Abstract
Normal control of breathing is characterized by maintenance of CO2 and O2 arterial pressures at constant levels by appropriate ventilatory responses to changes in CO2 production and O2 consumption. Abnormal development of this regulatory system during embryogenesis may produce early impairments in chemosensitivity, as in congenital central hypoventilation syndrome. The present study addresses the role of the mammalian achaete-scute homologous gene (Mash-1) in the development of respiratory control. We analyzed ventilatory responses to hypercapnia (8% CO2, 21% O2, 71% N2) and hypoxia (10% O2, 3% CO2, 87% N2) in newborn and adult Mash-1 heterozygous mice (Mash-1+/−) and their wild-type littermates (Mash-1+/+). Ventilation, breath duration, and tidal volume were measured using whole-body plethysmography. Ventilatory responses to hypercapnia were significantly weaker in newborn male Mash-1+/− compared with Mash-1+/+ mice as a result of a weaker breath-duration response. No differences were observed between adult Mash-1+/− and Mash-1+/+ mice. Our data suggest that Mash-1 may be involved in respiratory control development via mechanisms linked to the X chromosome.
Similar content being viewed by others
Main
Normal control of breathing is characterized by maintenance of CO2 and O2 arterial pressures at constant levels by appropriate ventilatory responses to changes in CO2 production and O2 consumption. These responses are generated chiefly by respiratory neuron networks within the brain stem and by central and peripheral chemoreceptors that provide input to these networks. Abnormal development of this regulatory system during embryogenesis may produce early impairment of chemosensitivity, as in CCHS (1).
The present study addresses the role of Mash-1 in respiratory control development. The rationale for studying the Mash-1 respiratory phenotype is based on two lines of evidence. First, ret gene mutations have been reported in two patients with CCHS and Hirschsprung's disease (2, 3). The ret proto-oncogene has been shown to code for a tyrosine kinase receptor expressed in vagal neural crest derivatives (4). Knockout for the ret gene has been achieved in mice by using homologous recombination (5). Newborn ret knockout mice (ret-/-) had a depressed ventilatory response to hypercapnia and died within a few hours after birth, whereas heterozygous ret+/− mice survived despite an altered response to hypercapnia (6). Second, a molecular link has been shown between Mash-1 and ret. Mash-1 has been reported to code for a basic helix-loop-helix transcription factor (7) and to be expressed in embryonic neurons before the ret gene in vagal neural crest derivatives (5, 8, 9) and in brain stem locus coeruleus neurons (10, 11). In vitro studies found that forced Mash-1 expression induced ret gene expression via expression of another transcription factor, Phox2a (12). Mash-1 activates Phox2a, which in turn directly activates genes coding for catecholaminergic neurotransmitter enzymes expressed in noradrenergic neurons (11, 12). Taken together, these two lines of evidence suggest that Mash-1 may be involved in respiratory control development.
We used the Mash-1 knockout mouse model (7) to investigate this possibility. Homozygous Mash-1-/- mice have multiple severe abnormalities including lack of autonomic gut wall innervation and die within 24 h after birth (7). This complicates specific respiratory function assessment. Heterozygous Mash-1+/− mice, however, seem to develop normally. We tested the hypothesis that heterozygous Mash-1+/− mice may exhibit impaired chemosensitivity responsible for altered responses to hypercapnia and/or hypoxia compared with their wild-type Mash-1+/+ littermates.
METHODS
Subjects
Mash-1 knockout mice (7) were maintained as heterozygotes on a 129SvEv × C57BL6/J hybrid background from heterozygous breed pairs generously supplied by F. Guillemot (Institut de Génétique et de Biologie Moléculaire et Cellulaire, CNRS/INSERM/Université Louis Pasteur, Strasbourg, France). The mice were housed at 24°C with a normal 12/12 h light/dark cycle with light onset at 0800 h and were given water and food ad libitum. Newborn mice were obtained by mating heterozygous females with wild-type males from the same 129SvEv × C57BL6/J hybrid background. Vaginal plugs were examined the next morning, and this day was counted as embryonic d 0 (E0). Pregnant mice were killed by dislocation of the neck on d 18.5 postcoitus (13), i.e. one day before normal littering (E19.5) for this genetic background. Embryos were delivered by cesarean section to control for gestational age. Each newborn was stimulated by pinching the thorax and placed among 1-d-old pups of a Swiss female within 3 min after its mother was killed. Half the Swiss newborns were killed before the cesarean section to facilitate acceptance and feeding of the Mash-1 newborns. Heterozygous newborns (Mash-1+/−) and their wild-type littermates (Mash-1+/+) were tested for ventilatory responses to hypercapnia and hypoxia. Mean time between delivery and ventilatory testing (i.e. the age of the newborns at testing time) was 99 ± 68 min.
