cytoarchitecture.
Cortical cell columns 400-μm wide in somatosensory cortex, region Par 1 as defined by Ziles and Wree (23), were selected for study, and two cortical columns per pup were examined. Tissue from one control pup from a dam that received BrdU on E13 could not be examined. Therefore, for the entire study, the brains from 143 pups (71 control and 72 toluene-treated) were analyzed. Sections were examined with an Olympus BHT microscope (Tokyo, Japan) fitted with a video camera. Each 400-μm-wide field was digitized with a personal computer and then analyzed with SigmaScan Pro software (Jandel Scientific, San Rafeal, CA). Cortical laminae II through VI were identified, and the total number of neurons per lamina (NeuN immunoreactive) together with the total number of BrdU-positive neurons per lamina was counted.
A disadvantage of the BrdU labeling method compared with [3H]-thymidine autoradiography is that it is difficult to discriminate between heavily labeled and lightly labeled cells. Therefore, some second and third generation cells in which the nuclear label has not been diluted completely to background levels were necessarily counted as BrdU positive. The advantages of BrdU immunohistochemistry are the rapid preparation of tissue specimens (several days rather than 1–3 mo) and the decreased expense with no need for darkroom processing (15). The initial report of the use of BrdU labeling to study the development of cortical cytoarchitecture noted that a disadvantage of the method was the reduced ability to identify the morphologic type of cell that was BrdU positive (15), but this problem was solved by developing the double labeling method using both BrdU and NeuN (21).
Data presentation and analysis.
Litter size, mean pup weight, and mean brain weight were determined for each of the 36 litters (18 control and 18 toluene-treated). The following values were obtained from each of the nine BrdU injection groups (E13–21) for both the control and toluene-treated animals: number of neurons (NeuN-positive cells) per cortical column (an index of neuronal packing density), number of neurons (NeuN-positive cells) for each cortical lamina, number of BrdU-positive neurons per cortical column, and number of BrdU-positive neurons for each cortical lamina. The number of neurons per cortical column and the number of neurons per cortical lamina for each pup analyzed were pooled for the control group and toluene-treated group. To evaluate the number of neurons generated on a particular day of gestation, the mean of the BrdU-positive neurons counted for each of the eight pups per BrdU injection group was determined (with the exception of the control E13 group, which included only seven pups). All numeric data are presented as mean ± SD. One-way ANOVA was used to compare the control and toluene-treated groups. A p value < 0.05 was selected for determining statistical significance. For the statistical analysis of the effects of toluene treatment on litter size, body weight, and brain weight, the litter was selected as the statistical unit. Because brain tissue from only four pups per litter was processed for immunohistochemistry, the pup was selected as the statistical unit for the analysis of the effects of toluene treatment on the number of neurons per cortical column and the number of neurons per cortical lamina.
RESULTS
Maternal exposure to toluene during d 6–21 of gestation did not affect litter size. Litter size in the control groups was 9.4 ± 2.8 pups, whereas the toluene-exposed group had 8.2 ± 2.8 pups per litter (F1, 34 = 2.61, NS). Prenatal toluene exposure did not affect either body weight or brain weight of the pups evaluated at 21 d of life. The mean pup weight per litter of the control pups was 42.68 ± 4.72 g, whereas the toluene-exposed group weighed 40.09 ± 4.90 g (F1, 34 = 2.03, NS). The mean brain weight per litter of the control group was 1.27 ± 0.06 g and that of the toluene-exposed group was 1.24 ± 0.06 g (F1, 34 = 1.72, NS).
Although toluene exposure did not affect litter size or the pup brain and body weights, prenatal toluene exposure did result in several changes in the cytoarchitecture of somatosensory cortex of the mature pups (Table 1). The total number of neurons in the entire 400-μm-wide cortical column (an index of neuronal packing density) was reduced by 12.6% in the toluene-exposed pups (p < 0.001). This reduction in neuronal packing was present in each cortical lamina, with the greatest reduction in layer IV (26.8%, p < 0.001).
BrdU-labeled neurons were easily identified in all sections examined. Gestational exposure to toluene resulted in several alterations in neurogenesis and neuronal migration. The cumulative number of BrdU-labeled neurons was substantially reduced in the toluene-exposed pups (Fig. 1). This reduction was noted in each cortical lamina, with the greatest reduction noted in laminae IV and VI (both 52%, data not shown).
