Abstract
Prematurely born children have reduced peak Vo2 compared with their peers, inferentially attributed to ventilatory limitation. The primary purpose of this study was to compare exercise ventilation and cardiac output in a sample of childhood survivors of lung disease of prematurity with those of a control group to elucidate reasons for lower peak Vo2. A secondary aim was to describe and compare the ventilatory response to incremental exercise. Thirty-two children, aged 8–9 y, were recalled for lung function and progressive exercise tests. Fifteen of them also performed submaximal exercise with measurement of cardiac output (indirect [CO2] Fick) and physiologic dead space. Results were compared with those of term-born, age- and sex-matched, control children. Pulmonary function tests showed mild airflow limitation. Peak Vo2 was lower in prematurely born children compared with control children, and was correlated with lean body mass. Their heart rate–Vo2 relationship and stroke volume were similar to that of term-born control children. Children with a history of bronchopulmonary dysplasia and hyaline membrane disease as infants exhibited greater exercise hyperpnea than did healthy control children, because of higher breathing frequency, and maintained lower end-tidal Pco2 during submaximal exercise. Physiologic dead space normalized for body weight was similar in preterm and term-born children. Lower peak Vo2 in this population is not caused by cardiopulmonary factors, but is best predicted by lean body mass. Ventilation did not limit exercise performance, although it appears that breathing during exercise is regulated differently in prematurely born children than in term-born children.
Similar content being viewed by others
Main
Numerous studies have reported pulmonary function abnormalities and exercise test results in children born prematurely with HMD, some of whom developed BPD (1–9). Most have found maximum aerobic capacity (peak Vo2) similar to that of control children (5, 6, 8, 9) and a tendency for children with BPD to experience exertional desaturation (2, 5, 7, 8). Typically, these studies reported ventilatory variables at maximal exercise, and based conclusions of ventilatory limitation on low breathing reserve at maximal exercise. Heldt et al. (4) studied HMD survivors and found normal HR and ventilatory responses to exercise in their population when compared with external control children. Santuz et al. (7) described lower minute volumes at all levels of exercise in BPD children compared with control children. Other than these, there has been very little study of the ventilatory response to exercise in survivors of lung disease of prematurity. Given the paucity of information on breathing during exercise in these patients, and the recognized limitations of estimating maximum voluntary ventilation as a predictor of maximal exercise ventilation (10), we believed the concept of ventilatory limitation in BPD could be a fallacy.
There has been very limited study of the circulation during exercise in childhood survivors of HMD or BPD. One report demonstrated a normal cardiac output–Vo2 relationship (8), whereas another indicated reduced effective pulmonary blood flow (11) in children with severe BPD. There is the potential for pulmonary vascular, as well as airway and parenchymal, injury as sequelae of prematurity (12, 13), and impaired pulmonary blood flow (i.e. cardiac output) could account for exercise limitation in these children. Moreover, the presence of airway obstruction itself leads to certain consequences during exercise that could affect cardiac performance. Using cystic fibrosis as a paradigm, stroke volume is lower in patients with cystic fibrosis and mild to moderate airway obstruction (14). Lean body mass had better predictive value for peak Vo2 in cystic fibrosis than did FEV1 (15), and the same could hold true for survivors of lung disease of prematurity.
The high-frequency ventilation trial (HIFI) was conducted in 1986–1987 (16), and the Winnipeg Health Sciences Centre was one of the participating centers. This provided the opportunity to assess outcome at age 8–9 y, in a relatively homogenous population of prematurely born children, cared for in very similar fashion. The primary aim of this study was to compare peak Vo2 and submaximal exercise cardiac output and ventilation in this population with that of healthy, matched, term-born control children to elucidate reasons for any differences in peak Vo2. A secondary aim was to better characterize their ventilatory response to exercise. We hypothesized that 1) children born prematurely who required mechanical ventilation for HMD would manifest a quantitatively normal ventilatory response to exercise, and would not be limited by ventilation; and 2) they would have low exercise stroke volume, resulting in a blunted cardiac output response to exercise. Thus, their exercise capacity would be limited by cardiac rather than by ventilatory factors.
