Abstract
Tacrolimus (FK506) is a potent immunosuppressive drug that, when complexed to a family of immunophilin proteins known as FK binding proteins, inhibits calcineurin in T lymphocytes. Although i.v. use of FK506 in pediatric transplant recipients has been linked to development of cardiomyopathies, its mechanism of cardiotoxicity has not been examined in a neonatal animal model. In our study the effects of FK506 were investigated in cardiac myocytes isolated from newborn piglets. The peak amplitude of electrically triggered fura-2 Ca2+ transients was increased in FK506-treated myocytes, but Ca2+ transient duration and baseline fura-2 Ca2+ ratios were not altered. 45Ca2+ uptake by digitonin-lysed piglet cells decreased at pCa ≤ 6.0, indicating that sarcoplasmic reticulum efflux channels were leaky. The results suggest that elevated release of sarcoplasmic reticulum Ca2+ during systole contributes to cardiotoxic effects observed in children.
Similar content being viewed by others
Main
FK506 is a potent immunosuppressive agent used primarily after liver or heart transplantation to treat graft rejection. FK506 binds to a family of FKBP to form a complex that inhibits calcineurin, a serine-threonine phosphatase required for T cell activation. In mammalian heart FKBP12.6 is associated with the ryanodine receptor isoform-2 in cardiac muscle SR(1,2). FKBP binding to the ryanodine receptor modifies SR Ca2+ efflux channel behavior by stabilizing the closed conformation of the channel (for review seeRef. 3). It is thought that FKBP12.6 enhances cooperation among the four subunits of the ryanodine receptor ensuring that channels open to the full conductance state and close tightly. Furthermore, Ca2+ flow is promoted unidirectionally from the SR lumen to the cytoplasm(3). Planar lipid bilayer studies indicate that tacrolimus binding to FKBP12.6 prevents its interaction with the ryanodine receptor, resulting in increased open probability of the efflux channel and flickering among subconductance states(4).
Recent reports have indicated that in pediatric patients, left ventricular hypertrophic cardiomyopathy is associated with i.v. administration of FK506(5,6). A correlation between blood tacrolimus levels and prolongation of the QT interval has also been noted in an adult patient who suffered near fatal cardiac arrhythmias, but congenital long QT syndrome may have been a predisposing factor(7). A study in rabbits found that cardiotoxic effects resulting from long-term i.v. administration of FK506 were dose dependent. Moreover, in both humans and rabbits cardiomyopathies regressed after removing or lowering the dose of the immunosuppressant(5,8).
Clinically, FK506 cardiotoxicity is most often observed in young children. However, studies examining the mechanism of action of FK506 have been primarily carried out with adult rat heart myocytes or using artificial planar lipid bilayers. Accordingly, our work was undertaken to evaluate the effects of FK506 in myocytes isolated from the ventricles of newborn piglet hearts. We have previously shown that neonatal piglet myocytes have a functioning SR and ryanodine receptors that contribute significantly to excitation-contraction coupling(9). Our results suggest that SR Ca2+ release channels (ryanodine receptors) become leaky in the presence of FK506 resulting in abnormal Ca transients.
METHODS
Myocyte isolation. Cardiac myocytes were isolated from the hearts of newborn (< 24 h) mixed breed piglets by a collagenase procedure(9). The use of all animals in this study has been approved by the Institutional Laboratory Animal Care and Use Committee at Ohio State University. Cardiac cell preparations were 86% viable and ATP content averaged 32 nmol/mg. Other properties are described in detail elsewhere(9,10).