Effect of prenatal exposure to toluene on the daily generation of neurons within somatosensory cortex. The cumulative number of neurons labeled by a single injection of BrdU given between E13 and E21 is plotted against the day of gestation. Prenatal toluene exposure resulted in a 43% reduction in the number of BrdU-labeled neurons.
Prenatal toluene treatment also affected the timing of neurogenesis. Depending upon the cortical lamina, the onset of neurogenesis was delayed by 1 to 2 d (Fig. 2). This was most apparent in the deeper layers (V and VI). Despite this delay in the onset of neurogenesis, prenatal toluene exposure did not prolong the overall course of neuronal generation. In both the control and toluene-treated animals, neurogenesis was substantially complete by gestational E19.
Effect of prenatal toluene exposure on the timing of neurogenesis in somatosensory cortex. For each cortical lamina, the number of BrdU-labeled neurons (neuronal birthdays) is plotted against the day of gestation. Depending upon the lamina, toluene exposure delayed neuronal generation by 1 to 2 d. For each lamina, the number of BrdU-labeled neurons was less in the toluene-exposed group, and toluene-exposure did not prolong the period of neuronal generation.
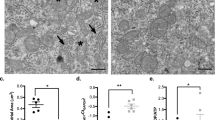
The generation of neurons within somatosensory cortex followed the expected inside-to-outside sequence, with layer VI neurons generated earlier and layer II-III neurons generated later during the period of E13 to E21 (Fig. 3). Although prenatal toluene exposure did not disturb the overall inside-to-outside gradient of neuronal generation, in particular the distribution of neurons generated on E15 was affected. In the toluene-treated pups, a substantial number of heterotopic BrdU-positive neurons were found within the white matter immediately below the column of somatosensory cortex (Fig. 4). By extending the 400-μm-wide column from cortical lamina VI down through the subcortical white matter, the number of BrdU-positive neurons within the white matter was counted. There were 48.8 ± 27.2 BrdU-labeled neurons within the white matter of the toluene-treated pups versus 16.3 ± 4.7 BrdU-positive neurons in the control group (F1, 14 = 11.1, p = 0.005). This pattern was present to a much lesser extent for neurons generated on E17 (3.9 ± 2.7 versus 0.9 ± 1.1;F1, 14 = 8.7, p = 0.01) but was not noted on any of the other days. On E14, the opposite pattern was found, with 3.1 ± 1.4 BrdU-positive neurons within the white matter of control animals and 0.9 ± 1.6 BrdU-positive neurons in the toluene-treated pups (F1, 14 = 8.4, p = 0.012).
Neuronal heterotopia within the subcortical white matter. Prenatal toluene exposure resulted in increased numbers of BrdU-labeled neurons labeled on E15 within the white matter. The upper and lower borders of the subcortical white matter are indicated by arrowheads. Control (A), toluene-exposed (B).
DISCUSSION
Children with toluene-abuse embryopathy are microcephalic and demonstrate significant clinical neurodevelopmental handicaps (4–10). This clinical teratologic syndrome shares some features with fetal alcohol syndrome (6), a condition that has been studied extensively both clinically and experimentally. Although neuropathologic specimens of patients with toluene-abuse embryopathy are not available, brains of patients with fetal alcohol syndrome have been evaluated, and marked alterations in cortical cytoarchitecture have been noted, including neuronal heterotopia and disrupted laminar organization (24). Although the mature brains of our toluene-exposed pups were not smaller than those of the control pups, prenatal toluene exposure did result in several abnormalities of cortical cytoarchitecture and neuronal generation. Previous cytoarchitectural studies of neuronal generation and migration using [3H]-thymidine labeling have demonstrated that prenatal ethanol exposure also disrupts the orderly pattern of neurogenesis (25–28). As the developmental effects of prenatal ethanol exposure were particularly apparent in somatosensory cortex (26, 29), we selected this region to study the effects of prenatal toluene exposure on cortical development.