Body weights were not significantly different between heterozygous Mash-1+/− newborns and their wild-type Mash-1+/+ littermates or between adult Mash-1+/− and Mash-1+/+ mice (Table 1). Adult mice were 7- to 9-wk old at the time of the study. Experimental protocols met the guidelines of the Institut National de la Santé et de la Recherche Médicale.
Genotyping
Genotype was determined by PCR (14). In newborns, genotyping was performed after respiratory testing by using DNA extracted from brain stem tissue. Adult mice were genotyped before respiratory testing. The sequences of PCR primers were 5′-GCCAGCTCATTCCTCCACTCA-3′ (5′ region of the PGKNeo gene) and 5′-GCAGTTGTAAGATGAACTAC-3′ (3′ region of the Mash-1 gene). The amplification program involved 35 cycles, each of which consisted of 3 min at 94°C, 2 min at 55°C, and 10 min at 72°C. PCR products were analyzed in 1% agarose gel.
Determination of Newborn Gender
Newborn gender was determined by PCR detection of the chromosome Y sry gene (14). Primer sequences were 5′-GAGAGCATGGAGGGCCAT-3′ and 5′-CCACTCCTCTGTGTACACT-3′. The amplification program involved 30 cycles, each of which consisted of 40 s at 96°C, 70 s at 65°C, and 6 min 40 s at 72°C. PCR products were analyzed in 2% agarose gel.
Ventilatory Measurements
Ventilatory parameters in newborn and adult mice were obtained noninvasively using whole-body barometric plethysmography based on Drorbaugh and Fenn's principle (15, 16). According to this principle, when an animal breathes in a nondistensible chamber, the pressure in the chamber increases during inspiration because of addition of water vapor to the inspired gas and to warming of the inspired gas from the temperature in the chamber to that in the alveoli; conversely, pressure decreases during expiration because of condensation of the water vapor and cooling of the expired gas. Measurement of these pressure changes can be used to calculate TTOT, VT, and VE (calculated as VT/TTOT).
Newborn mice.
The plethysmograph for newborn mice was composed of two 30-mL syringes, one containing the animal and the other serving as a reference for determination of pressure differences between the two syringes (pressure transducer EFFA, Le Pré-Saint-Gervais, France; range, ±0.1 mb). The syringes were immersed in a thermoregulated water bath that maintained the temperature inside the syringes between 28 and 30°C. Leakage through a catheter (time constant, 2 s) avoided slow pressure drifts between the two syringes. The pressure signal was filtered (bandwidth, 0.05–15 Hz at −3 dB), converted to a digital signal (MacAdios A/D 12-bits converter, GW Instruments, Somerville, MA) at a sample rate of 100 Hz (17), and used by a computer (PowerWave 604/150) to calculate TTOT (ms), VT (divided by body weight, μL/g), and VE (μL/s/g) (Software Superscope II, GW Instruments) on the basis of Drorbaugh and Fenn's equation (15, 16).
The plethysmograph was calibrated before each test by injecting 5 μL of air into the measurement chamber with a syringe and by introducing the corresponding pressure into Drorbaugh and Fenn's equation. The pressure rise in the measurement chamber induced by this injection was of the same order of magnitude as that resulting from inhalation of a VT by a newborn mouse. To avoid restraining the animals, body temperature was not recorded and was assumed to be stable at 37°C. Hypercapnic (8% CO2, 21% O2, 71% N2) and hypoxic (10% O2, 3% CO2, 87% N2) mixtures were introduced into the measurement chamber through a catheter (5-mL dead space) led through the thermostated bath to prevent drops in chamber temperature during the stimuli.
Adult mice.
