Abstract
Intermittent recordings of Doppler flow velocity and cardiac output are of value during intensive care of the sick newborn infant but result in repeated disturbance of the child. We describe a new device for making continuous precordial recordings of Doppler flow velocity from the pulmonary artery in healthy resting newborn infants. Optimal probe siting was evaluated in six babies, and signals were found to be best when the pulmonary artery was insonated from the mid left parasternum. Continuous recordings were made in 13 other babies. Pulmonary artery velocities and, by calculation, cardiac output were measured continuously over periods ranging from 24 to 60 min. Median right ventricular output ranged widely from 148 to 246 mL·kg−1·min−1. In contrast, for individual babies, the values were remarkably stable: the interquartile ranges varied from 13.2 to 29.9 mL·kg−1·min−1. The simultaneous display of signal power allowed independent assessment of artifactual changes in cardiac output. This technique is feasible in healthy term infants and now requires evaluation in the intensive care setting where it may provide useful information concerning trends and short-term variability in right ventricular output.
Similar content being viewed by others
Main
Noninvasive and detailed assessment of cardiopulmonary hemodynamics in the sick preterm infant is now possible using Doppler echocardiography. However, conventional techniques only allow intermittent observations resulting in repeated disturbance of the infant, which can itself change hemodynamic parameters. To date, no continuous method of measuring cardiac output noninvasively is available for use in the newborn. In contrast, in adult practice, cardiac output measurements have been made continuously using the partially invasive technique of transesophageal Doppler echocardiography (1, 2); routine use is recommended by some units to optimize management in adults with circulatory failure. Miniature transesophageal probes have been developed for use in infants and children (3, 4). However, sizes suitable for the newborn are not yet available.
Continuous Doppler recordings of neonatal cerebral blood flow (5–7) have been possible and, when combined with continuous monitoring of intra-arterial blood pressure (8), arterial oxygen saturation (9), and content, have provided valuable insight into the circulation not obtained through intermittent measurements.
Precordial Doppler echocardiographic signals are easier to obtain in the newborn than in the adult but, although Doppler signals from the aorta and pulmonary artery have been used to derive cardiac output in the newborn (10–15), no method of obtaining continuous recordings has been described. Because the use of such continuous data has an important role during neonatal intensive care, we have developed a new steerable and fixable precordial Doppler probe.
We aimed to evaluate the performance of this newly designed ultrasound probe in the measurement of precordial Doppler echocardiographic signals in healthy term infants and to record these signals continuously for up to 1 h and, thereby, to derive values for cardiac output.
METHODS
Doppler Machinery and Probe
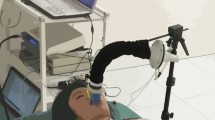
A 4-MHz pulsed-wave Doppler crystal was inserted into a specially designed steerable joystick device that was made in such a way that it could screw into a commercially available disposable adhesive transcutaneous fixation ring (Radiometer, Copenhagen) (Figs. 1 and 2). The signals are then transmitted to and processed by a nonimaging pulsed-wave Doppler system originally designed for use in peripheral vascular Doppler sonography (Dopstation, Scimed Ltd.).
The Doppler frequency-shift signals are displayed in real time and trend along with the power of the signal. Signal power gives an indication of the volume of moving blood within the pulsed-wave Doppler sample gate independently of the speed of blood sampled. As it is related to the emitted ultrasound frequency, the signal power is also known as “relative backscatter power” (16). A decrease in the power of the signal indicates that the sample volume has decreased and implies that the sample gate has moved out of the blood vessel that is being interrogated.
Safety of the 4-MHz Doppler transducer had been examined with respect to potential adverse biologic effects by use of a hydrophone and head amplifier. The ultrasound beam was visualized using a Schlieren system, which showed that the beam was tightly focused at 1.5 cm from the face of the transducer where the energy output of the transducer was highest. Energy output measured 2.26 mW/cm2. The American Institute of Ultrasound in Medicine recommends an acceptable safety level of 100 mW/cm2 (17). Ethical approval for the study of human infants with use of this system was obtained from the local Research Ethics Committee.
Study Population
Twenty-five healthy term infants were recruited from the postnatal wards after one or both parents gave informed consent. Infants were included in the study only if they were in a calm and resting state. Six of these 25 infants became unsettled either at the beginning or during the recording and were excluded. Each infant underwent a clinical cardiovascular examination together with a cross-sectional echocardiogram to exclude structural cardiac abnormality by use of the Hewlett-Packard Sonos 2500 ultrasound machine.
