Abstract
Fetal and maternal plasma noradrenaline responses to invasive procedures were determined in pregnancies of 18 to 37 wk gestation. Fetal umbilical venous blood sampling was performed either from the placental cord insertion, which is not innervated, or the intrahepatic vein, which is innervated, and thus may be more stressful for the fetus. Samples from diagnostic procedures, as well as from transfusion procedures, were compared between the two sites. Fetal plasma levels were significantly elevated in blood samples obtained from the intrahepatic vein compared with those from the placental cord insertion during diagnostic procedures [p < 0.05, geometric means and 95% confidence intervals (CI) were 0.67 nmol/L (0.43-1.04) and 0.36 nmol/L (0.25-0.54), respectively]. Plasma levels in samples taken before transfusion from the intrahepatic vein were also significantly higher than those from the placental cord insertion. After transfusion, there was a significant rise in fetal plasma noradrenaline levels at both sites; however, after transfusion through the intrahepatic vein, the rise was substantially greater than after transfusion through the placental cord insertion (p < 0.05, change, mean ΔNA, and 95% CI were 0.67 (0.37-1.22), and 0.20 (0.12-0.33), respectively). The ΔNA was significantly associated with the duration of the stimulus (the time the needle remained in situ) (p = 0.05, adjusted R2 = 0.48) and with gestational age. Maternal levels rose substantially and equally after transfusions at either site (mean ΔNA and 95% CI, 6.46 nmol/L, 1.74 to 11.18 and 9.49 nmol/L, 6.24 to 12.75 for the intrahepatic vein and placental cord insertion groups, respectively). There was no significant correlation between baseline fetal and maternal levels (r = 0.08, n = 41) or between ΔNA pre- and posttransfusion maternal and fetal values in either group. These results indicate that the fetus is capable of mounting an independent noradrenaline stress response to a needle transgressing its trunk from 18 wk gestation. The effect was observable in samples taken at a mean of 5.6 min after needling. The lack of correlation between maternal and fetal levels suggests that virtually no noradrenaline crosses the placenta directly, and that the observed fetal responses are not due to direct transport from the mother.
Similar content being viewed by others
Main
The aim of this study was to investigate fetal plasma noradrenaline responses to invasive procedures and compare them with those found previously for cortisol and β-endorphin and for blood flow redistribution. In a previous study, we have shown that the human fetus can mount a substantial cortisol and β-endorphin response to the stress associated with intrauterine needling(1). Concentrations in fetal plasma obtained during fetal blood sampling or intrauterine transfusions by needling the fetal IHV were compared with those obtained by the more conventional technique of needling the PCI. Sampling or transfusing blood using these two sites is similar in most respects. However, the PCI is not innervated, whereas the IHV is and must be accessed percutaneously during the procedure, which might generate a nociceptive and subsequent stress response in the fetus. Cortisol and β-endorphin did not increase within the first 10 min of sampling from either site. However, more prolonged needling during transfusions was associated with a significant increase in fetal plasma levels of both hormones when done at the IHV, whereas no such response was observed with transfusions via the PCI.
More recently, using a similar clinical model and color Doppler ultrasound, we found that invasive procedures involving puncture of the fetal trunk are associated with a significant decrease in the fetal middle cerebral artery pulsatility index(2), with no changes observed in procedures done via the PCI. This implies that fetal blood is preferentially redistributed to the fetal brain in response to intrauterine needling and seems similar to the "brain-sparing" effect observed in animal fetuses subjected to stresses such as hypoxia or hemorrhage(3,4). This response was more rapid than the change in fetal plasma cortisol or β-endorphin concentrations, with needling evoking an acute change in blood flow in procedures lasting as little as 4 min.
Noradrenaline responses in adults are generally much more rapid than those of cortisol. The current study was designed to test the hypothesis that invasive procedures via the IHV would produce an increase in fetal plasma noradrenaline, whereas those via the PCI would not. Further aims were to determine the degree and time course of the fetal plasma noradrenaline response both to needling and transfusion procedures via the two sites, determine the relationship with gestational age, and determine any relationship with maternal noradrenaline levels. It is known that the placenta is very rich in monoamine oxidase A(5) and COMT(6), which would efficiently metabolize noradrenaline, but it is not clear whether a small amount of maternal noradrenaline may still cross the placenta and have an effect on the fetus.