45Ca2+ uptake by sarcoplasmic reticulum. 45Ca2+ uptake by digitonin-lysed piglet cardiac myocytes was estimated according to methods described previously(9,11). Piglet myocytes were prepared by washing twice in medium containing (pH 7.2) 20 mM N,N-bis(2-hydroxyethyl)-2-aminoethanesulfonic acid, 11 mM glucose, 100 mM KCl, 16 µM rotenone, 0.2 mM EGTA, and 20 mM K2-oxalate. Cells were then treated with 10 µM oligomycin, 1 mM DTT, 10 mM phosphocreatine, and 0.2 U/mL creatine phosphokinase followed by cell permeabilization with 25 µg recrystallized digitonin/mg myocyte protein. Finally 10 mM MgATP was added to the cell medium, and where indicated, cells were also pretreated with 30 µM FK506. In a second set of experiments, 10 mM procaine and 30 µM recrystallized ruthenium red were also included to inhibit SR efflux channels. Lysed myocytes were warmed to 37°C for 5 min and then presented with 45Ca2+-EGTA (pCa 7.4-5.0) to initiate SR Ca2+ uptake. Myocytes were sampled after 2.5 min and 45Ca2+ uptake by cells estimated in acid extracts by liquid scintillation counting. Cellular protein was measured according to Lowry(12).
Ca2+-dependent fura-2 fluorescent ratio measurements. Isolated piglet myocytes were incubated with 2 µM fura 2-AM for 2 min and postincubated without fura 2-AM for at least 1 hr to facilitate ester hydrolysis. Fura 2-loaded cells were placed in a perifusion chamber mounted on the stage of a Nikon microscope and superfused with Krebs-Henseleit medium (pH 7.3, gassed with 95% O2: 5% CO2) containing 121 mM NaCl, 4.85 mM KCl, 1.2 mM MgSO4, 1.2 mM KH2PO4, 1 mM CaCl2, 25 mM NaHCO3, 5 mM pyruvate, 11 mM glucose and, where indicated, 30 µM FK506. Cells were field stimulated via parallel platinum electrodes, and fluorescence measurements, with excitation alternating between 340 and 380 nm, were obtained with a PTI filterscan system. Data are presented as the ratio of fluorescence intensity at 340 and 380 nm excitation. Peak height is defined as the difference of the fura-2 Ca2+ ratios recorded at baseline and peak fluorescence.
Because of unresolved issues having to do with compartmentation of fura-2(13), background fluorescence, and in vivo computation of Kd(14), attempts to calibrate the fura-2 signal yielded only rough approximations of intracellular Ca2+ levels. However, using a method previously described(15) with modifications in computing β′(16), we estimated that resting free [Ca2+]i of piglet myocytes was 130 ± 20 nM (n = 12 cells) and peak [Ca2+]i during electrical stimulation was 380 ± 40 nM. In those cells responding to FK506, peak [Ca2+]i increased to approximately 600 nM in the presence of the drug, a value comparable to the increase observed in electrically stimulated piglet myocytes treated with 100 nM isoproterenol.
Due to mitochondrial sequestering of the fura-2 dye (accounting for 20-30% of the total cellular dye), peak [Ca2+]i is probably underestimated(13). In addition to compartmentation issues and background fluorescence that hinder calibration of the signal, Hove-Madsen and Bers(14) have demonstrated that binding of fluorescent dyes to cellular proteins alters their calcium-binding properties, leading to uncertainties as to the in vivo value of the Kd. Despite the problems associated with calibration of the fura-2 signal, it is clear that changes in the fluorescence ratio do accurately reflect fluctuations in cytosolic calcium concentration occurring during a contraction-relaxation cycle.
Statistics. For Ca uptake studies, values are given as means ± SEM for n different myocyte preparations. Single cell results are presented as means ± SEM for n cells obtained from five to eight myocyte preparations. Values were compared using t test and significance was determined at p < 0.05.