The study was divided into two phases: the first in which different echocardiographic approaches were investigated in six infants, and the second in which continuous measurement of Doppler signals from the pulmonary artery was obtained in 13 infants.
Evaluation of Different Echocardiographic Approaches
Four echocardiographic windows, all of which have been used previously to assess cardiac output in adults (18–21), were evaluated to determine the most useful site for continuous recording of Doppler signals:1) the main pulmonary artery, insonated from the midhigh left parasternum;2) the ascending aorta, insonated from the suprasternal notch or high right parasternum;3) the tricuspid valve, insonated from the lower left sternal edge or apex; and 4) the mitral valve, insonated from the apex.
Main pulmonary artery.
Recordings from the main pulmonary artery were found to be the most stable of all sites investigated. However, during movement, the signal quality was reduced along with a decrease in the power of the signal but returned to the resting value after the infant had settled, provided the joystick position had not changed.
Ascending aorta.
Signals were difficult to obtain continuously from the ascending aorta because of poor adhesion of the mount, particularly in the suprasternal notch. The probe would require redesign to solve this problem. Signals from the high parasternum were obtained for short periods, but small movements of the infant's head deflected the probe, preventing longer-term recordings.
Tricuspid valve.
Recordings of flow across the tricuspid valve were sometimes obtainable for a few minutes with the probe at the lower left sternal edge, but, at the apex, signals were lost with respiration due to excessive ribcage movements during normal resting respiration.
Mitral valve.
Recordings of flow across the mitral valve from the probe positioned at the apex were only intermittently possible for the same reason as for tricuspid flow.
Continuous Recordings
Because recordings from the pulmonary artery were most promising, continuous recording of pulmonary artery flow for a period up to 1 h was attempted in the remaining 13 babies. The study was stopped earlier if the infant became too restless.
Pulmonary artery diameter.
Pulmonary artery diameter was measured in the standard parasternal short-axis view to allow derivation of right ventricular output from the Doppler flow integral. Measurements were taken on five occasions at the hinge points of the valve leaflets seen on the frozen image, using the scroll facility. The average of these five measurements was stored in the Dopstation and used as a constant for the continuous calculation of right ventricular output.
Positioning of the probe.
Initially, the best Doppler signal was obtained manually by using fine manipulation guided by the Doppler signal only, positioning the probe over the pulmonary artery, and searching for a strong stable flow pattern with flow away from the probe. Figure 3 shows the output from the Dopstation: the optimal signal had minimal spectral dispersion, a smooth outline, and the highest velocity within the pulmonary artery. The signals are displayed as frequency shift measured in kHz (top trace). Gain and filter settings were adjusted to minimize artifact and valve clicks. Power of the signals, or relative backscatter power, is displayed at the bottom half of the screen. The range was arbitrarily set between 0 and 64.
The adhesive transcutaneous fixation ring was then positioned at that particular site and the Doppler probe was screwed into it. Further fine adjustment of the direction of the ultrasound beam was achieved by manipulating the joystick within the adhesive ring and adjusting the depth of the pulse Doppler sample gate to give an optimal signal. The depths selected ranged between 2.3 and 2.8 cm. The length of the Doppler sample gate was fixed at 0.5 cm.
Analysis of signals.
The signals were processed by the Dopstation to display the data in real time and trend. The flow velocity integral was calculated by the Dopstation from the mean velocity, averaging the signals over 6-s epochs. This was automatically multiplied by the cross-sectional area of the pulmonary artery (derived from the previously measured pulmonary artery diameter) to give a 6-s flow volume. This was multiplied by 10 and adjusted for birth weight to give values in mL·kg−1·min−1.