METHODS
Subjects.
Study records were reviewed to identify children who had been enrolled in the high-frequency ventilation trial at the Winnipeg Health Sciences Centre in 1986–1987. The definitions for HMD and BPD for the present study were predetermined by those used in the HIFI trial. BPD was defined as a need for supplemental oxygen on the 28th postnatal and for >21 of 28 first days of life and compatible chest radiograph abnormalities at 28 postnatal days. All surviving participants, save those with significant psychomotor challenges and those for whom no current address could be found, were contacted. None of these children were receiving supplemental oxygen at the time of the study. Healthy control children were recruited from acquaintances of hospital personnel. Control subjects for pulmonary function and progressive exercise testing were recruited specifically for this study, whereas control subjects for cardiac output and dead space measurements were historical, from previous studies from this laboratory using identical methods (14, 17). Control subjects were selected primarily on the basis of similar sex and age as patients, and secondarily on matching height and weight, because all subjects were prepubescent. This study had been approved by the Faculty of Medicine Committee on the Use of Human Subjects in Research, and parents gave informed, written consent.
Protocol.
On arrival, all subjects first performed pulmonary function tests with measurement of static and dynamic lung volumes, maximal forced expiratory flow rates, and single-breath Dlco in a body box (6200 Autobox, Sensormedics, Yorba Linda, CA). Results were expressed as percent predicted using reference values (18–20). Patients who were born prematurely repeated the testing after inhalation of 200 μg of albuterol from a metered-dose inhaler to prevent exercise-induced bronchoconstriction (which might limit exercise capacity). None of the prematurely born children were receiving regular antiasthma treatment (e.g. inhaled corticosteroids).
All subjects then performed progressive exercise with step increments of 8 W each minute. They were strongly encouraged to pedal to exhaustion, manifested by falling pedal revolutions per minute despite visible signs of exhaustion, such as flushing and diaphoresis. After the test, patients from the HIFI trial were asked whether they wished to do a constant work-rate exercise test to measure cardiac output, which included blood gas measurement by finger prick. Only 15 prematurely born children agreed to this second test. They rested for 60–90 min until they felt ready to continue, then pedaled the ergometer for 6–8 min at a constant work rate of half maximum work capacity.
Apparatus and measurements.
Details of the conduct of exercise tests in this laboratory have been published previously (14, 17). Petco2 was measured over four to eight breaths at the midpoint of each minute during incremental exercise. Oxygen saturation was measured only in prematurely born children by pulse oximetry (Nelcor N-200, Hayward, CA) via ear-clip probe placed on the helix after rubbing with alcohol and local application of vasodilator creme. The highest level of oxygen uptake in the progressive test was considered peak Vo2 and expressed in milliliters per kilogram per minute. Maximum voluntary ventilation was computed as FEV1 ×35. The ventilatory anaerobic threshold was determined by the V-slope method (21). Cardiac output was measured during steady-state exercise by the indirect Fick (CO2) method with sampling of arterialized capillary blood gases, which also permitted computation of physiologic dead space by the Bohr equation. On completion of exercise, patients from the HIFI study underwent duplicate measurement of skinfold thickness over the triceps and the interscapular area to compute lean body mass (22).
Analysis.
