Abstract
Mice with mucopolysaccharidosis type VII (MPS VII) are devoid of β-glucuronidase and accumulate glycosaminoglycans in lysosomes resulting in bone dysplasia, learning disabilities, and decreased mobility. MPS VII males do not breed and, while MPS VII females occasionally mate with heterozygous males, they do not maintain their young postnatally. Heterozygous matings produce less than 25% MPS VII offspring, but until now it was unclear whether this results from prenatal or postnatal losses. The administration of recombinant β-glucuronidase from birth significantly reduces glycosaminoglycan storage in most tissues, increases life span, and improves the animal's cognitive ability and mobility. To determine whether reproductive failure is corrected by such therapy, male and female MPS VII mice were injected with enzyme at weekly intervals from birth to 5 wk of age (6xinj). Enzyme-replaced MPS VII mice bred when mated together. The 6xinj MPS VII males mated repeatedly until they were killed 135 d postinjection. All mated 6xinj MPS VII females gave birth to two litters, but maintained few of their young. Selective loss of MPS VII offspring was observed in matings between heterozygotes. Analysis of 379 preterm fetuses from heterozygous matings showed a frequency of 24.6% MPS VII pups, indicating that the decreased number of MPS VII pups produced by mating heterozygotes results from postnatal losses. The ovaries of young adult MPS VII mice have follicles and corpora lutea, and the testes generate sperm. Results suggest that the reproductive failure in MPS VII mice is related to impaired mobility and/or impaired cognitive function, and enzyme replacement restores mating capacity.
Similar content being viewed by others
Main
GUS (EC3.2.1.31) deficiency, MPS VII, belongs to a large family of heritable lysosomal storage diseases. The human disease is referred to as Sly syndrome (1). GUS, a lysosomal enzyme found in most mammalian tissues, is involved in the degradation of GAG. The deficiency of GUS in MPS VII mice results from a single nucleotide deletion in exon 10 which causes a frame shift leading to a premature stop codon in the guss transcript (2). The absence of GUS in the murine model allows build-up of GAG in lysosomes, and premature death (3). As in human Sly syndrome, the MPS VII mice have dysmorphic facies, corneal clouding, splenomegaly, short and thick long bones, and significantly reduced mobility. Neurologic defects in untreated MPS VII mice cause both cognitive and functional memory deficiencies (4). Because of the similarities between humans and mice with GUS deficiency, the MPS VII mice have become an important model for testing experimental treatments for MPS VII and other lysosomal storage diseases. Therapeutic strategies have included bone marrow transplantation, ERT, and gene therapy (5–10). We have previously shown that six weekly injections of GUS from birth normalizes bone growth, improves mobility, and dramatically reduces lysosomal storage in most tissues, including cortical neurons (7,11). ERT from birth also improves the performance of the MPS VII mice in activities dependent on spatial learning (12).
The MPS VII mice have reproductive failure that has not been evaluated in detail. Male MPS VII (gusmps/gusmps) mice do not breed and, while some MPS VII females breed when mated to heterozygous (gus+/gusmps) males, they are unable to maintain their young after birth (3). Potential reasons for the MPS VII reproductive failure include the following: 1) lysosomal storage disrupting gametogenesis or uterine function; 2) bone and joint disease causing reduced mobility and/or pelvic outlet restriction; 3) impaired behavioral response to a mating partner due to CNS disease; or 4) combinations of the above. This study was designed to 1) test the hypothesis that ERT, because it reduces bone dysplasia, improves mobility, and normalizes behavior, will have a beneficial effect on reproductive performance of MPS VII mice; and 2) examine testes, ovaries, and uteri of MPS VII mice for pathologic findings that might explain their reproductive failure. The MPS VII females and males were injected with enzyme either from birth to 5 wk of age (6xinj) or once at birth and again at 5 wk of age (2xinj). Matings were set up 1 wk after the final injection, and treated male and female MPS VII mice were tested for the ability to induce pregnancy or become pregnant, respectively. Treated MPS VII females were also tested for mating with heterozygous gus+/gusmps (+/mps) males, and treated MPS VII males were mated to both +/mps and wild type gus+/gus+ (+/+) partners following mating with treated females.