METHODS
Patients. Women with singleton pregnancies undergoing clinically indicated fetal blood sampling or transfusion between 18-37 wk gestation at the Centre for Fetal Care, Queen Charlotte's and Chelsea Hospital, were eligible to participate in the study. One twin pregnancy was also included, in which both fetuses satisfied the inclusion criteria as follows. All fetuses were appropriate for gestational age, were structurally normal on ultrasound, had end-diastolic frequencies present in the umbilical artery, and had no evidence of hydrops before the procedure. The operator chose the site of ultrasound-guided fetal blood sampling per routine clinical practice on the basis of technical factors and ease of approach. Randomization of the site of sampling was not considered appropriate because anatomical factors often dictate one approach in favor of the other. Fetal neuromuscular blockade was not used. One patient requested and received 5 mg diazepam orally. After the purity of the fetal samples was confirmed, full blood count (Coulter Counter; Coulter Electronics, Luton, UK) and blood gas analysis (Radiometer ABL 330, Copenhagen, Denmark) were done on all clinical samples. Fetuses with aneuploidy or severe anemia (Hb < 5 g/dL) were excluded. Procedures that were complicated or involved multiple fetal vessel punctures were also excluded.
From April 1995 to July 1996, 64 procedures were carried out on fetuses that satisfied these criteria. Two cases were complicated by dislodgement of the needle due to vigorous fetal movements. In one of them, blood was collected first from the IHV and subsequently from the PCI, and data were categorized among those from the IHV transfusion group. In the other, blood was collected only from the PCI after an unsuccessful attempt to collect from the IHV, and this case was categorized among those from the IHV group (no transfusion). In one case of fetocide for legal termination of pregnancy, intracardiac blood was collected.
Intravascular transfusion was performed on 11 patients with alloimmune anemia. Preparations of packed red blood cells or platelets were warmed to room temperature (25°C) before transfusion. Five patients underwent multiple transfusions including both the IHV and the PCI. For the main analysis in those cases, data from the pre- and posttransfusion samples from the first IHV transfusion were categorized with data from the rest of the IHV group, and similarly, data from the first PCI transfusion were categorized among data from the PCI group. Except for these five cases, all other fetuses were studied on a single occasion.
After collection of clinical samples, 0.5 to 2 mL of additional venous blood was withdrawn for research. The total volume of blood withdrawn was a maximum of 2 mL for fetuses younger than 18 wk, 4 mL for those between 18 and 22 wk, and 6 mL for those older than 22 wk. In all cases, this was less than 10% of the estimated fetoplacental volume.
All women signed written informed consent to the collection of additional samples for research purposes, as approved by the institutional ethics committee. Maternal samples (5 mL) were collected from the antecubital vein immediately before the procedure and 5 to 10 min after the end of the procedure. In all cases, the time elapsed from puncturing either the umbilical cord (in the PCI groups, TPCI) or the fetal trunk (in the IHV groups, TIHV) to collecting the blood sample from the target vessel was measured [values are expressed as min to 0.1 of a min (6 sec) accuracy]. In all cases, fetal and maternal samples were collected in chilled heparinized tubes and kept on ice until centrifugation. Plasma samples were stored at -70°C until assay.
Catecholamine assays. This was a modification of the original method by Brown and Jenner(7), based on the enzymatic conversion of catecholamines to their methylated derivatives, MA and NMA, using tritiated MA and NMA as the carrier molecules. COMT was prepared from rat livers(7), and the final preparation was freeze-dried and stored at -30°C until assay. Two d before removal of their livers, rats were given 100 mg/kg 6-hydroxydopamine i.p. to deplete endogenous catecholamine stores and reduce catecholamine content in the final enzyme preparation. We have found that this method of enzyme preparation reduces background noise to increase sensitivity when compared with a commercial COMT preparation (Sigma Chemical Co., Poole, UK).