Anthropometric variables between groups were tested for statistical significance using t test. Analysis of covariance (comparison of slopes) was performed to test for differences between groups in ΔVe/ΔVco2, ΔVo2/ΔHR, and ΔVo2/Δwork from progressive exercise tests. With work as the independent variable, repeated measures ANOVA was performed on each of ventilation, breathing frequency, tidal volume, and Petco2 as dependent variables. Post hoc testing of these results among the respective groups was performed by Fisher's least significant difference t statistic. Because this procedure requires balanced cells, analysis was restricted to workloads up to and including 64 W, which excluded one set of twins with HMD and one child with BPD. ANOVA was used to compare patient groups in terms of maximal exercise test results. Stepwise logistic regression was used to explore relationships between lung function and anthropometric variables as independent predictors and peak Vo2 as the dependent variable. Similarly, factors related to prematurity, such as birth weight, gestational age, severity of HMD, days of mechanical ventilation, mean airway pressure, and the ratio of Po2 to fraction of inspired oxygen were treated as continuous variables and examined as predictors of peak Vo2. Finally, the prematurely born infants were split into two groups—normal and low peak Vo2—with 35 mL·kg−1·min−1 as the cutoff. Neonatal complications that were tabulated during the course of the HIFI trial, such as air leak, patent ductus arteriosus, atelectasis, apnea, and intraventricular hemorrhage, were treated as discrete variables (present, not present). χ2 analysis was then used to determine whether perinatal events were overrepresented in the group with lower peak Vo2.
RESULTS
Thirty-two prematurely born children participated in this study (Table 1). They had a mean birth weight of 1165 g (range, 770-1850 g), a mean gestational age of 28.3 wk (range, 24–31 wk), and required an average of 19 d (range, 3–120 d) of mechanical ventilation: 9 d for children with HMD and 27 d for children with BPD. Details regarding their neonatal course and analysis of pulmonary function test results are presented in a companion paper. A total of 15 healthy control children were recruited to perform pulmonary function and maximal exercise tests, and were well matched with patients (Table 1).
In general, pulmonary function data demonstrated a mild obstructive pattern with reduced midexpiratory flow rates in children who had been born prematurely. Thirty-one percent (10 of 32) of patients had FEV1 <80% predicted, but only one child (who had HMD) had FEV1 <50% predicted. These findings were evenly distributed among all patients regardless of diagnosis (HMD or BPD). The Dlco was slightly but statistically significantly (p = 0.03) reduced only in BPD compared with control children (99 ± 17%versus 109 ± 19% predicted).
Progressive exercise test results are listed in Table 2. All prematurely born children performed the progressive exercise test, and 15 (10 BPD, five HMD) also underwent measurement of cardiac output and physiologic dead space during steady-state exercise. Children born prematurely had lower maximal aerobic power than did healthy control children. Peak Vo2 in patients ranged from 26.6 to 51.2 mL·kg−1·min−1, with seven of 31 (23%) patients having values <35 mL·kg−1·min−1, whereas only one of 16 control children had a peak Vo2 below this value. There was no difference in the ventilatory anaerobic threshold (expressed as percentage of peak Vo2, in milliliters per kilogram per minute, or liters per minute) between prematurely born and term-born children. There was no difference in the oxygen cost of work (ΔVo2/Δwork) in the progressive exercise test among groups. There was no correlation between FEV1 and peak Vo2 (nor between FEV1 and lean body mass) in univariate analysis. None of the adverse neonatal events recorded were found more frequently in patients with lower versus those with higher peak Vo2. Similarly, none of the perinatal variables, such as low birth weight, gestational age, severity of HMD, days of ventilatory support, mean airway pressure, or ratio of Po2 to fraction of inspired oxygen, bore any relationship to peak Vo2. Stepwise regression using lean body mass, FEV1, and sex as independent variables revealed lean body mass to be the only predictor of peak Vo2 (expressed in milliliters per minute) (r2 = 0.35, p = 0.004).