In addition to reproductive failure in MPS VII mice, we have observed that matings between +/mps mice result in only 17.1-19.8%, rather than the 25% expected MPS VII weanlings, indicating death of some MPS VII pups either in utero or postnatally. In a separate experiment, we analyzed the numbers of MPS VII mice present in utero at 18-19 dpc, 1-2 d preterm, to determine whether the less than expected number of MPS VII offspring from heterozygous matings was due to in utero or postnatal loss.
METHODS
Mice. We obtained +/+, +/mps, and mps/mps mice from B6.C-H2bm1/ByBir-+/mps matings. The stock is maintained by brother-sister matings (3). Newborn mice were identified using a fluorometric assay (13,14) that determines the amount of GUS activity in tissue acquired by toe clipping. Identification was confirmed in adults using a diagnostic PCR assay (14).
GUS treatment. Newborn mice were injected i.v. into the superficial temporal vein with 100 µL of buffer alone [10 mM Tris (pH 7.5), 150 mM NaCl, and 1 mM βglycerophosphate] or with 28 000 U of recombinant mouse GUS prepared as previously described (7,11,15). Units of GUS activity represent nanomoles of 4-methylumbelliferyl β-glucuronide substrate hydrolyzed per milligram of protein per hour. In the 6xinj MPS VII pups, the second dose was administered i.p. on d 7, and subsequent weekly doses were i.v. (tail vein). The 2xinj MPS VII mice were injected i.v. at birth and at the 5th wk postnatally.
Breeding protocol. Male/female pairings were established 1 wk after the last enzyme injection and continued for 4 mo. The initial matings were between 6xinj MPS VII parents, between 2xinj MPS VII parents, and between +/mps males and 6xinj or 2xinj MPS VII females. Females were monitored daily for vaginal plugs, to detect when mating had occurred and to identify d 0 of pregnancy. Once females had mated, they were caged separately and body weights were recorded daily. The removed female was subsequently replaced with two untreated females: a +/+ and a +/mps. Thereafter, each untreated female that mated was removed and replaced with a female of like genotype. When females gave birth, the numbers of pups were recorded and the fate of the pups was checked daily until weaning at 28 d. When their pups were weaned, the enzyme-treated MPS VII mothers were returned to identically treated MPS VII males, and the untreated females were removed. Statistical analyses to determine mean, SEM, and probability were performed using SigmaStat211 software from Jandel Scientific (San Rafael, CA). Probability was assessed by t tests, ANOVA, and χ2.
In utero frequency of MPS VII offspring. Matings between untreated +/mps parents were established, and pregnant females were identified by vaginal plugs. Pregnant females were killed 18-19 dpc, 1-2 d before term, and pups were removed by cesarean section. The genotypes of the 379 pups were determined by biochemistry and PCR.
Histopathology. Four 6xinj MPS VII males and four 6xinj MPS VII females, two 2xinj MPS VII males and one 2xinj MPS VII female all used in the breeding experiment above were killed at 6 mo of age (135 d after their last injection). Gonads were fixed in 2% glutaraldehyde, 4% paraformaldehyde, postfixed in osmium and embedded in Spurr's resin, sectioned at 0.5 µm, and stained with toluidine blue. The uteri were fixed in neutral buffered formalin, embedded in paraffin, routinely sectioned, and stained with hematoxylin and eosin. Tissues were examined by light microscopy.
Testes were also obtained from a separate ERT regimen (12) in which the six postnatal injections were either with buffer or 28 000 U of recombinant mouse GUS. These mice were tested functionally and were killed at 12 wk of age, 45 d after their last enzyme injection. Mice in this series included nine buffer-treated normal mice, six buffer-treated MPS VII mice, nine GUS-treated normal mice, and 10 GUS-treated MPS VII mice. Ovaries and uteri were also obtained from three untreated MPS VII female mice at each of three time points: 2, 4, and 8 mo of age. Gonads and uteri were fixed in neutral buffered formalin, embedded in paraffin, sectioned, and stained with hematoxylin and eosin for light microscopy.
Three MPS VII mice born of 6xinj MPS VII parents (35 d after their last enzyme injection) and three MPS VII mice born to an untreated +/mps mother mated to a 6xinj MPS VII father were perfused 28 d postnatally with 2% glutaraldehyde, 4% paraformaldehyde. Tissues were prepared as described for the 6xinj MPS VII ovaries above, and sections of brain from mice in the two litters were evaluated. In addition, liver, spleen, eye, and rib from one of the weanlings from the heterozygous mother and archival tissues from other MPS VII weanlings from heterozygous matings were compared with the tissues from weanlings of 6xinj MPS VII mothers.