RESULTS
Continuous measurements were obtained in 13 infants (aged 13 h to 4.5 d with birth weights ranging from 2890 to 3880 g). The median total recording time was 40 min (range, 24–60 min). During these recordings, movement interfered briefly with the recording to the extent that the signal was either decreased or lost transiently, typically during a wriggle or stretch. However, once the infant settled down again to its original position, the signal returned. Figure 4 shows a typical trace: the top graph represents right ventricular output measured as mL/min recorded over 1 h. The power of the signal (relative backscatter power) is recorded simultaneously over the same period and is displayed at the bottom of the screen. The vertical lines marked the times when the infant moved. If the signal did not restabilize, this was clearly indicated by an altered wave form with reduced velocity and a reduction in the power of the signal, indicating that the decrease in cardiac output was, in fact, artifactual. Signal power decreased sharply during exaggerated movement. This was clearly identifiable from considering the trace. Across the study population, periods during which the signal decreased because of movement were associated with an average decrease in power signal of 28%. This usually occurred when the probe was knocked or the wire pulled during a brief episode of restlessness. The probe was then readjusted to obtain the optimal signal and to return the power of the signal to starting values. The arrows on the trace demarcate the periods of artifactual decrease in cardiac output (Fig. 4).
Printer output of Dopstation showing trending of right ventricular (RV) output (mL/min;top trace) and power of the signal (arbitrary units;bottom trace) over time (62-min screen). Cardiac output expressed in mL·kg−1·min−1 was derived after the recording. The vertical lines are markers denoting infant movement and are associated with a decrease in signal power. Data between arrows were excluded.
Median length of recording was 40 min, varying from 24 to 60 min. Episodes of movement identified by observation in which there was a simultaneous decrease in signal power have been excluded. The percentage of the recording that was excluded together with the length of recording achieved for each infant is given in Table 1. Between 15 and 53% of the total recording time was lost because of movement artifact.
Median right ventricular output was 180 mL·kg−1·min−1 (range, 148–246 mL·kg−1·min−1). Figure 5 shows the median and interquartile ranges for individual children (baby A–M). Whereas the values for median right ventricular output varied widely, for each individual child, the 6-s epochs showed much less variation with interquartile ranges varying only between 13.2 and 29.9 mL·kg−1·min−1, or between 8.3 and 14.5% of the median value.
DISCUSSION
Blood pressure measurement alone is a poor indicator of adequacy of cardiac output. It is well recognized that newborn infants are often able to maintain systemic blood pressure close to normal until cardiovascular collapse approaches. Further, in the preterm infant, there is wide variation in normal blood pressure values (22, 23) associated with adequate peripheral perfusion. Thus, the potential clinical value of a system for recording cardiac output continuously in the newborn is great, particularly if this can be achieved noninvasively. For example, there is increasing evidence that low left and right ventricular output measured with Doppler echocardiography is associated with poor outcome in asphyxiated infants or those with persistent hypoxemia (24, 25).
The system evaluated in this article represents, to our knowledge, the first attempt to record precordial Doppler echocardiographic signals continuously at any age. Newborn infants, especially those with hyaline membrane disease, have good ultrasonic windows because of the relative lack of inflated lung anterior to the heart (air being largely impervious to ultrasound), making this age group potentially the most suitable for such a system. However, we found it impracticable to record flow patterns continuously from the ascending aorta or either inlet valve in unsedated infants. We have the impression that the difficulty with obtaining ascending aortic flow was related mostly to the design of the probe, particularly to its size and shape, which may be possible to overcome in the future. However, we suspect that continuous recordings from the apex will never be possible because of ribcage movement.
The stability of the signals from the pulmonary artery relates to a number of factors. Pulmonary arterial blood flow is directed from the front of the chest toward the back. Thus, the probe can be placed on the infant's chest so it is away from the head and neck but, at the same time, still in line with blood flow. Respiratory movement did not noticeably affect alignment with flow. In contrast, respiration alters alignment with flow through the inlet valves when they are insonated from a fixed part of the chest near the apex of the heart.
The demonstration that continuous recording of right ventricular output was possible in healthy infants for up to 1 h suggests that the technique has considerable potential use in intensive care. Ultimately, we aim to use this system in unwell infants, particularly hypoxemic infants in the first days of life, most of whom will be receiving muscle relaxants or sedation and will be less likely to move the probe themselves. Thus, interference and movement artifacts should be even less of a problem.
The steerable joystick system was very effective in maintaining the recording of Doppler signals from the pulmonary artery in healthy term infants studied when asleep or resting quietly. Even with this method, it was difficult to maintain a stable probe position in the infant who was restless, feeding, or in any other position than prone. This may be somewhat improved by subtle refinements in manufacture of the probe, including making the wire attachment to it lighter than at present, although we doubt recordings will be feasible in the very active infant.