Fetal or maternal plasma samples (200-500 µL) were mixed with cold 10% vol/vol 0.1 M HCl and centrifuged at 25 000 × g for 20 min at 4°C. Portions (50-70 µL) from each sample were transferred in triplicate to polypropylene tubes. A buffer solution (pH 8.3), consisting of 3.6 mg/mL COMT preparation, 2.1 mg/mL reduced glutathione, Tris (0.8 M), MgCl2 (0.25 M), ethylene glycol-bis[β-aminoethyl ether]-N, N,N',N'-tetracetic acid (80 mM), and 1% (vol/vol) of 5% o-benzylhydroxylamine, was divided into two parts: 300 µCi of S-[3H]-adenosyl-L-methionine was added per mL of buffer to one portion, and to the other, 400 nCi of S-[14C]-adenosyl-L-methionine/mL and 400 ng/mL each of noradrenaline-HCl and adrenaline-HCl were added. We added 25 µL of the 3H mixture to two tubes of each triplicate, and 25 µL of the 14C mixture was added to the third tube. All tubes were incubated at 25°C for 90 min in a shaking water bath. Unlabeled S-adenosyl-L-methionine and unlabelled NMA and MA were then added to the samples, which were then semi-purified by extraction with ethyl acetate. The aqueous layer was then applied onto appropriate thin layer chromatography silica plates (Whatman Inc., Clifton, NJ), which were then developed in chloroform/methanol/70% (vol/vol) ethylamine (32:6:4 vol/vol). After extraction of the radiolabeled methylated derivatives, adrenaline and noradrenaline contents were estimated simultaneously as previously described(7). Incomplete recovery was corrected within each batch by running a number of selected samples both with and without a spike of a known amount of added noradrenaline and adrenaline standard. The intra- and inter-assay coefficients of variation for noradrenaline were 11% and 13%, respectively. The limit of sensitivity for the assay was 0.12 nmol/L (20 pg/mL).
Pilot data showed that baseline fetal adrenaline levels were either very low (approximately 10% of noradrenaline values) or undetectable, and the inter- and intraassay coefficients of variation were unacceptably high (> 15%). The study was thus confined to noradrenaline.
STATISTICAL ANALYSIS
Nonnormal data for fetal plasma noradrenaline concentration and the respective maternal values in the blood sampling group without transfusion were logarithmically transformed. Blood gases (PO2, PCO2) and pH levels are known to change during gestation and therefore were expressed as gestation-independent z scores(8). Unpaired t testing was used to compare values at the two sites and paired t tests for comparisons within groups. Associations with gestational age and stimulus duration were examined by standard linear regression, whereas the influence of blood gases, pH levels, gestational age, duration of the stimulus, and the net volume transfused were investigated using multiple regression. All calculations were performed using MINITAB (version 10.5XTRA) statistical software.
RESULTS
A summary of patient characteristics and blood gas values is given in Table 1. A summary of the noradrenaline data are given in Table 2.
Fetal Samples
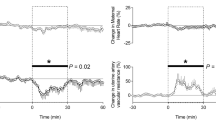
Fetal blood sampling without transfusion. Fetal plasma noradrenaline concentrations (Fig. 1) were higher in samples obtained via the IHV than the PCI (2-tailed test, p = 0.04; IHV: n = 14, geometric mean; 95% CI: 0.67 nmol/L; 0.43-1.04 nmol/L; PCI: n = 11, geometric mean 0.36 nmol/L, 95% CI 0.25-0.54). There was no evidence of bradycardia after the procedure. The time interval from puncturing the fetal trunk to collecting the blood sample in the IHV group (tIHV) was significantly longer than in the PCI group (tPCI, defined as the interval from puncturing the umbilical cord to collecting the blood sample) (p = 0.01; mean for tIHV = 19.4 min, 95% CI 11.3-27.5; mean for tPCI = 6.1 min, 2.2-10.1). The four fetuses (Fig. 1) whose noradrenaline levels exceeded 1.27 nmol/L were 18, 18, 24, and 35 wk gestational age, and tIHV for them were 25, 35, 30, and 29 min, respectively. However, there was no significant association between fetal noradrenaline concentrations and tIHV. Regression analysis revealed to significant associations between noradrenaline concentrations in the IHV or PCI groups and the gestational age, PO2, PCO2, or pH levels.