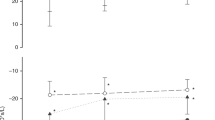
The ventilatory response to incremental exercise below the anaerobic threshold, when expressed as ΔVe/ΔVco2, was similar in all groups. However, ventilation was characterized by more rapid respiratory rates in prematurely born children, such that exercise hyperpnea was greater during submaximal exercise (Fig. 1), resulting in lower Petco2. Findings were similar in both BPD and HMD children, although statistically significant differences occurred only in the BPD group. Physiologic dead space during constant work-rate exercise was 3.4 ± 1.4 mL/kg (mean ± SD) in BPD and 3.9 ± 1.2 mL/kg in HMD survivors, not significantly different from the 3.9 ± 0.45 mL/kg found in historical control children. There were no differences in Petco2, minute volume, or gas exchange ratio at maximal exercise among the groups. It is noteworthy that four of 18 control children had values for the ratio of ventilation at maximal exercise to maximum voluntary ventilation above unity, whereas none of the prematurely born children exceeded this limit. Oxygen saturation was 99 ± 1% at rest in prematurely born children (identical in BPD and HMD) and fell to a mean of 96 ± 2% in heavy exercise. The greatest fall (6%) in oxygen saturation, to a nadir of 93% at maximal exercise, was seen in an HMD patient (FEV1 = 47% predicted), and another four patients (two BPD) experienced a 4% drop in saturation. The occurrence of desaturation bore no relationship to reduced Dlco.
Box plot of peak Vo2 in healthy control children (CNT) and in children who had been born prematurely, split according to diagnosis (HMD or BPD). The horizontal line is the median value, the box represents the first and third quartiles, and the error bars represent the 10th and 90th percentiles. Mean values were significantly higher (p < 0.05) in control children (see Table 2).
The cardiac response to exercise showed a similar heart rate–Vo2 relationship in each group of children (Table 2). Oxygen pulse at maximal exercise was lower, although not significantly so, in survivors of HMD or BPD. There was a significant correlation between the slope of ΔVo2/Δwork and lean body mass in the preterm children (r2 = 0.26, p = 0.002). Historical control children used for comparison of stroke volume and dead space consisted of 14 8- to 10-y-old healthy children. According to the respective protocols, prematurely born patients exercised at 50% of their maximum work capacity (1.5 ± 0.2 W/kg), whereas control children exercised at 1.4 ± 0.2 W/kg. Because healthy control children were slightly taller (140 ± 6 cm) and heavier (35 ± 8 kg) than subjects in the present study, dead space was normalized for body weight, and stroke volume was expressed as a percent of predicted (23). Stroke volume during moderate, constant work-rate exercise was 98% predicted in survivors of HMD or BPD and 95% predicted in control children.
DISCUSSION
Childhood survivors of premature birth who required mechanical ventilation and oxygen supplementation have, on average, peak Vo2 within the normal range, but slightly lower than matched control children. Although they did not appear to have any significant cardiopulmonary limitation to exercise, they exhibited certain adaptations in their cardiopulmonary responses to exercise, distinct from term-born children. In our study, patients with BPD demonstrated a ventilatory response that was quantitatively greater than that of control subjects at submaximal workloads. Yet, none of the patients in the present study exceeded their maximum voluntary ventilation, something commonly seen in patients whose exercise capacity is believed to be limited by ventilation. Their physiologic dead space, computed by sampling of arterialized capillary blood gases, was no different from that of control children. Resting spirometry provided no predictive value in determining peak Vo2 in univariate analysis. Indeed, these findings provide little support for a ventilatory limitation to exercise in this population in the absence of exercise-induced bronchoconstriction.
There were qualitative differences in the ventilatory response to exercise in prematurely born children, particularly those with BPD, compared with term-born control children: higher respiratory rate with normal tidal volume. We can only speculate on the reason for the exaggerated tachypnea, but believe that it can be deduced from the lung pathology of BPD. There is small airway obstruction, and the parenchyma shows areas of overdistension adjacent to areas of atelectasis and fibrosis (particularly of the alveolar septa) (24). The quasi-static pressure-volume curve is not shifted down and to the right as might be expected (1), but another study reported reduced dynamic compliance in school-age children with BPD (9). To our knowledge, there has been no study of dynamic hyperinflation during exercise in this population, but it is logical to presume this would occur in survivors of lung disease of prematurity, both because of airway obstruction (25) and because of reduced upstream pressure (1). If dynamic hyperinflation developed during exercise, it would preferentially affect the more slowly emptying, i.e. emphysematous, compartments, which would then divert tidal air into the more fibrotic, stiffer compartments (these would likely be rapidly emptying because of good upstream pressure). Thus, as ventilatory demand increases, a person with BPD would have to overcome both increased resistive and elastic loads. Rather than raise tidal volume, ventilation is maintained by higher breathing frequencies.