RESULTS
Enzyme-treated MPS VII parents can mate repeatedly. The 6xinj MPS VII mice appeared clinically robust, had markedly improved bone growth, and were difficult to distinguish from +/+ and +/mps control mice. Data in Table 1 clearly indicate that 2xinj and 6xinj MPS VII males and females mated, as evidenced by the presence of a vaginal plug. The treated males mated throughout the entire 4 mo of the experiment even though their mobility declined during the last few weeks. The health and mobility of GUS-treated MPS VII females were comparable with those of similarly treated males.
The average number of days to plug is presented in Table 1. When a comparison is made of days to plug for 6xinj females (5.0 d), +/mps females (3.8 d), and +/+ (4.0 d) females mated to 6xinj males (A, B, and C, respectively), no significant difference was observed (p = 0.120). A similar comparison cannot be made between groups of females mated to 2xinj males because only two successful matings occurred between 2xinj males and a single 2xinj female remated after her first pregnancy. The increase in the number of days to plug observed in the matings with 2xinj females (E and H) may be explained by small sample number and a large SEM within these groups. It is interesting that vaginal plugs were observed when three untreated MPS VII mothers were mated to three +/+ males (data not shown). These females took an average 5.0 d to plug (SEM = 0.6), similar to +/+ and +/mps mothers. In our MPS VII colony, untreated MPS VII males fail to mate with either untreated MPS VII, +/mps, or +/+ females.
All 6xinj MPS VII females were impregnated twice by their 6xinj MPS VII male partners (A), but one of these females miscarried late in her second pregnancy. Of the 2xinj MPS VII females mated to 2xinj MPS VII males (E), one mated a second time but died before giving birth and the other mouse of this group was not tested for mating because she died inexplicably after the last enzyme injection. As noted above, three untreated MPS VII mothers mated with +/+ males, although only one of these matings resulted in a productive pregnancy leading to a live birth.
All of the treated females that were mated to 6xinj or 2xinj MPS VII males became pregnant. The initial weights of the treated MPS VII females (6xinj mean wt = 19.8 g and 2xinj mean wt = 19.0 g) recorded the day vaginal plugs were observed were not significantly different from initial weights of +/mps and +/+ mothers (mean wt =21.1 g) (p = 0.506). Untreated MPS VII mothers had initial weights (mean wt =17.5 g) significantly less (p = 0.0457) than control mothers. Weight gain during pregnancy was more dependent on the female genotype than on the number of GUS injections the mother received. Both 6xinj and 2xinj MPS VII females averaged gains of 14.5 g, whereas the +/mps and +/+ mothers averaged gains of 18-19 g (p = 0.0345 for 6xinj mothers and p = 0.00896 for 2xinj mothers). Only one of three untreated MPS VII females became pregnant by +/+ males, and her weight gain was 9.6 g (data not shown).
Matings between enzyme-treated MPS VII parents are productive. Table 2 describes the outcome of the pregnancies from the various matings. Although treated MPS VII mothers became pregnant and carried pups to term, these mothers had difficulty maintaining their young in the first few hours immediately after birth. Pups were frequently born at night, and counting of pups born was done early in the morning. Therefore, pups lost at or soon after birth, and subsequently devoured by the mother, were not recorded or genotyped. Partially eaten pups were often noted and were included in the count of total pups born but were not genotyped. Comparison of mean number of pups born to 6xinj MPS VII (A) parents versus those born to +/mps (B) and +/+ (C) mothers mated to 6xinj MPS VII fathers showed a statistically significant difference (p = 0.00475). In an all-pairwise comparison (Dunn's method), p < 0.05 occurred only in comparisons between A versus B and A versus C. When comparing the mean number of pups born to 6xinj and 2xinj MPS VII mothers mated to +/mps fathers (D and H, respectively), a significant difference was observed (p = 0.0430) indicating that the 2xinj mothers were worse than 6xinj mothers at caring for their young. As expected, when comparing 6xinj (B) and 2xinj (F) fathers mated to +/mps mothers, no significant difference in number of pups born was observed (p = 0.341). Similarly, no difference was observed when 6xinj fathers (C) or 2xinj fathers (G) were mated to +/+ mother (p = 0.7616).