When the results of our study are compared with those of experiments designed to examine the effects of prenatal ethanol exposure on neurogenesis, several differences in the effects of these two developmental toxins are apparent. The present experiment demonstrated that prenatal toluene exposure caused a delay in the onset of neurogenesis of somatosensory cortex and that the neuronal packing of the mature cortex was reduced. Although prenatal ethanol exposure also resulted in a delay in the onset of neuronal generation, a late surge in neuronal births was demonstrated (25, 27). In addition, although the normal inside-to-outside (radial) gradient of neuronal generation (14) was apparent in the toluene-treated animals, prenatal ethanol treatment disrupted this gradient. Specifically, the late surge of neuronal generation seen in the brains of the ethanol-treated pups resulted in mature neurons that were located aberrantly throughout the deeper laminae of the cortical column rather than in the superficial layers (25, 27). This resulted in a decrease in the number of neurons in lamina II and an increase in the number of neurons in each of the deeper laminae (25). In subsequent studies, it was demonstrated that prenatal ethanol exposure resulted in a miniaturization of the brain. The microcephalic brains from ethanol-treated pups had fewer neurons, but there were no difference in either the laminar volumes or neuronal packing density (29). Although the somatosensory cortex of the brains of the toluene-exposed pups also contained fewer neurons, these brains were not microcephalic. This suggests that brain size must have been preserved by some compensatory mechanism such as glial hyperplasia or hypertrophy of glia and the neuropil. Such cellular changes have been shown in the brains of rats exposed to ethanol during certain periods of development. Specifically, in rats exposed to ethanol prenatally, increases in both glial somatic volume and neuropil volume have been demonstrated (29), whereas postnatal ethanol exposure during the developmental period of the brain growth spurt (30) resulted in a transient astrogliosis (31).
Similar to the reduction in somatosensory cortex neuronal number induced by prenatal toluene exposure seen in the present study, inhalation toluene exposure during P1–28 (100 or 500 ppm for 12 h/d) has been shown in pups examined on P28 to result in a reduction of the volume of the dentate granule cell layer of the hippocampus, whereas other hippocampal layers were unaffected (32). However, in animals that were killed on P120, these effects were no longer present, suggesting late postnatal compensatory growth of dentate granule cells (33). In the rat, the dentate granule cells undergo neurogenesis and postmitotic differentiation during the period of exposure, whereas hippocampal pyramidal cells are relatively mature at the time of birth (14). Dentate granule cells continue to demonstrate some degree of neurogenesis and morphogenesis throughout the juvenile period (34). These observations together with the results from the present study suggest that, on a histologic level, the developmental toxicity of toluene is most apparent on neurons that undergo neurogenesis only during the interval of toluene exposure.
Few neuronal migrational abnormalities in somatosensory cortex were demonstrated in the pups exposed prenatally to toluene. Whereas prenatal exposure to ethanol resulted in extensive aberrant migration of late-generated neurons (15, 25), prenatal toluene exposure resulted in some degree of neuronal heterotopia with excessive BrdU-positive neurons labeled on E15 and E17 found within the white matter. Neurons labeled on E15 were located within each of the cortical laminae. Therefore, it is difficult to conclude the likely migrational destination of the numerous heterotopic neurons within the white matter of the E15 toluene-treated pups. Neurons labeled on E17 were generally located in the more superficial cortical laminae. Therefore, the heterotopic neurons within the white matter of the E17 pups may have resulted from failed neuronal migration to laminae II-III and IV. The greater number of BrdU-positive neurons within the white matter of the E14 control animals was likely due to the overall delay of the onset of neuronal generation in the toluene-treated animals. It has been demonstrated that prenatal exposure to ethanol results in premature transformation of radial glia into astrocytes (35). This has led to the suggestion that the aberrant migration of the late-generated neurons in brains of rats exposed prenatally to ethanol is secondary to the premature degradation of radial glial networks (35, 36). However, as prenatal toluene exposure resulted in the development of neuronal heterotopia somewhat earlier during the period of neurogenesis, another mechanism may underlie this abnormality in neuronal migration.