The latter could explain why the preterm children elected to increase respiratory rate rather than tidal volume, but one must still explain why they did so to the extent of causing hypocapnia compared with term-born control children. Santuz et al. (7) did not comment on Pco2 or dead space, but the fact that they reported lower values for Ve/Vo2 in their cohort of prematurely born children suggests that arterial Pco2 might have been elevated. Bader et al. (5) reported elevated transcutaneous Pco2 at maximal exercise. Thus, we were surprised to find consistently significantly lower Petco2 in children with a history of BPD, and similar (although not statistically significant) findings in those with past HMD. It is possible that the prematurely born children regulate their Pco2 around a lower set point and therefore must increase minute volume, although we are not aware of any data supporting this. Another hypothesis is that this group of children has some additional ventilatory stimulation during exercise causing them to breathe to the point of lowering arterial Pco2. Neither of these seem likely, inasmuch as one would have expected differences in ΔVe/ΔVco2 between the prematurely born children and term-born control children. Careful examination of Figure 2 reveals that control children exhibited the slight rise in Petco2 typically seen during mild to moderate incremental exercise, whereas the preterm children did not. This transient respiratory acidosis is believed to reflect the slightly slower kinetics of Ve compared with Vco2 (26). Santuz et al. (7) observed slower kinetics of Vo2 and Ve in their group of BPD patients. If these (Vco2 and Ve) kinetics were concordant, then the transient elevation in Petco2 normally found in progressive exercise below the anaerobic threshold might not occur. We are not aware of any study of the kinetics of the ventilatory response to exercise in children born prematurely. It is believed that the carotid body is directly involved in determining the kinetics of the ventilatory response to exercise (27). Interestingly, delayed postnatal resetting of the peripheral chemoreceptors has been shown in preterm infants with BPD (28). There might well be persistent, subtle differences in peripheral chemoreceptor function in older children who were born prematurely. The fact remains that these childhood survivors of pulmonary disease of prematurity manifested lower Petco2 compared with term-born, healthy control children, implying that regulation of exercise ventilation differs.
Graphs of the ventilatory response to incremental exercise. Only workloads of 64 W are shown, because subject numbers dropped markedly above this load. Minute volume was statistically significantly lower only in the BPD group (p = 0.04). Petco2 was significantly lower in both HMD (p = 0.05) and BPD (p = 0.03) groups compared with term-born control (cnt) children. Respiratory rate was statistically significantly lower in the BPD children (p = 0.005), but not in the HMD children (p = 0.06), compared with control children. Tidal volume did not differ among groups (p = 0.2).
There are very few published reports on exercise cardiac output in this population, although our results corroborate recent findings by Jacob et al. (8) of normal exercise cardiac output in children with BPD. The normal stroke volume and HR found in these two studies suggest that no substantial circulatory abnormality is a likely cause for the lower peak Vo2 observed. Mitchell and Teague (11) used acetylene rebreathing and found reduced effective pulmonary blood flow during exercise in children with BPD. Caution should be exercised in presuming that this is equivalent to reduced cardiac output, because this technique is influenced by ventilatory inhomogeneity (slow versus fast compartments) and could therefore give falsely low results. This is an important area for study because of the presence of pulmonary hypertension in infants with BPD (11) and the possibility of abnormal development of the pulmonary vascular bed in infants with HMD (12). In this context, the Dlco results merit consideration. The Dlco was marginally reduced in the BPD patients compared with control children, although this result was of dubious clinical significance. Previous studies of Dlco in children and young adults who had been born prematurely have shown conflicting results, with normal or low values reported (29, 30). Taken together, the findings in this study imply that the pulmonary vascular bed has developed enough to allow recruitment to accommodate the increased cardiac output caused by exercise.