The plethysmograph for adult mice was composed of three superimposed cylindrical Plexiglas chambers with a capacity of 0.6, 3.6, and 3.6 L, respectively. The upper chamber was for gas admission and mixing, the middle chamber served as a reference for pressure measurements, and the lower chamber contained the animal. Contrary to the setup for newborns, a 1500-mL/min flow of dry air (Bronkhorst Hi-Tec airflow stabilizer, Uurlo, Holland) was delivered through the admission chamber and then divided into two 750-mL/min flows through the reference and measurement chambers, respectively. This flow avoided CO2 and water accumulation and maintained a constant temperature inside the plethysmograph, which allowed long measurement periods. The positive pressure inside the plethysmograph created by this flow was <0.4 mm Hg, a value associated with a <0.1% error in VT measurements (16). The pressure difference between the reference and animal chambers was measured using a pressure transducer (EFFA, Le Pré-Saint-Gervais, France; range, ±0.1 mb), filtered (bandwidth 0.4–15 Hz at −3 dB), and converted into a digital signal as for the newborns. The plethysmograph was calibrated before each experiment by injecting 100 μL of air into the measurement chamber via a syringe. Body temperature was not recorded and was assumed to be stable at 37°C (17–20). Fractional CO2 and O2 concentrations inside the measurement chamber were measured continuously (Arelco CO2/O2 analyzer, Fontenay-sous-Bois, France) by sampling 100 mL/min from the outflow of the plethysmograph. The temperature inside the measurement chamber was monitored continuously using a thermistor probe. The mean temperature increase was <0.6°C, a value that has been shown to cause an error in VT measurements of <1% (16). Relative humidity was not measured and was assumed to be near zero because the plethysmograph was purged continuously by a dry airflow. However, exploratory measures indicated that the water content of expired gas and urine may have increased humidity up to 15% (corresponding to a partial pressure of water below 4 mm Hg) when the animal was in the cage, thus leading to a VT measurement error of <4% (16).
Procedure
Newborn mice.
Each newborn mouse was placed inside the measurement syringe and allowed a 1-min familiarization period. Breathing-pattern measurements were done by 1) flushing the syringe with 60 mL of air (this took 45 s);2) recording ventilatory data in air for 90 s;3) flushing the syringe with 60 mL of air (45 s);4) injecting 60 mL of the hypercapnic or hypoxic mixture (45 s); and 5) recording breathing variables for 90 s. The time interval between the two stimuli was, therefore, 3 min 45 s. To control for possible aftereffects of a given stimulus (e.g. arousal and aversion due to hypercapnia or hypoxia), the order of stimulation was counterbalanced across the mice: air, hypercapnia, air, hypoxia in half the pups and air, hypoxia, air, hypercapnia in the other half. After the last recording, the newborn was extracted from the syringe, weighed, and killed by neck section. There are, therefore, no data on the survival rate of Mash-1+/− newborns.
Adult mice.
The heterozygous and wild-type mice groups were matched for gender, time of day the experiment was performed (morning, midday, or evening), and order of stimuli (hypercapnia first or hypoxia first). The adult mice were tested according to a previously validated protocol (21). Briefly, each mouse was weighed and placed in a cylindrical wire-mesh cage (9 cm long and 3.5 cm in diameter) secured to the floor of the measurement chamber to avoid movement artifacts. After a 30-min familiarization period, the respiratory stimuli were administered by replacing the airflow through the plethysmograph by a hypercapnic or hypoxic flow (with the same compositions as for the newborns, i.e. 8% CO2, 21% O2, 71% N2 and 10% O2, 3% CO2, 87% N2, respectively) delivered by two cylinders. The switch from air to the hypoxic or hypercapnic mixture was controlled by the computer via electrovalves, and the gas mixtures were allowed to flow into the plethysmograph for 15 min. Steady states of gas concentrations were obtained 10 min after electrovalve opening. Each test consisted of a 15-min air period, a 15-min hypercapnic or hypoxic period, and a 15-min air period. Because of the residual CO2 level inside the plethysmograph (0.5%), the fractional CO2 concentration was 8.5% during hypercapnic stimulation and 3.5% during hypoxic stimulation. Previous studies have shown that normocapnia is maintained in rats exposed to 9% O2 + 4.3% CO2 (22) and in anesthetized dogs exposed to 10% O2 + 3% CO2 (22), suggesting that arterial CO2 pressures remained approximately within the normal range during hypoxic stimulation in the present study.
Data Reduction
Newborn mice.
Ventilatory data free from movement artifacts were selected visually by discarding portions of the tracings without individualized breaths or with large drifts exceeding twice the mean amplitude of the volume signal. TTOT, VT, and VE were first averaged over the selected tracing segments. When several tracing segments were selected for a given condition, we used the weighted mean of the means for each segment. Numbers of breaths and cumulated duration of ventilatory tracings used for the calculations are indicated in Table 2. Baseline breathing variables were those measured during the first air period, because the second air period was influenced by the aftereffects of the first stimulus.