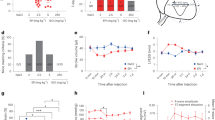
45Ca2+-uptake by digitonin-lysed myocytes. As depicted in Figure 1A, in the absence of inhibitors of the SR Ca2+ efflux channels, oxalate-supported 45Ca2+-uptake by permeabilized myocytes increased as a function of free Ca until [Ca2+] reached approximately 0.6 µM (pCa 6.2). At this point Ca2+-induced Ca2+ efflux from the SR is stimulated and the estimated SR Ca2+ content reflects the equilibrium between active uptake via the Ca pump and loss through the efflux channels. In the presence of 30 µM FK506, SR 45Ca2+ accumulation is reduced compared with control at pCa less than 6.2. At pCa 5.8 SR 45Ca2+ in FK506-treated digitonin lysed myocytes is only 75% of control values (Fig. 1B). In a previous publication we reported that when efflux channels are blocked with procaine and ruthenium red, 45Ca2+ uptake continues to rise reaching a maximum of 285 nmol 45Ca2+/mg/min at pCa 5.0(9). FK506 did not alter the effects of procaine and ruthenium red on SR Ca uptake (Fig. 2).
45Ca2+ uptake in digitonin-lysed piglet cardiac myocytes treated with FK506. Oxalate-supported 45Ca2+ uptake was estimated in myocytes permeabilized with digitonin in the presence of mitochondrial inhibitors, an ATP regenerating system and the appropriate Ca-EGTA buffer (pCa 7.2-5.3) as described in "Methods." Squares, Control; circles, lysed myocytes treated with 30 µM FK506 for 30 min before Ca uptake assay. A, SR accumulation in nmol 45Ca2+/mg/min; B, Ca uptake by FK506-treated cells expressed as % of control. Points are mean ± SEM for six preparations of piglet myocytes. *p < 0.007 compared with control.
Effect of FK506 on intracellular Ca2+ transients. FK506 did not consistently evoke changes in the amplitude of electrically triggered intracellular Ca2+ transients of newborn piglet myocytes. Cells from five (of a total of eight) myocyte preparations, showed increased peak amplitudes of steady state fura-2 ratio fluorescence recordings in the presence of 30 µM FK506 (Fig. 3 and Table 1, maximum amplitude = 160 ± 9% of control, n = 11 cells, p < 0.003 versus nontreated cells), whereas in other preparations, piglet myocytes did not respond to FK506 or had slight declines in peak amplitude (amplitude = 94 ± 3% of control, n = 15, p = 0.2 versus nontreated cells). The duration of the fura-2 Ca2+ transient and resting fura-2 Ca2+ ratios were not altered by FK506 even in cells displaying a positive inotropic response (table 1).
Positive inotropic effect of FK506 on Ca2+ transients in a piglet myocyte. Typical recordings of fura-2 Ca2+ transients under control conditions and in the presence of 30 µM FK506. Traces are steady state signal averaged fluorescence transients from a fura-2 loaded myocyte. y axis is the ratio of fluorescence intensity at 340 and 380 nm excitation in arbitrary units; distance between tics on the x axis is 2 s.
DISCUSSION
In the presence of FK506 accumulation of 45Ca2+ by digitonin-lysed neonatal piglet myocytes was reduced at [Ca2+] of more than 1 µM. This result supports planar lipid bilayer studies showing that FK506 prolonged the mean open lifetime of efflux channels and caused the appearance of different subconductance states(4). Furthermore, in resting adult rat cardiac myocytes treated with the immunosuppressant, McCall et al. observed an increased frequency of occurrence of Ca2+ sparks(17), whereas Xiao et al.(4), using higher concentrations of FK506, found that the duration of spontaneous Ca2+ sparks was prolonged.
Published studies also are in conflict regarding FK506 effects on baseline intracellular Ca2+ levels. Both a global loss of resting SR [Ca2+]i(17) or no alterations in resting [Ca2+]i and caffeine releasable SR Ca2+ content(4) have been reported. Furthermore, in stimulated adult rat myocytes, diastolic intracellular Ca2+ was unchanged(17,18) or increased(4) after exposure to FK506. In our studies baseline fura-2 Ca2+ ratios in electrically stimulated piglet myocytes were not significantly increased in the presence of FK506.