The pathophysiologic mechanisms by which toluene produces developmental abnormalities in the fetus as well as acute and chronic encephalopathy in abusers and after industrial intoxications are poorly understood. Due to its lipid solubility, this solvent is rapidly distributed to regions of the brain that have a high lipid content (37); this includes the developing CNS of the fetus (38). Clinically, in patients with chronic toluene encephalopathy, white matter atrophy, loss of oligodendroglia, and gliosis were noted at autopsy (39). Therefore, toluene may have a direct neurotoxic effect on glia. Experimentally, toluene has been shown to either increase or decrease levels of specific glial markers, depending on the brain region studied. For example, glial acidic fibrillary protein levels were reduced in the thalami of adult rats exposed to 1000 ppm toluene for 6 h daily for 3 or 7 d (40), whereas a dose-dependent increase in the glial markers α-enolase, creatine kinase-B, and β-S100 protein, suggesting gliosis, was found particularly in the cerebella of rats exposed to up to 1000 ppm toluene for 8 h/d, 6 d/wk for 16 wk (41). Even though the toluene exposures in the above studies were administered to mature rats, these results lend support to the suggestion that glial hypertrophy or hyperplasia may compensate for the neuronal loss demonstrated in our study.
Toluene has also been shown to affect neurotransmitter systems and neuroendocrine function, particularly of the hypothalamic-pituitary-adrenocortical axis. For example, adult rats exposed via inhalation to toluene at 1000 ppm for 6 h/d for 4 d had increased median eminence norepinephrine levels and turnover (42). In another study, toluene administered in drinking water for 28 d to adult mice resulted in a dose-dependent increase in hypothalamic norepinephrine and vanillylmandelic acid levels along with increased levels of circulating adrenocorticotropic hormone and corticosterone (43). The toluene-induced increases in adrenocorticotropic hormone and corticosterone are of particular interest. Glucocorticoid receptor gene expression is first noted in the rat brain after E13 (44), a point during gestation when the animals in our study were exposed to toluene. It has been demonstrated that dexamethasone given to pregnant dams during late gestation produces a permanent increase in hepatic glucocorticoid receptor gene expression of the adult offspring as well as glucose intolerance (45). In another investigation, stress paradigms administered to adult rats led to a down-regulation of glucocorticoid receptors in hippocampus and cerebellum (46). Given that toluene exposure leads to elevated corticosterone levels, it is possible that some of the teratologic effects of toluene are due to a disruption of the maternal hypothalamic-pituitary-adrenocortical axis and an alteration of the development and function of fetal glucocorticoid receptors.
The primary effect of prenatal toluene exposure on the cytoarchitecture of somatosensory cortex is a diffuse reduction in neuronal number. This change could be due to a reduction in neuronal generation and/or enhanced neuronal apoptosis. These experiments have demonstrated that prenatal toluene exposure results in a delay and shortening of the period of neuronal generation as well as a reduction in cumulative generation of neurons, both of which could contribute to this reduction in neuronal number. Further studies of the effects of prenatal toluene exposure on the cellular organization of cortex will need to focus on glial development, the possible role of enhanced neuronal apoptosis, and the effects of this developmental toxin on other brain regions. As our previous work (12, 13) together with the present study demonstrated that prenatal toluene-induced generalized growth retardation (including microcephaly) resolved by P21, studies of the developmental effects of postnatal toluene exposure during the period of the brain growth spurt, the final days of gestation through the second week of postnatal life (30), are also needed to better characterize the teratogenic effects of this abused solvent on the CNS.