Adult mice.
TTOT, VT, and VE were averaged over successive 15-s periods during the last 3 min of each phase, during which inspired fractions of inspired gas have been shown to remain constant (21). Movement artifacts were small because the mice were restrained, and therefore all the values were considered for analysis.
Statistics
Breathing patterns in newborns and adults were evaluated using separate ANOVA for each population (newborns and adults), for each stimulus (hypoxia and hypercapnia), and for each variable (TTOT, VT, and VE) (Superanova Software, Abacus Concepts, Berkeley, CA). Between-subject factors were group (two levels, heterozygous Mash-1+/−versus wild-type Mash-1+/+) and order of stimuli (two levels, hypercapnia followed by hypoxia or the reverse), and the within-subject factor was gas (two levels, air versus hypercapnia or air versus hypoxia). In newborns, age (in minutes) was added as a cofactor, and, in adults, time of testing (three levels: morning, midday, afternoon) was added as a between-subject factor.
The main effect for gas reflected the ventilatory response to a given stimulus. Consequently, the differences between heterozygous Mash-1+/− and wild-type Mash-1+/+ mice with respect to these responses were evaluated on the basis of the group-by-gas interaction. In addition, gender effects were evaluated on the basis of the gender-by-gas and group-by-gas-by-gender interactions. Data are summarized as the group mean ± SD in the text and as the mean ± SEM in the Figures. Differences were considered significant when p was <0.05.
RESULTS
Newborn mice.
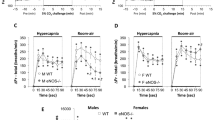
Comparisons of VE and TTOT between heterozygous Mash-1+/− and wild-type Mash-1+/+ newborn mice yielded different results in males and females (Figs. 1 and 2). Baseline VE was significantly lower in heterozygous Mash-1+/− females than in Mash-1+/+ females [F (1,20) = 6.24, p = 0.02], whereas the corresponding difference in males was not significant. Consistent with these findings, there was a significant gender-by-genotype interaction for VE and TTOT [F (1, 39) = 7.27, p = 0.01 and F (1,39) = 5.81, p = 0.02, respectively]. Differences for VT were not significant.
Ventilatory responses to hypercapnia (panel A) and hypoxia (panel B) in female wild-type Mash-1+/+ and heterozygous Mash-1+/− newborn mice. Baseline VE was significantly lower in heterozygous Mash-1+/− (n= 17) than in Mash-1+/+ females (n= 5). The VE response to hypercapnia was significantly stronger in female Mash-1+/− than in female Mash-1+/+ newborns.
The VE response to hypercapnia was significantly weaker in male Mash-1+/− than in male Mash-1+/+ newborn mice (30% ± 67 versus 119% ± 86) as a result of an impaired TTOT response (−27% ± 14 versus 14% ± 36;Fig. 1, panel A). These findings were supported by significant gender-by-genotype-by-gas interactions for VE [F (1,35) = 8.71, p = 0.005] and TTOT [F (1,35) = 5.41, p = 0.02] and by the results of partial comparisons between male Mash-1+/− and male Mash-1+/+ newborns for VE [F (1,17) = 4.43, p = 0.05] and (marginally) for TTOT [F (1,17) = 3.68, p = 0.07].
Female newborns displayed the opposite effects:Mash-1+/− mice showed a significantly stronger VE increase in response to hypercapnia than Mash-1+/+ mice [56% ± 56 versus 32% ± 52;Fig. 2, panel A;F (1,18) = 4.73;p = 0.04]. The differences for VT and TTOT were not significant. However, this was probably due to the lower baseline VE in Mash-1+/− females than in Mash-1+/+ females mentioned above.
The VE response to hypoxia was weaker in male Mash-1+/− than in male Mash-1+/+ newborns (30% ± 82 versus 69% ± 36) as a result of an impaired TTOT response (−20% ± 13 versus 6% ± 29;Fig. 1, panel B). Consistent with this difference, we also found significant gender-by-genotype-by-gas interactions for VE and TTOT [F (1,35) = 5.46, p = 0.02] and [F (1,35) = 5.64, p = 0.02] as well as a smaller VE increase and a smaller TTOT decrease in male Mash-1+/− than in male Mash-1+/+ mice [F (1,17) = 3.69, p = 0.07 and F (1,17) = 3.82, p = 0.06]; however, these last two effects were not significant. Ventilatory responses to hypoxia were not significantly different between Mash-1+/− and Mash-1+/+ female newborns (Fig. 2, panel B). The effects of age were not significant for either ventilatory variable in either condition.