The majority of preparations of newborn piglet myocytes treated with FK506 had higher Ca2+ transient peak amplitudes, results that agree with findings in adult rat myocytes(4,17,18). Although the positive inotropic action of FK506 is thought to arise from its ability to alter the open probability of the ryanodine receptor(4), duBell et al.(18) provide evidence that FK506 inhibits outward K+ currents, thereby prolonging the action potential and secondarily changing Ca2+ flux. However, 45Ca2+ uptake studies in digitonin-lysed piglet cardiac myocytes clearly indicate that Ca2+ gating through SR release channels is compromised by FK506 treatment.
In some piglet cardiac cells exposed to FK506 for up to 40 min, electrically triggered Ca2+ transients were not altered. Although we have shown that ryanodine receptors are present in piglet myocytes and Ca2+ transient peak height is reduced by ryanodine(9), it is possible that efflux channel coupling to the SR or interactions with FKBP are not completely develop at this stage. Accordingly, 45Ca2+ uptake in digitoninlysed adult rat heart myocytes was much more sensitive to ryanodine than piglet myocytes (adult rat heart cells, K0.5 = 1 µM, maximal inhibition of net Ca uptake = 95%(11); piglet cardiac myocytes, K0.5 = 10 µM, maximal ryanodine inhibition = 50% (Hohl CM, unpublished). Moreover, human atrial myocytes isolated from 3-d- to 4-yr- old patients displayed Ca channel-gated release of Ca2+ from the SR already at 3 d, yet there was an age-dependent increase in the rate of Ca2+ release, indicating an increased efficiency of Ca2+ signaling in the more mature myocytes(19). Rats and rabbits also show age-dependent increases in ryanodine receptor function and SR contributions to excitation-contraction coupling(20–22).
In conclusion, we have demonstrated that Ca2+ handling by the SR and electrically triggered Ca2+ transients are altered in newborn piglet myocytes exposed to FK506. These changes are consistent with extensive calcification of cardiac tissue described in a tacrolimus-treated transplant recipient(23) and may be linked to the appearance of cardiomyopathies in children treated with this immunosuppressant.
Abbreviations
- FK506:
-
tacrolimus
- FKBP:
-
FK binding protein
- SR:
-
sarcoplasmic reticulum
- pCa:
-
-log M [Ca2+]
References
Timerman AP, Onoue H, Xin HB, Barg S, Copello J, Wiederrecht G, Fleischer S 1996 Selective binding of FKBP 12:6 by the cardiac ryanodine receptor. J Biol Chem 271: 20385–20391.
Lam E, Martin MM, Timerman AP, Sabers C, Fleischer S, Lukas T, Abraham RT, O'Keefe SJ, O'Neill EA, Wiederrecht GJ 1995 A novel FK506 binding protein can mediate the immunosuppressive effects of FK506 and is associated with the cardiac ryanodine receptor. J Biol Chem 270: 26511–26522.
Marks AR 1997 Intracellular calcium-release channels: regulators of cell life and death. Am J Physiol 272: H597–H605.
Xiao RP, Valdivia HH, Bogdanov K, Valdivia C, Lakatta EG, Cheng H 1997 The immunophilin FK506-binding protein modulates Ca2+ release channel closure in rat heart. J Physiol 500: 343–354.
Atkison P, Joubert G, Barron A, Grant D, Paradis K, Seidman E, Wall W, Rosenberg H, Howard J, Williams S 1995 Hypertrophic cardiomyopathy associated with tacrolimus in paediatric transplant patients. Lancet 1: 894–896.
Chang RK, Alzona M, Alejos J, Jue K, McDiarmid SV 1998 Marked left ventricular hypertrophy in children on tacrolimus (FK506) after orthotopic liver transplantation. Am J Cardiol 81: 1277–1280.
Hodak SP, Moubarak JB, Rodriguez I, Gelfand MC, Alijani MR, Tracy CM 1998 QT prolongation and near fatal cardiac arrhythmia after intravenous tacrolimus administration: a case report. Transplantation 66: 535–537.
Nomoto S, Fujiwara H, Ban T, Ohara K 1994 Cardiotoxicity of long-term intravenous administration of FK506 in rabbits: dose relationship and recovery after discontinuance. Transplant Proc 26: 855–857.