Abbreviations
- BrdU:
-
5′-bromodeoxyuridine
- NeuN:
-
neuronal nuclear antigen
- E:
-
embryonic day
- P:
-
postnatal day
References
Carroll E 1977 Notes on the epidemiology of inhalants. NIDA Res Monogr 15: 14–27
Padilla E, Padilla A, Morales A 1979 Inhalant, marihuana, and alcohol abuse among barrio children and adolescents. Int J Addict 14: 945–964
Comstock EG, Comstock BS 1977 Medical evaluation of inhalant abusers. NIDA Res Monogr 15: 54–80
Arnold GL, Kirby RS, Langendoerfer S, Wilkins-Haug L 1994 Toluene embryopathy: clinical delineation and developmental follow-up. Pediatrics 93: 216–220
Goodwin TM 1988 Toluene abuse and renal tubular acidosis in pregnancy. Obstet Gynecol 71: 715–718
Hersh JH, Podruch PE, Rogers G, Weisskopf B 1985 Toluene embryopathy. J Pediatr 106: 922–927
Hersh JH 1989 Toluene embryopathy: two new cases. J Med Genet 26: 333–337
Pearson MA, Hoyme HE, Seaver LH, Rimsza ME 1994 Toluene embryopathy: delineation of the phenotype and comparison with fetal alcohol syndrome. Pediatrics 93: 211–215
Streicher HZ, Gabow PA, Moss AH, Kono D, Kaehny WD 1981 Syndromes of toluene sniffing in adults. Ann Intern Med 94: 758–762
Toutant C, Lippmann S 1979 Fetal solvents syndrome. Lancet 1: 1356
Gospe SM Jr, Saeed DB, Zhou SS, Zeman FJ 1994 The effects of high-dose toluene on embryonic development in the rat. Pediatr Res 36: 811–815
Gospe SM Jr, Zhou SS, Saeed DB, Zeman FJ 1996 Development of a rat model of toluene-abuse embryopathy. Pediatr Res 40: 82–87
Gospe SM Jr, Zhou SS 1998 Toluene abuse embryopathy: longitudinal neurodevelopmental effects of prenatal exposure to toluene in rats. Reprod Toxicol 12: 119–126
Bayer SA, Altman HJ 1995 Neurogenesis and neuronal migration. In: Paxinos G (ed) The Rat Nervous System, 2nd Ed. Academic Press, San Diego, CA, pp 1041–1078
Miller MW, Nowakowski RS 1988 Use of bromodeoxyuridine-immunohistochemistry to examine the proliferation, migration, and time of origin of cells in the central nervous system. Brain Res 57: 44–52
Soriano E, Del Rio J 1991 Simultaneous immunocytochemical visualization of bromodeoxyuridine and neural tissue antigens. J Histochem Cytochem 39: 255–263
Zeman FJ 1968 Effects of maternal protein restriction on the kidney of the newborn young of rats. J Nutr 94: 111–116
Taubeneck MW, Daston GP, Rogers JM, Gershwin ME, Ansari A, Keen CL 1995 Tumor necrosis factor-α alters maternal and embryonic zinc metabolism and is developmentally toxic in mice. J Nutr 125: 908–919
Gospe SM Jr, Al-Bayati MAS 1994 Comparison of oral and inhalation exposures to toluene. J Am Coll Toxicol 13: 21–32
Pellegrino LJ, Pellegrino AS, Cushman AJ 1979 A Stereotaxic Atlas of the Rat Brain, 2nd Ed. Plenum Press, New York, NY
Zhou SS, Gospe SM Jr 1998 Double labeling of proliferating neurons with anti-BrdU and anti-NeuN: an improved immunohistochemical technique utilizing microwave irradiation. J Histotechnol 21: 201–204
Mullen RJ, Buck CR, Smith AM 1992 NeuN, a specific nuclear neuronal protein in vertebrates. Development 116: 201–211
Ziles K, Wree A 1995 Cortex: areal and laminar structure. In: Paxinos G (ed) The Rat Nervous System, 2nd Ed. Academic Press, San Diego, CA, pp 649–685
Clarren SK 1986 Neuropathology in fetal alcohol syndrome. In: West JR (ed) Alcohol and Brain Development. Oxford University Press, New York, NY, pp 158–166
Miller MW 1986 Effects of alcohol on the generation and migration of cerebral cortical neurons. Science 233: 1308–1311
Miller MW 1987 Effect of prenatal exposure to alcohol on the distribution and time of origin of corticospinal neurons in the rat. J Comp Neurol 257: 372–382
Miller MW 1988 Effect of prenatal exposure to ethanol on the development of cerebral cortex: I. Alcohol Clin Exp Res 12: 440–449
Miller MW 1992 Effects of prenatal exposure to ethanol on cell proliferation and neuronal migration. In: Miller MW (ed) Development of the Central Nervous System: Effects of Alcohol and Opiates. Wiley-Liss Inc, New York, NY, pp 47–69
Miller MW, Potempa G 1990 Numbers of neurons and glia in mature rat somatosensory cortex: effects of prenatal exposure to ethanol. J Comp Neurol 293: 92–102