Intrauterine transfusions. Samples collected at the beginning of the procedure from the IHV had significantly higher noradrenaline concentrations than those from the PCI (p = 0.04, see Table 2 and Fig. 2). The tIHV was not significantly different from the tPCI (mean IHC: 5.6 min, PCI: 2.8 min). Gestational age was not significantly different between the two groups nor was any of the acid-base parameters or pH levels in pretransfusion samples.
Noradrenaline concentrations increased significantly after transfusion in both the PCI and the IHV group (values shown in Table 1, paired t test for both groups, p < 0.005). The increase (ΔNA) in the IHV transfusion group was significantly higher than the increase in the PCI transfusion group (p = 0.002); mean Δ for the IHV group (n = 7) 0.67, 95% CI 0.37-1.22; mean Δ for the PCI (n = 10) group 0.20 (0.12-0.33). Regression analysis revealed that the duration of the stimulus (i.e. the time interval from puncturing the fetal trunk to collecting the last sample), but not gestational age, was associated with the rise in noradrenaline concentration in the IHV group (regression of ΔNA on ΔtIHV, adjusted R2 = 0.48, p = 0.05). There was no significant difference in blood gas PO2, PCO2, or pH levels before or after transfusion in either group; also, there was no significant association between noradrenaline levels and PO2, PCO2, or pH levels. Transfusion via the IHV resulted in an increase in noradrenaline levels in all fetuses, including the youngest one (25 wk, 1.5-fold increase in noradrenaline levels). Fetal plasma noradrenaline levels increased 2.1-fold in the patient who received diazepam orally.
Regression analysis of pooled data (n = 21, logarithmically transformed), including both pretransfusion samples and fetal blood samplings without transfusion in the IHV groups, indicated that the duration of stimulus (tTOTAL) exerted a significant influence on "basal" noradrenaline levels. A quadratic regression model provided the best noradrenaline response curve (adjusted R2 = 0.27, p = 0.026). Using multiple regression analysis for the same data set, we found that not only time but also gestational age, albeit at a much smaller extent, contributed to the variation in noradrenaline levels.
When all cases in the transfusion group are considered, i.e. including those in which more than one sampling procedure was performed, the ΔNA in fetal plasma between the two sites remained highly significant (p = 0.001, number of procedures, IHV = 12, PCI = 16, mean Δ between the two sites = 0.29 nmol/L, 95% CI 0.18-0.44).
Maternal Samples
Fetal blood sampling only. Mean baseline plasma maternal levels of noradrenaline were much higher than fetal values in both the PCI and the IHV groups, as shown in Table 2 and Figures 1–3. There was no significant difference in noradrenaline concentrations between PCI and IHV procedures (IHV: mean 4.03 nmol/L, 95% CI 3.14-4.91 nmol/L; PCI: 4.70 nmol/L, 95% CI 3.55-5.85 nmol/L; p = 0.31).
Transfusions. Two mothers objected to the collection of their blood samples (one in each group). Maternal noradrenaline rose significantly with transfusion in both the PCI and IHV groups (Fig. 2) (p < 0.005 for the PCI group and p = 0.01 for IHV group, see also Table 1). However, ΔNA was similar in both groups (Table 1, p = 0.21). There was no significant correlation between either pre- or posttransfusion fetal and maternal values or the fetal ΔNA and the maternal ΔNA in either PCI or IHV group nor any correlation when data from both PCI and IHV groups were pooled together. Also, maternal concentrations did not correlate with gestational age in either PCI or IHV groups. There was not any pretransfusion blood sample taken from the patient who received diazepam, but the posttransfusion value was 14.90 nmol/L.