An important future development that will allow this or similar systems to be used routinely will be the automatic detection of changes in position of the Doppler sampling gate. The continuous recording of signal power in this study confirms that decreases in power occur with excessive movement and correlate with decreases in the Doppler-shift signal. True decreases in the mean Doppler-shift frequency (i.e. due to decreasing stroke volume) would be associated with an unchanged power of the signal, as the volume of blood within the sample gate remains constant. The best cutoff point for such an automated system is not yet clear and requires further study.
The absolute values for right ventricular output presented here are much less important than the serial continuous data. Variation in the baseline value can be expected due to error in the measurement of the pulmonary artery diameter. Indeed, the data may better be presented as “minute distance” derived from the Doppler signal [minute distance (cm/min) = flow velocity integral (cm) × heart rate (beats per minute)] (26, 27). This parameter is directly proportional to cardiac output and does not include measurement of pulmonary artery diameter. In future, refinement of the software will allow continuous display of minute distance.
Our data show remarkable stability in right ventricular output in the resting infant. In newborns undergoing intensive care, it is known that cardiac output can change up to 3-fold in response to changes in pulmonary blood flow due to fluctuating pulmonary vascular resistance. It, therefore, seems likely that the technique will be sensitive enough to detect significant change in cardiac output.
From the data presented here, it is not possible to differentiate the separate influences of stroke volume and heart rate on the variability of right ventricular output because the system currently analyzes 6-s epochs, and beat-to-beat counting by the system is unreliable. This additional information could be of important physiologic and clinical interest. We are in the process of developing an ECG gated beat-to-beat counting system.
It is, of course, interesting to speculate whether this system will be of value in neonatal intensive care. Potential future problems with the system in intensive care include interference from ventilator noise and disturbance of the system during handling. Further, turbulence in the pulmonary artery from left-to-right shunting may disturb the Doppler wave form in preterm infants recovering from respiratory failure. The technique would also be limited by factors affecting the echocardiographic window, such as chronic lung disease or pulmonary interstitial emphysema. One may, however, anticipate that it will find a place in the hemodynamic monitoring of larger newborns with hypoxemia or birth asphyxia who typically have low cardiac output (25). Such infants do not have turbulent flow in the pulmonary artery from left-to-right shunting and would clearly benefit from a system to monitor cardiac output.
The present study has shown that continuous recording of right ventricular output is possible in resting healthy infants for up to 1 h. It is, therefore, the first to record cardiac output continuously by a noninvasive means in the human infant. Therefore, it has considerable potential in the study of physiology of the healthy newborn circulation, particularly if further refinements to the probe and its lead attachments allow it to be more stable in the more active infant. The study also provides normative data for comparison with infants undergoing intensive care, where the system is currently being evaluated and further developed.
References
Singer M, Clarke J, Bennett ED 1989 Continuous hemodynamic monitoring by esophageal Doppler. Crit Care Med 17: 447–452
Singer M, Bennett ED 1991 Non-invasive optimization of left ventricular filling using Doppler. Crit Care Med 19: 1132–1137
Murdoch IA, Marsh MJ, Tibby SM, McLuckie A 1995 Continuous haemodynamic monitoring in children: use of transoesophageal Doppler. Acta Paediatr 84: 761–764
Sloth E, Hasenkam JM, Sorensen KE, Pederson J, Olsen KH, Hansen OK 1996 Pediatric multiplane transesophageal echocardiography in congenital heart disease: new possibilities with a miniature probe. J Am Soc Echocardiogr 9: 622–628