Loss of pups was significantly higher among treated MPS VII females. The percentage of total pups born that survived to weaning, regardless of the father's genotype, was 91.2% among the +/+ mothers (C, G), 78.1% among the +/mps mothers (B, F), 27.3% among the 6xinj MPS VII mothers (A, D), and 25.9% among the 2xinj MPS VII mothers (E, H). Comparison of numbers of surviving pups born to 6xinj MPS VII parents (A) versus +/mps mothers (B) and +/+ (C) mothers sired by 6xinj MPS VII fathers showed a statistically significant difference (p < 0.01). In the all-pairwise comparison of this group, p < 0.05 occurred only in comparisons between A versus B and A versus C.
There was selective loss of MPS VII pups postnatally. The mean number of surviving MPS VII/total surviving pups was used to calculate the observed percentages for each mating type. No MPS VII pups of the 50% expected, survived in matings between enzyme-injected MPS VII females and +/mps males (D, H). The expected frequency of MPS VII pups surviving to weaning for +/mps females mated to 6xinj (B) or 2xinj (F) MPS VII males was 50%. The observed frequencies for these matings were 34.7% and 36.8%, respectively. Chi-square analysis on observed and expected numbers showed p < 0.01, indicating significant and selective loss of the MPS VII pups.
MPS VII pups from heterozygote matings are not lost prenatally. In our experience, +/mps matings yield only 17.1-19.8% MPS VII weanlings rather than the 25% expected from such matings. These values are generated from examination of two independent periods of colony maintenance records. In the first period, 1179 pups were examined at weaning and 233 were identified as MPS VII (19.8%) (3). In a subsequent second period, 1216 pups were genotyped and 208 were MPS VII (17.1%). To answer the question of whether this loss in MPS VII pups occurs in utero, we examined the genotype of 379 fetuses from +/mps matings delivered by cesarean section at 18-19 dpc. Of these, 24.6% were found to be MPS VII by PCR and biochemistry. Chi-square analysis was used to calculate p = 0.0059, indicating this observed frequency of MPS VII pups at 1 to 2 days before birth was not significantly different from the 25% expected from +/mps matings. We conclude that MPS VII pups born to +/mps mothers are lost between birth and weaning.
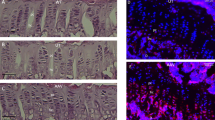
Ovaries, testis, and uteri from enzyme-treated MPS VII mice show lysosomal storage. Follicles were present in all of the untreated MPS VII mice and corpora lutea were identified in four of the six untreated MPS VII mice examined at 2 and 4 mo of age (Fig. 1A), but corpora lutea were not seen in the three 8-mo-old untreated females. The untreated females also showed storage in interstitial cells (Fig. 1C) comparable with treated females examined 4 mo postinjection (Fig. 1D). The older untreated mice had ceroid-containing cells in the ovarian stroma, and all had uterine storage similar to that seen in the treated MPS VII females. The ovaries from enzyme-treated MPS VII breeding females examined 4 mo after the last enzyme injection all showed follicles and corpora lutea indicating probable ovulation (Fig. 1B), along with scattered clusters of interstitial cells distended with clear vacuoles (Fig. 1D). In these mice, the endometrial stroma contained cells with vacuolated cytoplasm (Fig. 1F) that was not present in the normal females (Fig. 1E). The treated MPS VII mice also had macrophages localized between the uterine muscle layers that were distended with storage.
Ovaries from untreated MPS VII females at 4 mo of age showed follicles (open arrow) and corpora lutea (arrowhead) (bar = 385 µm) (A). Ovaries from 5-mo-old MPS VII breeding females treated with 6xinj GUS also contained both follicles (open arrow) and corpora lutea (arrowhead) (bar = 385 µm) (B). Ovarian interstitium from untreated MPS VII females had clusters of vacuolated cells containing storage material (bar = 24 µm) (C). Also, clusters of interstitial cells containing lysosomal storage (arrow) were observed in the ovarian interstitium of 6xinj MPS VII breeding females (bar = 24 µm) (D). The endometrium and myometrium from normal mice showed no evidence of lysosomal storage (bar = 62 µm) (E). The endometrium and myometrium from 6xinj MPS VII breeding females contained multiple cells distended by vacuoles (arrow) (bar = 64 µm) (F). The testis from a male MPS VII mouse treated with buffer showed interstitial foam cells (arrowhead) and maturing germinal epithelium (bar = 59 µm) (G). Similarly, 6xinj had interstitial foam cells (arrowhead) indicating lysosomal storage in the interstitium (bar = 24 µm) (H, and at higher magnification in I). In addition, seminiferous tubules in testis of 6xinj MPS VII males contained normal maturing germinal epithelium with spermatids in the lumens (bar = 64 µm) (H). A, B, E, F, G, and H were stained with hematoxylin and eosin. C, D, and I were stained with tolouidine blue.