Baseline maternal noradrenaline concentrations for the whole sample showed almost no correlation with baseline fetal concentrations (r = 0.08, n = 41) (Fig. 3) nor was there a correlation with gestational age.
DISCUSSION
This study shows that a potentially stressful stimulation involving transgression of the fetal trunk can result in an acute increase in circulating fetal noradrenaline. This was observed in the samples obtained by fetal blood sampling, baseline samples obtained before transfusion, and in the samples obtained after transfusion. In all cases there was a significant difference in increase between procedures carried out through the IHV and those at the PCI, thus supporting our original hypothesis.
Although in general there was a significant correlation with the duration of the stimulus, a significant increase was obtained in the pretransfusion IHV group compared with the PCI group, when the mean duration was only 5.6 min. This suggests that the noradrenaline response time can be under the 10 min observed for cortisol and β-endorphin(1) but comparable in time course to the responses seen in the blood flow redistribution study(2).
The youngest fetuses studied were 18 wk, and two of these in the IHV blood-sampling group showed markedly elevated levels compared with the PCI group (Fig 1). The youngest fetuses in the IHV transfusion group (25 wk) also showed definite responses. This study thus confirms that circulating noradrenaline is present in the human fetus from as early as 18 wk gestation and, even at that stage, seems to reflect a functionally active system.
This is the first study of its type, examining acute fetal noradrenaline responses in utero. Previous studies have examined plasma noradrenaline levels in samples from normal and hypoxic fetuses from 16 to 36 wk gestation and demonstrated an increase with hypoxia(9,10). Their baseline values were within the same range as ours (0.78, 0.1-3 nmol/L, median and range). They also found an increase in values with gestational age.
The total lack of correlation between baseline maternal and fetal levels (Fig 3) and between the ΔNA after transfusion is of interest. It suggests that no significant amounts of noradrenaline passes through the placenta from mother to baby. An increase of 0.27 nmol/L, which would almost double basal fetal plasma levels, would represent only 3% of a maternal level of 9 nmol/L. However, the placenta is very rich in COMT and monoamine oxidase, which metabolize noradrenaline(5,6). Early studies suggested that appreciable amounts of noradrenaline might cross the placenta. Labeled noradrenaline was found in the blood of fetuses with lethal abnormalities after i.v. injection of 14C-D,L-noradrenaline to the mother immediately before delivery(11). Similar results were obtained from experiments in animals(12) and on perfused human placentas(13). However these experiments used the D-L racemic mixture of noradrenaline and the D-form may not have been metabolized.
The current study also shows that there is a pronounced maternal response to the experience of blood transfusion (Fig 2). Most women find the procedures for fetal blood transfusion stressful. This, as to be expected, is equally evident with transfusion at both sites, during which noradrenaline levels increased approximately to the same extent.
The cause for the small increase in fetal levels after transfusion at the PCI remains unclear. It is unlikely to be the result of noradrenaline spill-over from the maternal circulation, as discussed above. It may be a direct effect of transfusion. It is not caused by a technical artefact due to before and after sample being assayed in different runs, because pre- and postsamples from the same subject were assayed together. One possible explanation would be that the large rise in maternal noradrenaline concentrations might cause placental vasoconstriction, as demonstrated in animal models(14–16), and this in turn could reduce placental blood flow leading to transient fetal hypoxemia(17) and reactive catecholamine secretion.
The source of the increased fetal noradrenaline observed here is not certain nor is its functional significance. In adults, plasma noradrenaline primarily represents a spill-over from the release from sympathetic neurones, whereas the adrenal gland predominantly releases adrenaline. In the fetus the position is less clear. The fetal adrenal gland contains approximately equal adrenaline and noradrenaline levels, at least until midgestation(18). Our pilot data confirmed previous findings that fetal adrenaline levels in the plasma are very low(9,10). Greenough et al.(9) found that plasma adrenaline was not detectable in all fetuses, was in general less than 10% of total catecholamines, showed no correlation with gestational age, and demonstrated no evidence of a response to hypoxemia. An early study found that in preterm babies the adrenaline response to delivery is greater than in those at term(19), but more recent evidence has shown a greater catecholamine response in term than in preterm infants(20,21). Noradrenaline levels, but not adrenaline concentrations, were significantly higher in plasma derived from the umbilical cord of babies delivered vaginally compared with those delivered by elective cesarean section at term(22). It is not known at what gestational ages the noradrenaline and adrenaline compartments of the adrenal become reactive, and to what type of stimulus. It would be of interest to investigate adrenaline responses in a future study, using a more sensitive assay method.