The number of valid breaths was significantly higher in Mash-1+/+ than in Mash-1+/− newborn mice during CO2 exposure, especially in males [main effect of genotype:F (1, 38) = 6.17, p= 0.018 and gender-by-genotype interaction:F (1,38) = 7.38, p= 0.009] because of their higher breathing frequencies. A similar trend was observed during O2 exposure [F (1,38) = 4.32, p= 0.045, gender-by-genotype interaction NS]. The difference was not significant during air exposure. Recording durations were significantly longer in Mash-1+/+ than in Mash-1+/− mice during CO2 and O2 exposures, especially in males [main effects for genotype:F (1,38) = 6.14, p= 0.018 and F (1,38) = 4.33, p= 0.044, respectively; interaction gender-by-genotype:F (1,38) = 7.38, p= 0.001; interaction for O2: NS]. These differences were caused by the fact that Mash-1+/+ newborn mice showed fewer movement artifacts than Mash-1+/− newborn mice, resulting in a smaller loss of valid ventilatory tracings.
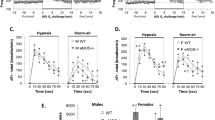
Adult mice.
Neither baseline VE nor ventilatory responses to hypercapnia or hypoxia were significantly different between Mash-1+/+ and Mash-1+/− adults overall or within each gender (Figs. 3 and 4). Baseline TTOT and VT were higher in females than in males [main effect for gender, F (1,49) = 10.91, p = 0.001 and F (1,49) = 5.78, p = 0.02, respectively], but baseline VE values were not significantly different. Females showed a stronger VE response to hypercapnia than males, independently from genotype (Fig. 3, A and Fig. 4, A); this effect was due to a stronger TTOT response [F (1,50) = 5.39, p = 0.02 and F (1,50) = 7.49, p = 0.008, respectively]. The ventilatory response to hypoxia did not show significant gender differences.
DISCUSSION
The main finding from this study is that male newborn mice displayed weaker ventilatory responses to hypercapnia than their wild-type littermates. A similar trend (although not significant) was observed for the response to hypoxia. This difference was caused mainly by a smaller increase in breathing frequency of Mash-1+/− male newborns in response to both stimuli. Baseline breathing patterns were not significantly different between male Mash-1+/+ and Mash-1+/− newborn mice. Among female newborns, the Mash-1+/+ animals had significantly higher baseline VE values, resulting in a weaker hypercapnic response compared with Mash-1+/− mice (however, the validity of this finding is limited by the small number of female Mash-1+/+ newborns). None of the differences noted between Mash-1+/− and Mash-1+/+ newborns was present in adult mice.
Limitations of ventilatory measures in newborn mice.
Baseline VE values in newborn wild-type mice in the present study were similar to those reported by Burton et al. (6) (4.5 versus 3.9 μL/s, respectively); our value would probably have been even closer to theirs had we performed testing on the first day of life, as they did, rather than on E18.5 (neither gender nor exact age was determined in their study). Notwithstanding these comparable VE values, in our study, frequency was twice as high and VT was halved compared with the values reported by Burton et al. (6). These differences may be ascribable to the use of head-out plethysmography in the study by Burton et al. (6) versus whole-body plethysmography in our study. In head-out plethysmography, the head of the newborn mouse emerges from the measurement chamber through an opening that is sealed around the neck; this may increase ventilatory resistance in the trachea, thus slowing the respiratory rate.
In contrast, our baseline VE values were considerably lower than those reported by Kuwaki et al. (13) in mouse pups born at E18.5 by cesarean section (4.5 versus 33 μL/s, respectively). They were also lower than those reported by Jacquin et al. (23) (18.3 μL/s). These differences may be ascribable in part to differences in experimental conditions. In particular, the temperature of the measurement chamber was between 23 and 27°C in the study by Kuwaki et al. (13), 31°C in that by Jacquin et al. (23), and 28°C in our study. In addition, it should be pointed out that the VE differences across these studies are due primarily to differences in VT, a parameter whose determination using Drorbaugh and Fenn's equation can be affected by numerous sources of errors. For example, in the present study, the animal was placed in a dry chamber and the partial pressure of water inside the measurement chamber was considered to be zero, neglecting the humidity generated by the animal. This has been shown to cause an underestimation of VT (16). In the study by Kuwaki et al. (13), the gas within the chamber was assumed to be saturated with water vapor, an approach that has been shown to cause an overestimation of VT (16).