Potential biases in this study could have been introduced in recruitment of patients and control children in that we did not elicit information regarding habitual level of physical activity in study subjects. However, this was performed by Santuz et al. (7), and no differences between control children and BPD patients were found that might have confounded their interpretation. The values of peak Vo2 attained by our control children were clearly in the midrange of normal, implying our control subjects were not more physically fit than average. Another limitation of this study was the failure to measure skinfold thickness in healthy control children, which would have allowed comparison between the two groups of subjects. We expected that prematurely born children would have shown cardiopulmonary limitation to exercise, but our findings do not support this at all. This leaves so-called “peripheral factors,”i.e. muscle mass and metabolism, to account for the lower peak Vo2. The equivalency of ΔVo2/Δwork and respiratory quotient among groups argues against differences in metabolism. It had already been shown that children with BPD had lower lean body mass compared with matched control children (2). The dose-response relationship found between lean body mass and peak Vo2 supports our contention that the reason for lower peak Vo2 in survivors of lung disease of prematurity is that they have less metabolically active muscle.
Abbreviations
- BPD:
-
bronchopulmonary dysplasia
- HMD:
-
hyaline membrane disease
- FEV1:
-
forced expiratory volume in the first second
- D lco:
-
diffusing capacity of the lung for carbon monoxide
- Vo2:
-
oxygen uptake
- Petco2:
-
partial pressure of end-tidal carbon dioxide
- Ve:
-
minute ventilation
- Vco2:
-
carbon dioxide production
- HR:
-
heart rate
- HIFI:
-
high-frequency ventilation trial
References
Jacob SV, Coates AL, Lands LC, MacNeish CF, Riley SP, Hornby L, Outerbridge EW, Davis GM, Williams RL 1998 Long-term pulmonary sequelae of severe bronchopulmonary dysplasia. J Pediatr 133: 193–200.
Giacoia GP, Venkataraman PS, West-Wilson KI, Faulkner MJ 1997 Follow-up of school age children with bronchopulmonary dysplasia. J Pediatr 130: 400–408.
Gross SJ, Ianuzzi DM, Kveselis DA, Anbar RD 1998 Effect of preterm birth on pulmonary function at school age: a prospective controlled study. J Pediatr 133: 188–192.
Heldt GP, McIlroy MB, Hansen TN, Tooley WH 1980 Exercise performance of the survivors of hyaline membrane disease. J Pediatr 96: 995–999.
Bader D, Ramos AD, Lew CD, Platzker ACG, Stabile MW, Keens TG 1987 Childhood sequelae of infant lung disease: exercise and pulmonary function abnormalities after bronchopulmonary dysplasia. J Pediatr 110: 693–699.
Baraldi E, Zanconato S, Zorzi C, Santuz P, Benini F, Zachello F 1991 Exercise performance in very low birth weight children at the age of 7–12 years. Eur J Pediatr 150: 713–716.
Santuz P, Baraldi E, Zaramella P, Filippone M 1995 Factors limiting exercise performance in long-term survivors of bronchopulmonary dysplasia. Am J Respir Crit Care Med 152: 1284–1289.
Jacob SV, Lands LC, Coates AL, Davis GM, MacNeish CF, Hornby L, Riley SP, Outerbridge EW 1997 Exercise ability in survivors of severe bronchopulmonary dysplasia. Am J Respir Crit Care Med 155: 1925–1929.
Parat S, Moriette G, Delaperche MF, Escourrou P, Denjean A, Gaultier C 1995 Long term pulmonary functional outcome of bronchopulmonary dysplasia and premature birth. Pediatr Pulmonol 20: 289–296.
Babb TG, Rodarte JR 1993 Estimation of ventilatory capacity during submaximal exercise. J Appl Physiol 74: 2016–2022.
Mitchell SH, Teague WG 1998 Reduced gas transfer at rest and during exercise in school age survivors of bronchopulmonary dysplasia. Am J Respir Crit Care Med 155: 1925–1929.