The buffer-treated (Fig. 1G) and the 6xinj MPS VII males killed 7 wk after the last enzyme injection had similar gonadal histology. The enzyme-treated MPS VII males had scattered vacuolated cells in the testicular interstitium (Fig. 1, H and I), but germinal epithelial cell maturation and spermatogenesis had occurred (Fig. 1H).
Lysosomal storage is similar in pups born to heterozygous and treated MPS VII mothers. The extent of lysosomal storage in the brain, including the neocortical neurons, glial cells, hippocampal neurons, cerebellum and leptomeninges, retina, cornea, and viscera was similar in MPS VII weanlings whether the mother was +/mps or MPS VII and treated with GUS. Thus there is no evidence that the genotype of the mother, which could conceivably affect delivery of GUS across the placenta to the fetus or through the mother's milk, influences the storage of the offspring.
DISCUSSION
The results indicate that enzyme treatment of MPS VII parents restores the ability to breed and allows productive pregnancies but has no long-lasting effect on histopathology of reproductive tissues. In addition, we show that MPS VII pups survive in utero but succumb between birth and weaning more often than their +/+ and +/mps littermates. Postnatal losses are even more extreme when the mother is MPS VII (3), though slightly less so if the MPS VII mother is enzyme-treated.
Although untreated MPS VII females do mate with +/+ males, only rarely are such matings productive. It appears from females produce sperm and ovulate, respectively, and that the females reaccumulate uterine storage to the level seen in the untreated MPS VII females by 4 mo after the last enzyme treatment. The fact that enzyme-treated MPS VII males actively pursue females in estrus and that this change in behavior leads to productive matings regardless of the genotype of the female demonstrates that enzyme treatment restores normal mating behavior.
Prior results with ERT from birth in MPS VII mice have demonstrated an improvement in the histopathology of bones, joints, and cortical neurons and an increase in mobility (7,11). the same clinical improvement was apparent in the current experiments. It is not clear whether the normal mating behavior after ERT is due to improvements in CNS or improvements in bones and joints that allow greater mobility. It does appear that even when the mobility has begun to decline posttreatment, at a time when visceral storage returns (11), the males continue to mate. It is known that performance of MPS VII mice in the Morris water maze post-ERT is as good as that of normal mice (12), although parenteral injections of GUS do not reduce GAG storage in all regions of the brain (11). Nevertheless, improved CNS function may contribute to the improved mating performance. Given the positive results of ERT on reproductive performance, it will be of interest to examine the effect of other therapies, such as gene therapy (16), direct enzyme injection in the adult brain, or hematopoietic stem cell transplantation after lethal irradiation of MPS VII mice with shielded gonads.
The fact that all ERT females mate productively, give birth to young, and maintain at least a small portion of their pups to weaning contrast with the reproductive failure characteristic of untreated MPS VII females. As seen in Table 2, observations of average numbers of pups born seem to indicate that 6xinj females and 2xinj females still have difficulty maintaining their young (A, D and E, H). The success of a second pregnancy appears to be enzyme dose-related. The 2xinj mothers have increased difficulty during a second pregnancy, possibly because the bone and joint deformities, pelvic structure, and joint elasticity are less improved than in the 6xinj females. The loss of young postnatally might be prevented by fostering cesarean-derived pups from ERT matings on +/+ mothers if the loss is due to the MPS VII mother's inability to maintain her young.