The wide variability in breathing parameter values reported in the literature raises questions about the validity of whole-body plethysmography in newborn mice. Although the basic physical principles of this method logically extend to the small-sized device used for newborn mice, the measures obtained by this method have not been validated against pneumotachography. It follows that the absolute VT and VE values reported in this study should be considered with caution. However, the limitations of plethysmography do not affect the validity of the differences between Mash-1+/+ and Mash-1+/− newborns shown in our study, first, because these differences mainly concerned TTOT, which is reliably measured by whole-body plethysmography (contrary to VT), and second, because any measurement errors affected Mash-1+/+ and Mash-1+/− animals to the same extent. For these same reasons, it is unlikely that the differences were produced by the respiratory effects of cesarean section because this procedure affects VT rather than TTOT, at least in humans (24).
The difference in ventilatory behavior between wild-type Mash-1+/+ and heterozygous Mash-1+/− newborns.
The fact that male Mash-1+/− newborn mice displayed weaker VE responses to hypercapnia (and to a lesser extent to hypoxia) than their male wild-type Mash-1+/+ littermates supports the hypothesis that Mash-1 may play a qualitatively similar role in respiratory control network development to that played by ret. On the other hand, the stronger response to hypercapnia in female Mash-1+/− than Mash-1+/+ newborns seems to militate against this hypothesis. However, this stronger response was probably a result of the high baseline VE and TTOT values in the group of Mash-1+/+ females, which was composed of only five pups. If this was indeed the case, the weak VE responses to hypercapnia of Mash-1+/+ female newborns may have been due to sampling variations. This possibility is further supported by a comparison of findings during the second air period in the hypercapnic versus hypoxic test. This period was not used for baseline level assessment because the aftereffects of the first stimulation tend to cause arousal; this explains the increased VE values during the second air period in the Mash-1+/− females and in the male newborns of both genotypes. In contrast, female Mash-1+/+ newborns had lower VE values during the second than during the first air period, suggesting that inaccuracies occurred in baseline breathing-pattern evaluation in this group. Because gender was not determined in previous breathing-pattern studies in newborn mice (6, 13, 25, 26), published data are not of assistance in resolving this issue.
However, another explanation for gender effects may be Mash-1 modulation of an X-chromosome gene. The X-linked effect of Mash-1 mutation in male and female newborns may be related to the hybrid genetic background of the mice (129SvEv × C57BL6/J). The animals used in our study may have had two distinct alleles for a putative X-chromosome gene regulated by the Mash-1 gene product, one from the 129vEv background and the other from the C57BL6/J background. These two gene alleles may have different levels of expression; for instance, one may be a “low expression” allele and the other a “normal expression” allele. Newborn females may compensate for the defect by random X inactivation. In contrast, a newborn male group may include mice with the low expression allele and mice with the normal expression allele. This hypothesis could be tested by segregating these two alleles by back-crossing mice from the hybrid population used in this study with either 129SvEv mice or C57BL6/J mice. This method may provide a means of identifying the X-chromosome gene whose expression is modified by Mash-1.
Adult mice.
None of the ventilatory differences observed between newborn Mash-1+/+ and Mash-1+/− mice was present in adult mice: adult Mash-1+/+ and Mash-1+/− mice were identical in terms of weight and ventilatory function. Two hypotheses can be put forward to explain this finding. Postnatal impairment of ventilatory responses to hypercapnia (and possibly to hypoxia) in Mash-1+/− mice may have been overcome during development. Although we are not aware of published reports of recovery from early chemosensitivity impairment, this possibility cannot be discarded. Alternatively, the only Mash-1+/− mice to survive to adulthood may be those with the mildest impairments, and, as a result, the adult Mash-1+/− mice included in our study may have been a survival-selected sample with normal respiratory function. Only a longitudinal study from birth to adulthood could clarify this issue.
Possible neurologic mechanisms inducing hypoventilation.
Mash-1 is expressed in the peripheral and central nervous systems including the brain stem, as shown by in situ hybridization (27). Two lines of argument support a direct role of Mash-1 expression in the differentiation of neurons involved in breathing control. First, Mash-1 plays a key role in promoting the differentiation of the peripheral sympathetic and parasympathetic autonomic neurons. This has been established by knockout experiments and manipulation of isolated progenitors (7, 12). It follows that Mash-1+/− animals may have functional abnormalities of brain stem vagal neural crest derivatives such as the vagus nerve and nuclei. Second, Mash-1 may also direct neuron development in brain stem networks, as suggested by a recent study in which the locus coeruleus region of Mash-1-/- embryo brain stems was found to lack noradrenergic neurons (11). Noradrenergic neurons are present in Mash-1+/− embryos, which does not preclude subtle phenotypic abnormalities (11).