Dobbing J 1981 The later development of the brain and its vulnerability. In: Davis JA, Dobbing J (eds) Scientific Foundations of Paediatrics, 2nd Ed. William Heinemann Medical Books Ltd, London, pp 744–759
Goodlett CR, Leo JT, O'Callaghan JP, Mahoney JC, West JR 1993 Transient cortical astrogliosis induced by alcohol exposure during the neonatal brain growth spurt in rats. Dev Brain Res 72: 85–97
Slomianka L, Edelfors S, Ravn-Jonsen A, Rungby J, Danscher G, West MJ 1990 The effect of low-level toluene exposure on the developing hippocampal region of the rat: histological evidence and volumetric findings. Toxicology 62: 189–202
Slomianka L, Rungby J, Edelfors S, Ravn-Jonsen A 1992 Late postnatal growth in the dentate area of the rat hippocampus compensates for volumetric changes caused by early postnatal toluene exposure. Toxicology 74: 203–208
Kaplan MS, Bell DH 1983 Neuronal proliferation in the 9-month-old rodent - radioautographic study of granule cells in the hippocampus. Exp Brain Res 52: 1–5
Miller MW, Robertson S 1993 Prenatal exposure to ethanol alters the postnatal development and transformation of radial glia to astrocytes in the cortex. J Comp Neurol 337: 253–266
Guerri C, Renau-Piqueras J 1997 Alcohol, astroglia, and brain development. Mol Neurobiol 15: 65–81
Gospe SM Jr, Calaban MJ 1988 Central nervous system distribution of inhaled toluene. Fund Appl Toxicol 11: 540–545
Ghantous H, Danielsson BRG 1986 Placental transfer and distribution of toluene, xylene, and benzene, and their metabolites during gestation in mice. Bio Res Pregnancy Perinatol 7: 98–105
Kornfeld M, Moser AB, Moser HW, Kleinschmidt-Demasters B, Nolte K, Phelps A 1994 Solvent vapor abuse leukoencephalopathy. J Neuropathol Exp Neurol 53: 389–398
Little AR Jr, Gong A, Singh U, El-Fawal H, Evans HL 1998 Decreases in brain glial fibrillary acidic protein (GFAP) are associated with increased serum corticosterone following inhalation exposure to toluene. Neurotoxicology 19: 739–747
Huang J, Asaeda N, Takeuchi Y, Shibata E, Hisanaga N, Ono J, Kato K 1991 Dose-dependent effects of chronic exposure to toluene on neuronal and glial cell marker proteins in the central nervous systems of rats. Br J Ind Med 49: 282–286
Andersson K, Fuxe K, Toftgård R, Nilsen OG, Eneroth P, Gustafsson JÅ 1980 Toluene-induced activation of certain hypothalamic and median eminence catecholamine nerve terminal systems of the male rat and its effects on anterior pituitary hormone secretion. Toxicol Lett 5: 393–398
Hsieh GC, Sharma RP, Parker RDR 1991 Hypothalamic-pituitary adrenocortical axis activity and immune function after oral exposure to benzene and toluene. Immunopharmacology 21: 23–32
Kitraki E, Kittas C, Stylianopoulou F 1997 Glucocorticoid receptor gene expression during rat embryogenesis. Differentiation 62: 21–31
Nyirenda MJ, Lindsay RS, Kenyon CJ, Burchell A, Secki JR 198 Glucocorticoid exposure in late gestation permanently programs rat hepatic phosphoenolpyruvate carboxykinase and glucocorticoid receptor expression and causes glucose intolerance in adult offspring. J Clin Invest 101: 2174–2181
Kitraki E, Karandrea D, Kittas C 1998 Long-lasting effects of stress on glucocorticoid receptor gene expression in the rat brain. Neuroendocrinology 69: 331–338
Acknowledgements
The authors thank Dr. M.W. Miller for his assistance with the development of certain histologic techniques and for critical review of the manuscript and Robert Munn for assisting with digital photomicroscopy.
Author information
Authors and Affiliations
Additional information
Supported in part by Grant R03-DA0665 from the National Institute on Drug Abuse and by funds from the Children's Miracle Network Research Program and the Hibbard E. Williams Research Program, University of California, Davis.
This work was presented at the Annual Meeting of the Pediatric Academic Societies, May 1999, in San Francisco, California.
Rights and permissions
About this article
Cite this article
Gospe, S., Zhou, S. Prenatal Exposure to Toluene Results in Abnormal Neurogenesis and Migration in Rat Somatosensory Cortex. Pediatr Res 47, 362–368 (2000). https://doi.org/10.1203/00006450-200003000-00013
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1203/00006450-200003000-00013