Abman SH, Sondheimer HW 1992 Pulmonary circulation and cardiovascular sequelae of BPD. In: Weir EK, Archer SL, Reeves JT (eds) Diagnosis and Treatment of Pulmonary Hypertension. Futura Publishing, Mount Kisco, NY, 155–180.
Hislop AA, Haworth SG 1990 Pulmonary vascular damage and the development of cor pulmonale following hyaline membrane disease. Pediatr Pulmonol 9: 152–16.
Pianosi P, Pelech AN 1996 Stroke volume during exercise in cystic fibrosis. Am J Respir Crit Care Med 153: 1105–1109.
Lands LC, Heigenhauser GJF, Jones NL 1992 Analysis of factors limiting maximal exercise performance in cystic fibrosis. Clin Sci 83: 391–397.
HIFI Study Group 1989 High frequency oscillatory ventilation compared with conventional mechanical ventilation in the treatment of respiratory failure in preterm infants. N Engl J Med 320: 88–93.
Pianosi P, Garros D 1996 Comparison of impedance cardiography with indirect Fick (CO2) method of measuring cardiac output in healthy children during exercise. Am J Cardiol 77: 745–749.
Polgar G, Promadhat V 1971 Pulmonary Function Testing in Children: Techniques and Standards. WB Saunders, Philadelphia, PA, 88–201.
Bucci G, Cook CD, Barrie H 1961 Studies of respiratory physiology in children: V. J Pediatr 58: 820–828.
Morris JF, Koski A, Breese J 1975 Normal values and evaluation of forced expiratory flow. Am Rev Respir Dis 111: 755–762.
Beaver WL, Wasserman K, Whipp BJ 1986 A new method for detecting anaerobic threshold by gas exchange. J Appl Physiol 60: 2020–2027.
Slaugther MH, Lohman TG, Boileau RA, Horswill CA, Stillman RJ, VanLoan MD, Bemben DA 1988 Skinfold equations for estimation of body fatness in children and youth. Hum Biol 60: 709–723.
Godfrey S, Davies CTM, Wozniak E, Barnes CA 1971 Cardio-respiratory response to exercise in normal children. Clin Sci 40: 419–431.
Stocker JT 1986 Pathologic features of longstanding “healed” bronchopulmonary dysplasia. Hum Pathol 17: 943–961.
Pellegrino RV, Brusasco V, Rodarte JR, Babb TG 1993 Expiratory flow limitation and regulation of end-expiratory lung volume during exercise. J Appl Physiol 74: 2552–2558.
Wasserman K, Whipp BJ 1983 Coupling of ventilation to pulmonary gas exchange during non-steady state work in men. J Appl Physiol 54: 587–593.
Whipp BJ 1994 Peripheral chemoreceptor control of exercise hyperpnea in humans. Med Sci Sports Exerc 26: 337–347.
Caldner NA, Williams BA, Smyth J, Boon AW, Kumar R, Hanson MA 1994 Absence of respiratory chemoreflex responses to mild hypoxia in infants who have suffered bronchopulmonary dysplasia. Pediatr Res 35: 677–381.
Hakulinen AL, Järvenpää A-L, Turpeinen M, Sovijärvi A 1996 Diffusing capacity of the lung in school-aged children born very preterm with and without bronchopulmonary dysplasia. Pediatr Pulmonol 21: 353–360.
Northway WH Jr, Moss RB, Carlisle KB, Parker BR, Popp RI, Pitlick PT, Eichler I, Lamm RL, Brown BW Jr 1990 Late pulmonary sequelae of bronchopulmonary dysplasia. N Engl J Med 323: 1793–1799.
Author information
Authors and Affiliations
Additional information
Supported by grants from the Children's Hospital of Winnipeg Research Foundation.
Rights and permissions
About this article
Cite this article
Pianosi, P., Fisk, M. Cardiopulmonary Exercise Performance in Prematurely Born Children. Pediatr Res 47, 653–658 (2000). https://doi.org/10.1203/00006450-200005000-00016
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1203/00006450-200005000-00016