Because other groups of brain stem neurons express Mash-1, the possibility exists that Mash-1 may promote the development of neurons involved in the bulbopontine and suprapontine drive to breathing. Taken together, these data support the hypothesis that the impaired ventilatory responses to hypercapnia (and the similar trend observed for hypoxia) seen in our study may be ascribable to a modification in Mash-1 gene expression responsible for abnormal development of subsets of brain stem neurons and/or vagal neural crest derivatives including central and peripheral chemoreceptors.
Putative link with Ret expression.
The sequential expression of Mash-1 followed by the ret gene may account for the impaired VE phenotype found in both ret+/− newborns (6) and male Mash-1+/− newborns (present study). However, Mash-1 expression abnormalities may occur in brain stem cell subsets that do not express ret but do induce the impaired VE phenotype. Discrimination between these two features could be achieved by a study of ret and Mash-1 double mutants.
Finally, the altered hypercapnic responses (and the corresponding trend of the hypoxic responses) observed in male Mash-1+/− newborns may be linked to changes in wakefulness and behavior. On the one hand, Mash-1 and ret genes control the development of noradrenergic neurons in the locus coeruleus (10, 11). Possibly, altered control of wakefulness by the locus coeruleus in heterozygous newborns may affect the breathing pattern. On the other hand, the impaired ventilatory responses in male Mash-1+/− newborns may trigger unpredictable behavioral responses to chemical stimuli, which may explain the longer movement artifacts in the ventilatory tracings of these animals during CO2 and O2 exposures. Conceivably, group differences in motor activity may have induced group differences in breathing pattern, although our data do not prove this hypothesis.
In conclusion, our data support the hypothesis that Mash-1 may be involved in the development of chemically mediated breathing control and may be linked to the X chromosome. Because Mash-1 is expressed upstream of ret, it may be of interest to look for Mash-1 mutations in patients with CCHS.
Abbreviations
- CCHS:
-
congenital central hypoventilation syndrome
- Mash-1 :
-
mammalian achaete-scute homologous gene
- ret :
-
rearranged during transfection
- TTOT:
-
breath duration
- VT:
-
tidal volume
- VE:
-
ventilation
References
Gozal D, Gaultier C (eds) 1996 Proceedings from the First International Symposium on the Congenital Central Hypoventilation Syndrome, New Orleans. Pediatr Pulmonol 23: 133–168
Amiel J, Salomon R, Attié T, Pelet A, Trang H, Mokhtari M, Gaultier C, Munnich A, Lyonnet S 1998 Mutations of the RET-GDNF signaling pathway in Ondine's curse. Am J Hum Gen 62: 715–717
Sakai T, Wakizaka A, Matsuda H, Nirasawa Y, Itoh Y 1988 Point mutation in exon 12 of the receptor tyrosine kinase proto-oncogene RET in Ondine-Hirschsprung syndrome. Pediatrics 101: 924–925
Pachnis V, Mankoo B, Costantini F 1993 Expression of the c-RET proto-oncogene during mouse embryogenesis. Development 119: 1005–1017
Schuchardt A, D'agati V, Larsson-Blomberg L, Costantini F, Pachnis V 1994 Defects in the kidney and enteric nervous system of mice lacking the tyrosine kinase receptor RET. Nature 367: 380–383
Burton MD, Kawashima AA, Brayer JA, Kazemi H, Shannon DC, Schuchardt A, Costantini F, Pachnis V, Kinane TB 1997 RET proto-oncogene is important for the development of respiratory CO2 sensitivity. J Auton Nerv Syst 63: 137–143
Guillemot F, Lo LC, Johnson JE, Auerbach A, Anderson DJ, Joyner AL 1993 Mammalian achaete-scute homolog 1 is required for the early development of olfactory and autonomic neurons. Cell 75: 463–476
Durbec PL, Larsson-Blomberg LB, Schuchardt A, Costantini F, Pachnis V 1996 Common origin and developmental dependence on c-ret of subsets of enteric and sympathetic neuroblasts. Development 122: 349–358