The less-than-expected number of MPS VII pups from heterozygous matings has been a concern to investigators interested in producing MPS VII mice. Here we show that this loss does not occur before delivery and thus presumably occurs at or after birth. Whether this loss is due to the selective killing of MPS VII pups by +/mps mothers or the reduced fitness of the pups that are eaten after their death is not clear. Certainly, some wild type pups are eaten by their +/+ mothers, but the loss of MPS VII mice appears to be selective. On the other hand, both postnatal enzyme treatment and marrow transplantation (data not shown) rescue the MPS VII pups that survive long enough to be treated. Few treated MPS VII mice die between treatment begun at birth and weaning. It is still not clear, therefore, why some mice survive whereas some of their MPS VII littermates succumb. Inasmuch as the untreated newborn MPS VII mice that survive long enough to be examined have little lysosomal storage and are phenotypically indistinguishable from +/+ littermates, storage accumulation is not likely to be a factor in the postnatal deaths. It would be interesting to know whether some newborn MPS VII pups are more severely affected than those that survive and are eliminated by their mothers who distinguish them because of these features. Some MPS VII human fetuses are hydropic at birth, and some are even diagnosed with prenatal hydrops (17–21). In fact, neonatal hydrops may be the most common manifestation of MPS VII that has until recently gone undiagnosed. Whether lysosomal storage in the placenta impairs its role in supporting murine MPS VII fetuses is unknown. Lysosomal storage has been described in the placenta in humans with MPS VII (22). A systematic examination of the placenta in murine MPS VII has not yet been reported.
Finally, the demonstration that ERT postnatally improves breeding performance and reproduction in the MPS VII mice is extremely useful. The production of sufficient MPS VII mice for experimentation is costly because heterozygous breedings have been required and survival of the MPS VII pups varies between 17.1% and 19.8%. The ability to generate 100% MPS VII offspring is especially valuable for studies aimed at treating MPS VII fetuses. For example, our attempts at bone marrow transplantation and hematopoietic stem cell gene transfer therapy to MPS VII mice in utero have been hampered by the low frequency of MPS VII survivors. Enzyme therapy of MPS VII males and females makes it possible for us to plan pregnancies in which 100% of the fetuses will be MPS VII.
Abbreviations
- GUS:
-
β-glucuronidase
- MPS VII:
-
mucopolysaccharidosis type VII
- GAG:
-
glycosaminoglycan
- ERT:
-
enzyme replacement therapy
- 6xinj:
-
six enzyme injections
- 2xinj:
-
two enzyme injections
- dpc:
-
days postconception
References
Sly WS, Quinton BA, McAlister WH, Rimoin DL 1973 β-Glucuronidase deficiency: report of clinical, radiologic, and biochemical features of a new mucopolysaccharidosis. J Pediatr 82: 249–257
Sands MS, Birkenmeier EH 1993 A single-base-pair deletion in the β-glucuronidase gene accounts for the phenotype of murine mucopolysaccharidosis type VII. Proc Natl Acad Sci U S A 90: 6567–6571
Birkenmeier EH, Davisson MT, Beamer WG, Ganschow RE, Vogler CA, Gwynn B, Lyford KA, Maltias LM, Wawrzyniak CJ 1989 Murine mucopolysaccharidosis type VII: characterization of a mouse with β-glucuronidase. J Clin Invest 83: 1258–1266
Chang PL, Lambert DT, Pisa MA 1993 Behavioural abnormalities in a murine model of a human lysosomal storage disease. Neuroreport 4: 507–510
Birkenmeier EH, Barker JE, Vogler CA, Kyle JW, Sly WS, Gwynn B, Levy B, Pegors C 1991 Increased life span and correction of metabolic defects in murine mucopolysaccharidosis type VII after syngeneic bone marrow transplantation. Blood 78: 3081–3092
Sands MS, Barker JE, Vogler C, Levy B, Gwynn B, Galvin N, Sly WS, Birkenmeier EH 1993 Treatment of murine mucopolysaccharidosis type VII by syngeneic bone marrow transplantation in neonates. Lab Invest 68: 676–686
Sands MS, Vogler C, Kyle JW, Grubb JH, Levy B, Galvin N, Sly WS, Birkenmeier EH 1994 Enzyme replacement therapy for murine mucopolysaccharidosis type VII. J Clin Invest 93: 2324–2331
Wolfe JH, Sands MS, Barker JE, Gwynn B, Rowe LB, Vogler CA, Birkenmeier EH 1992 Reversal of pathology in murine mucopolysaccharidosis type VII by somatic cell gene transfer. Nature 360: 749–753
Marechal V, Naffakh N, Danos O, Heard JM 1993 Disappearance of lysosomal storage in spleen and liver of mucopolysaccharidosis VII mice after transplantation of genetically modified bone marrow cells. Blood 82: 1358–1365
Vogler C, Sands MS, Galvin N, Levy B, Thorp C, Barker JE, Sly WS 1998 Murine mucopolysaccharidosis type VII: the impact of therapies on the clinical coarse and pathology in a murine model of lysosomal storage disease. J Inherit Metab Dis 21: 575–586
Vogler C, Sands MS, Levy B, Galvin N, Birkenmeier EH, Sly WS 1996 Enzyme replacement with recombinant β-glucuronidase: impact of therapy during the first six weeks of life on subsequent lysosomal storage, growth and survival. Pediatr Res 39: 1050–1054
O'Connor L, Erway L, Vogler C, Sly W, Nicholes A, Grubb J, Holmberg S, Sands MS 1998 Improvements in behavior and hearing following enzyme replacement therapy for murine mucopolysaccharidosis type VII. J Clin Invest 101: 1394–1400
Fisher HD, Gonzales-Noriega A, Sly WS 1980 β-Glucuronidase binding to human fibroblast receptors. J Biol Chem 255: 5069–5074
Wolfe JH, Sands MS 1996 Murine mucopolysaccharidosis type VII: a model system for somatic gene therapy of the central nervous system. In: Lowenstein PR, Enquist LW (eds) Protocols for Gene Transfer in Neuroscience: Towards Gene Therapy of Neurological Disorders. New York: John Wiley & Sons Ltd., 253–274.