Hohl CM, Livingston B, Hensley J, Altschuld RA 1997 Calcium handling by sarcoplasmic reticulum of neonatal swine cardiac myocytes. Am J Physiol 273: H192–H199.
Livingston BE, Altschuld RA, Hohl CM 1996 Metabolic compartmentalization in neonatal swine myocytes. Pediatr Res 40: 59–65.
Wimsatt DK, Hohl CM, Brierley GP, Altschuld RA 1990 Calcium accumulation and release by the sarcoplasmic reticulum of digitonin-lysed adult mammalian ventricular cardiomyocytes. J Biol Chem 265: 14849–14857.
Lowry OH, Rosebrough NJ, Farr AL, Randall RJ 1951 Protein measurement with the folin phenol reagent. J Biol Chem 183: 265–272.
Spurgeon HA, Stern MD, Baartz G, Raffaeli S, Hansford RG, Talo A, Lakatta EG, Capogrossi MC 1990 Simultaneous measurement of Ca2+, contraction and potential in cardiac myocytes. Am J Physiol 258: H574–H586.
Hove-Madsen L, Bers DM 1992 Indo-1 binding to protein in permeabilized ventricular myocytes alters its spectral and Ca binding properties. Biophys J 63: 89–97.
Li Q, Altschuld RA, Stokes BT 1987 Quantitation of intracellular free calcium in single adult cardiomyocytes by fura-2 fluorescence microscopy: calibration of fura-2 ratios. Biochem Biophys Res Commun 147: 120–126.
Haworth RA 1989 Quantitation of intracellular free calcium in single myocytes by Fura-2 fluorescence microscopy. Cell Calcium 10: 263–264.
McCall E, Li L, Satoh H, Shannon TR, Blatter LA, Bers DM 1996 Effects of FK-506 on contraction and Ca2+ transients in rat cardiac myocytes. Circ Res 79: 1110–1121.
duBell WH, Wright PA, Lederer WJ, Rogers TB 1997 Effect of the immunosuppressant FK506 on excitation-contraction coupling and outward K+ currents in rat ventricular myocytes. J Physiol 501: 3: 509–516
Hatem SN, Sweeten T, Vetter V, Morad M 1995 Evidence for presence of Ca2+ channel-gated Ca2+ stores in neonatal human atrial myocytes. Am J Physiol Heart Circ Physiol 268: H1195–H1201.
Seguchi M, Harding JA, Jarmakani JM 1986 Developmental change in the function of sarcoplasmic reticulum. J Mol Cell Cardiol 18: 189–195.
Fisher DJ, Tate CA, Phillips S 1992 Developmental regulation of the sarcoplasmic reticulum calcium pump in the rabbit heart. Pediatr Res 31: 474–479.
Wibo M, Bravo G, Godfraind T 1991 Postnatal maturation of excitation-contraction coupling in rat ventricle in relation to the subcellular localization and surface density of 1,4-dihydropyridine and ryanodine receptors. Circ Res 68: 662–673.
Atkison PR, Joubert GI, Guiraudon C, Armstrong R, Wall W, Asfar S, Grant D 1997 Arteritis and increased intracellular calcium as a possible mechanism for tacrolimus-related cardiac toxicity in a pediatric transplant recipient. Transplantation 64: 773–775.
Acknowledgements
The authors thank James Hensley for technical assistance. FK506 was kindly provided by Fujisawa Pharmaceutical Co. (Deerfield, IL).
Author information
Authors and Affiliations
Additional information
Supported by National Institutes of Health Grant HL-52793.
Rights and permissions
About this article
Cite this article
Hohl, C., Altschuld, R. FK506 Alters Sarcoplasmic Reticulum Calcium Release in Neonatal Piglet Cardiac Myocytes. Pediatr Res 46, 316–319 (1999). https://doi.org/10.1203/00006450-199909000-00011
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1203/00006450-199909000-00011