Natarajan D, Grigoriou M, Marcos-Gutierrez CV, Atkins C, Pachnis V 1999 Multipotential progenitors of the mammalian enteric nervous system capable of colonising aganglionic bowel in organ culture. Development 126: 157–168
Morin X, Cremer H, Hirsch MR, Kapur RP, Goridis C, Brunet JF 1997 Defects in sensory and autonomic ganglia and absence of locus coeruleus in mice deficient for the homeobox gene Phox2a. Neuron 18: 411–423
Hirsch MR, Tiveron MC, Guillemot F, Brunet JF, Goridis C 1998 Control of noradrenergic differentiation and Phox2a expression in MASH-1 in the central and peripheral nervous system. Development 125: 599–608
Lo L, Tiveron MC, Anderson DJ 1998 Mash-1 activates expression of the paired homeodomain transcription factor Phox2a, and couples pan-neuronal and subtype-specific components of autonomic neuronal identity. Development 125: 609–620
Kuwaki T, Cao WH, Kurihara Y, Kurihara H, Ling GY, Onodera M 1996 Impaired ventilatory responses to hypoxia and hypercapnia in mutant mice deficient in endothelin-1. Am J Physiol 270:R1279–R1286
Hogan B, Beddington R, Costantini F, Lacy E 1994 Manipulating the Mouse Embryo: Laboratory Manual. Cold Spring Harbor Laboratory Press, Plain View, NY, pp 1–497
Drorbaugh JE, Fenn WO 1955 A barometric method for measuring ventilation in newborn infants. Pediatrics 16: 81–86
Epstein MAF, Epstein RA 1978 A theoretical analysis of the barometric method for measurement of tidal volume. Respir Physiol 32: 105–120
Tankersley CG, Fitzgerald V, Kleeberger SR 1994 Differential control of ventilation among inbred strains of mice. Am J Physiol 267:R1371–R1377
Tankersley CG, Fitzgerald RS, Levitt RC, Mitzner WA, Ewart SL, Kleeberger SR 1997 Genetic control of differential baseline breathing pattern. J Appl Physiol 82: 874–881
Tankersley CG, Fitzgerald RS, Mitzner WA, Kleeberger SR 1993 Hypercapnic ventilatory responses in mice differentially susceptible to acute ozone exposure. J Appl Physiol 75: 2613–2619
Tankersley CG, Kleeberger SR, Russ B, Schwartz A, Smith P 1996 Modified control of breathing in genetically obese (ob/ob) mice. J Appl Physiol 81: 716–723
Dauger S, Nsegbe E, Vardon G, Gaultier C, Gallego J 1998 The effects of restraint on ventilatory responses to hypercapnia and hypoxia in mice. Respir Physiol 112: 215–225
Pepelko WE, Dixon GA 1975 Arterial blood gas in conscious rats exposed to hypoxia, hypercapnia, or both. J Appl Physiol 38: 581–587
Jacquin TD, Borday V, Schneider-Maunoury S, Topilko P, Ghilini G, Kato F 1996 Reorganization of pontine rhythmogenic neuronal networks in Krox-20 knockout mice. Neuron 17: 747–758
Ronca AE, Abel RA, Alberts JR 1996 Perinatal stimulation and adaptation of the neonate. Acta Paediatr 416: 8–15
Erickson JT, Conover JC, Borday V, Champagnat J, Barbacid M, Yancopoulos G, Katz DM 1996 Mice lacking brain-derived neurotrophic factor exhibit visceral sensory neuron losses distinct from mice lacking NT4 and display a severe developmental deficit in control of breathing. J Neurosci 16: 5361–5371
Mortola JP 1984 Breathing pattern in newborns. J Appl Physiol 56: 1533–1540
Guillemot F, Joyner AL 1993 Dynamic expression of the murine achaete-scute homologue Mash-1 in the developing nervous system. Mech Dev 42: 171–185
Acknowledgements
The authors thank Dr. François Guillemot, LGCM, Strasbourg, France, for the kind gift of Mash-1+/− founders.
Author information
Authors and Affiliations
Additional information
C.M. and V.N., respectively, were supported by fellowships from Naître et Vivre and Ligue Nationale contre le Cancer. C.M. and S.R were supported by Fondation pour la Recherche Médicale.
Rights and permissions
About this article
Cite this article
Dauger, S., Renolleau, S., Vardon, G. et al. Ventilatory Responses to Hypercapnia and Hypoxia in Mash-1 Heterozygous Newborn and Adult Mice. Pediatr Res 46, 535 (1999). https://doi.org/10.1203/00006450-199911000-00008
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1203/00006450-199911000-00008