Grubb JH, Kyle JW, Cody LB, Sly WS 1993 Large scale purification of phosphorylated recombinant human β-glucuronidase from over expressing mouse L cells. FASEB J 7: 1255a( abstr)
Wolfe JH, Deshmane SL, Fraser NW 1992 Herpesvirus vector gene transfer and expression of β-glucuronidase in the central nervous system of MPS VII mice. Nature Genet 1: 379–382
Nelson A, Peterson L, Frampton B, Sly WS 1982 Mucopolysaccharidosis VII (β-glucuronidase deficiency) presenting as nonimmune hydrops fetalis. J Pediatr 101: 574–576
Irani D, Kim HS, El-Hibri H, Dutton RV, Beadet A, Armstrong D 1983 Postmortem observations on β-glucuronidase deficiency presenting as hydrops fetalis. Ann Neurol 14: 486–490
Lissens W, Dedobbeleer G, Foulon W, De Catte L, Charels K, Goossens A, Liebaers I 1991 β-Glucuronidase deficiency as a cause of prenatally diagnosed non-immune hydrops fetalis. Prenat Diagn 11: 405–410
Kagie MJ, Kleijer WJ, Huijmans JG, Maaswinkel P Mooy, Kanhai HH 1992 Beta-glucuronidase deficiency as a cause of fetal hydrops. Am J Med Genet 42: 693–695
Molyneux AJ, Blair E, Coleman N, Daish P 1997 Mucopolysaccharidosis type VII associated with hydrops fetalis: histopathological and ultrastructural features with genetic implications. J Clin Pathol 50: 252–254
Nelson J, Kenny B, O'Hara D, Harper A, Broadhead D 1993 Foamy changes of placental cells in probable β-glucuronidase deficiency associated with hydrops fetalis. J Clin Pathol 46: 370–371
Acknowledgements
The authors thank Drs. John Eppig and Wesley Beamer for critical review of the manuscript, Dr. Patricia Farrar for reviewing the gonadal histology, and Brenda Sprague for both mouse colony maintenance and performing the in utero experiments.
Author information
Authors and Affiliations
Additional information
Supported by an NSRA DK09570 to B.W.S.; R01s DK41082, DK27726, and HL49525 to J.E.B.
Rights and permissions
About this article
Cite this article
Soper, B., Pung, A., Vogler, C. et al. Enzyme Replacement Therapy Improves Reproductive Performance in Mucopolysaccharidosis Type VII Mice But Does Not Prevent Postnatal Losses. Pediatr Res 45, 180–186 (1999). https://doi.org/10.1203/00006450-199902000-00004
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1203/00006450-199902000-00004
This article is cited by
-
Morphologic description of male reproductive accessory glands in a mouse model of mucopolysaccharidosis type I (MPS I)
Journal of Molecular Histology (2020)
-
Growth and endocrine function in patients with Hurler syndrome after hematopoietic stem cell transplantation
Bone Marrow Transplantation (2008)
-
Clinical response to persistent, low‐level β‐glucuronidase expression in the murine model of mucopolysaccharidosis type VII
Journal of Inherited Metabolic Disease (2007)
-
Prevention of systemic clinical disease in MPS VII mice following AAV-mediated neonatal gene transfer
Gene Therapy (2001)