Possible fetal sources for the increased plasma noradrenaline after IHV needling include both the adrenal and extra-adrenal chromaffin tissue(23), as well as sympathetic postganglionic neurones. Our data provide no direct evidence for a fetal sympathetic, as opposed to adrenal, response.
The function of the response is unclear. Noradrenaline is essential for mouse fetal development(24) and may well have other functions, including perhaps a mediation of the "brain sparing effect" or redistribution of blood flow in response to, for example, hypoxemia(25). A fetal noradrenaline response is certainly very important by birth(26,27) when, among other adaptation processes, it switches the mature lung from active Cl--fluid secretion to active Na+-fluid absorption in preparation for breathing in air(28). The responses observed here are much smaller than those observed at birth(20,29) but so is the degree of trauma likely to be involved.
In summary, this study has demonstrated that the fetus from 18 to 37 wk gestation can mount an acute noradrenaline response to intrauterine needling via the IHV, and that this is not due to direct transport from the mother.
Abbreviations
- CI:
-
confidence intervals
- COMT:
-
catechol-O-methyl transferase
- IHV:
-
intrahepatic vein
- MA:
-
metadrenaline
- NMA:
-
normetadrenaline
- PCI:
-
placental cord insertion
References
Giannakoulopoulos X, Sepulveda W, Kourtis P, Glover V, Fisk NM 1994 Fetal plasma cortisol and beta-endorphin response to intrauterine needling. Lancet 344: 77–81.
Teixeira J, Fogliani R, Giannkoulopoulos X, Glover V, Fisk NM 1996 Fetal haemodynamic stress response to invasive procedures. [letter] Lancet 347: 624
van Huisseling H, Muijsers GJ, de Haan J, Hasaart TH 1992 The acute response of the umbilical artery pulsatility index to changes in blood volume in fetal sheep. Eur J Obstet Gynecol Reprod Biol 43: 149–55.
Calvert SA, Widness JA, Oh W, Stonestreet BS 1990 The effects of acute uterine ischemia on fetal circulation. Pediatr Res 27: 552–556.
Glover V, Sandler M 1986 Clinical chemistry of monoamine oxidase. Cell Biochem Funct 4: 89–98.
Barnea ERM, Maclusky NJP, Decherney AHM, Naftolin FM 1988 Catechol-O-methyl transferase activity in the human term placenta. Am J Perinatol 5: 121–127.
Brown MJ, Jenner DJ 1981 Novel double-isotope technique for enzymatic assay of catecholamines, permitting high precision, sensitivity and plasma sample capacity. Clin Sci 61: 591–598.
Nicolaides KH, Economics DL, Soothill PW 1989 Blood gases, pH, and lactate in appropriate- and small-for-gestational age fetuses. Am J Obstet Gynecol 161: 996–1001.
Greenough A, Nicolaides KH, Lagercrantz H 1990 Human fetal sympathoadrenal responsiveness. Early Hum Dev 23: 9–13.
Okamura K, Watanabe T, Tanigawara S, Endo H, Iwamoto M, Murotsuki J, Yajima A 1990 Catecholamine levels and their correlation to blood gases in umbilical venous blood obtained by cordocentesis. Fetal Diagn Ther 5: 147–152.
Sandler M, Ruthven CRJ, Contractor SF, Wood C, Booth RT, Pinkerton JHM 1963 Transmission of noradrenaline across the human placenta. Nature 197: 598–600.
Morgan CD, Sandler M, Panigel M 1972 Placental transfer of catecholamines in vitro and in vivo. Am J Obstet Gynecol 112: 1068–1075.