Evans DH, Schlindwein FS, Levene MI 1989 An automatic system for capturing and processing ultrasonic Doppler signals and blood pressure signals. Clin Phys Physiol Meas 10: 241–251
Fenton AC, Evans DH, Levene MI 1990 On-line cerebral blood flow velocity and blood pressure measurement in neonates: a new method. Arch Dis Child 65: 11–14
Anthony MY, Evans DH, Levene MI 1991 Cyclical variation in cerebral blood flow. Arch Dis Child 66: 12–16
Evans DH, Lark GM, Archer LNJ, Levene MI 1985 The continuous measurement of intra-arterial pressure in the neonate: method and accuracy. Clin Phys Physiol Meas 7: 179–184
Strauss AW, Escobedo M, Goldring D 1974 Continuous monitoring of arterial oxygen tension in the newborn. J Pediatr 85: 254–261
Alverson DC, Eldridge M, Dillon T, Yabek SM, Berman WJ 1982 Non-invasive pulsed Doppler determination of cardiac output in neonates and children. J Pediatr 101: 46–50
Alverson DC, Eldridge MW, Johnson JD, Aldrich M, Angelus P, Berman WJ 1984 Non-invasive measurement of cardiac output in healthy preterm and term newborn. Am J Perinatol 1: 148–151
Walther FJ, Kim DH, Ebrahimi M, Siassi B, Ramadan NA, Ananda AK 1985 Pulsed Doppler determinations of cardiac output in neonates: normal standards for clinical use. Pediatrics 76: 829–833
Scholler GF, Ceelmajer JM, Wight CM, Bauman AE 1987 Echo Doppler assessment of cardiac output and its relation to growth in normal infants. Am J Cardiol 60: 1112–1116
Alverson DC, Aldrich M, Angelus P, Backstrom C, Werner S 1987 Longitudinal trends in left ventricular cardiac output in healthy infants over the first year of life. J Ultrasound Med 6: 519–524
Mandelbaum-Isken VH, Linderkamp O 1991 Cardiac output by pulsed Doppler in neonates using apical window. Pediatr Cardiol 12: 13–16
Evans DH, McDicken WN, Skidmore R, Woodcock JP 1989 Doppler Ultrasound: Physics, Instrumentation, and Clinical Applications. John Wiley, Chichester, 114–143
AIUM Biological Effects Committee, American Institute of Ultrasound in Medicine. 1987 Statement on mammalian in vivo ultrasonic biological effects. J Clin Ultrasound 5: 2–4
Nishimura RA, Callahan MJ, Schaff HV, Ilstrup DM, Miller FA, Tajik AJ 1984 Non-invasive measurement of cardiac output by continuous-wave Doppler echocardiography: initial experience and review of the literature. Mayo Clin Proc 59: 484–489
Huntsman LL, Stewart DK, Barnes SR, Franklin SB, Colocousis JS, Hessel EA 1983 Non-invasive Doppler determination of cardiac output in man: clinical validation. Circulation 50: 1394–1400
Chandraratna PA, Nanna M, McKay C, Nimalasuriya A, Swinney R, Elkayam U 1984 Determination of cardiac output by transcutaneous continuous-wave ultrasonic Doppler computer. Am J Cardiol 53: 234–237
Fisher DC, Sahn DJ, Friedman MJ, Larson D, Valdes-Cruz LM, Horowitz S 1983 The mitral valve orifice method for non-invasive 2-dimensional echo Doppler determination of cardiac output. Circulation 67: 872–877
Weindling AM 1989 Blood pressure monitoring in the newborn. Arch Dis Child 64: 444–447
Earley A, Fayer P, Ng S, Shinebourne EA, de Swiet M 1980 Blood pressure in the first 6 wk of life. Arch Dis Child 55: 755–757
Evans N, Kluckow M 1996 Early determinants of right and left ventricular output in ventilated preterm infants. Arch Dis Child 74:F88–F94
Skinner JR, Hunter S, Hey EN 1996 Haemodynamic features at presentation in persistent pulmonary hypertension of the newborn and outcome. Arch Dis Child 74:F26–F32
Skinner JR, Boys RJ, Heads A, Hey EN, Hunter S 1996 Estimation of pulmonary arterial pressure in the newborn: study of the repeatability of four Doppler echocardiographic techniques. Pediatr Cardiol 17: 360–369
Childs C, Goldring S, Tann W, Hillier VF 1998 Suprasternal Doppler ultrasound for assessment of stroke distance. Arch Dis Child 79: 251–255
Acknowledgements
The authors thank SciMed Ltd. for technical assistance in the development of the system. We also thank Alan E. Nicklin for the construction of a prototype of the steerable probe.
Author information
Authors and Affiliations
Additional information
Support provided by The Sir Jules Thorn Charitable Trust.
Rights and permissions
About this article
Cite this article
Tsai-Goodman, B., Thorne, G., Whittingham, T. et al. Development of a System to Record Cardiac Output Continuously in the Newborn. Pediatr Res 46, 621 (1999). https://doi.org/10.1203/00006450-199911000-00021
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1203/00006450-199911000-00021