Saarikoski S 1974 Fate of noradrenaline in the human foetoplacental unit. Acta Physiol Scand Suppl 421: 36–48.
Stevens AD, Lumbers ER 1995 Effects of intravenous infusions of noradrenaline into the pregnant ewe on uterine blood flow, fetal renal function, and lung liquid flow. Can J Physiol Pharmacol 73: 202–208.
Gu W, Jones CT, Parer JT 1985 Metabolic and cardiovascular effects on fetal sheep of sustained reduction of uterine blood flow. J Physiol Lond 368: 109–129.
Gu W, Jones CT 1986 The effect of elevation of maternal plasma catecholamines on the fetus and placenta of the pregnant sheep. J Dev Physiol 8: 173–186.
Stevens AD, Lumbers ER 1995 Effects of intravenous infusions of noradrenaline into the pregnant ewe on uterine blood flow, fetal renal function, and lung liquid flow. Can J Physiol Pharmacol 73: 202–208.
Wilburn LA, Jaffe RB 1988 Quantitative assessment of the ontogeny of metenkephalin, norepinephrine and epinephrine in the human fetal adrenal medulla. Acta Endocrinol (Copenh) 118: 453–459.
Newnham JP, Marshall CL, Padbury JF, Lam RW, Hobel CJ, Fisher DA 1984 Fetal catecholamine release with preterm delivery. Am J Obstet Gynecol 149: 888–896.
Greenough A, Lagercrantz H, Pool J, Dahlin I 1987 Plasma catecholamine levels in preterm infants. Effect of birth asphyxia and Apgar score. Acta Paediatr Scand 76: 54–59.
Schwab KO, Breitung B, von Stockhausen HB 1996 Inappropriate secretion of umbilical plasma catecholamines in preterm compared to term neonates. J Perinat Med 24: 373–380.
Moftaquir-Handaj A, Barbe F, Barbarino-Monnier P, Aunis D, Boutroy MJ 1995 Circulating chromogranin A and catecholamines in human fetuses at uneventful birth. Pediatr Res 37: 101–105.
Battaglia G 1969 Ultrastructural observations of the biogenic amines in the carotid body and aortic-abdominal bodies of the human fetus. Z Zellforsch Mikrosk Anat 89: 590–607.
Thomas SA, Matsumoto AM, Palmiter RD 1995 Noradrenaline is essential for mouse fetal development. Nature 374: 643–646.
Gleason CA, Hamm C, Jones MD Jr 1990 Effect of acute hypoxemia on brain blood flow and oxygen metabolism in immature fetal sheep. Am J Physiol 258:H1064–H1069.
Lagercrantz H, Bistoletti P 1977 Catecholamine release in the newborn infant at birth. Pediatr Res 11: 889–893.
Walters DV, Olver RE 1978 The role of catecholamines in lung liquid absorption at birth. Pediatr Res 12: 239–242.
O'Brodovich HM 1996 Immature epithelial Na+-channel expression in one of the pathogenetic mechanisms leading to human neonatal respiratory distress syndrome. Proc Assoc Am Physicians 108: 345–355.
Padbury JF, Roberman B, Oddie TH, Hobel CJ, Fisher DA 1982 Fetal catecholamine release in response to labor and delivery. Obstet Gynecol 60: 607–611.
Author information
Authors and Affiliations
Additional information
This work was supported by Wellbeing and the Henry Smith Charity.
Rights and permissions
About this article
Cite this article
Giannakoulopoulos, X., Teixeira, J., Fisk, N. et al. Human Fetal and Maternal Noradrenaline Responses to Invasive Procedures. Pediatr Res 45, 494–499 (1999). https://doi.org/10.1203/00006450-199904010-00007
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1203/00006450-199904010-00007
This article is cited by
-
Analgesia for fetal pain during prenatal surgery: 10 years of progress
Pediatric Research (2021)
-
„Environmental enrichment“ und Schwangerschaft
Der Gynäkologe (2020)
-
Effect of combined music and touch intervention on pain response and β-endorphin and cortisol concentrations in late preterm infants
BMC Pediatrics (2017)