Abstract
Hepatocyte proliferation and differentiation occur simultaneously during late mammalian gestation. We hypothesized that regulation of hepatocyte growth and differentiation would be coordinated in late gestation fetal hepatocyte cultures such that proliferation would be most active in a population of less well-differentiated cells. Cultured fetal hepatocytes (embryonic d 19 and 21; E19 and E21) were studied using double staining immunofluorescent microscopy. Differentiation was assessed as staining for α-fetoprotein (AFP), three markers of enzymic differentiation (glucokinase [GK], phosphoenolpyruvate carboxykinase [PEPCK], and carbamoyl phosphate synthase [CPS]), and a hepatocyte cell-cell adhesion molecule (C-CAM). Proliferation was assessed using immunocytochemical detection of proliferating cell nuclear antigen (PCNA) or 5-bromo-2′-deoxy-uridine (BrdU) incorporation into DNA. Fetal hepatocyte cultures consisted of a heterogeneous population of cells, slightly more than half of which were proliferative under defined, growth factor-free conditions. These cultures were heterogeneous for AFP expression. There was no correlation between the expression of AFP and PCNA or AFP and S-phase entry (BrdU staining) during the first 48 h in culture. Similar results were obtained in staining for the enzymic differentiation markers and C-CAM. In addition, the differentiation status of cultured fetal hepatocytes was unrelated to a presumed indicator of mature growth regulation, mitogenic responsiveness to transforming growth factor α (TGFα), and hepatocyte growth factor (HGF). Finally, absence of any correlation between proliferation and differentiated phenotype was supported by in vivo studies using staining for PCNA, AFP, CPS, and PEPCK in liver sections. These results indicate that the developmental program governing differentiation of late gestation fetal rat hepatocytes is independent from mechanisms controlling proliferation.
Similar content being viewed by others
Main
During the latter stages of mammalian development, cells and tissues undergo the morphologic and functional differentiation required to support the transition from fetal to postnatal life. A well-characterized model system for perinatal metabolic adaptation is liver development in the rat(1,2). As liver mass triples during the last three days before term in the rat, functional differentiation simultaneously occurs at a rapid rate. The induction of enzymes of glycogen metabolism and gluconeogenesis provide the metabolic mechanisms required for glucose homeostasis after birth. Other enzyme systems allow the neonatal liver to assume the clearance and detoxification functions provided by the placenta during prenatal life.
The coordination of differentiation and growth control is clear when dealing with model systems for cell types that achieve terminal differentiation and a complete loss of proliferative potential. Examples, including PC12 pheochromocytoma cells(3), 3T3-L1 preadipocytes(4), and mouse myoblasts(5), incorporate density-induced growth arrest into protocols used to induce terminal differentiation in vitro. Unlike the aforementioned cell types, mature mammalian hepatocytes maintain the ability to proliferate. In addition, the induction of hepatocyte proliferation in response to liver injury or loss of liver mass is not accompanied by dedifferentiation(6).
Late gestation fetal rat hepatocytes are capable of a high rate of proliferation under defined culture conditions in the absence of growth factors(7,8). Our prior studies have demonstrated that hepatocytes isolated on embryonic d 19 (E19, term being E21) represent two subpopulations; hepatocytes that are actively proliferating in a nonsynchronous manner when isolated, and hepatocytes that are quiescent at the time of isolation and remain quiescent in vitro under defined, growth factor-free conditions(8). Proliferation of fetal hepatocytes in vivo declines as term approaches(8) such that E21 rat hepatocytes are growth arrested when isolated. However, when placed in culture without growth factors, they enter the cell cycle and proceed through a synchronous wave of DNA synthesis, consistent with in vivo growth inhibition at term.
The experimental model of late gestation fetal rat hepatocytes in primary culture has been used extensively to study molecular mechanisms involved in the control of differentiation. The decision to use this in vitro model for the present studies was based on the potential it provided for mechanistic studies on the relationship between cell cycle control and induction of differentiation. Our studies were designed to test the hypothesis that fetal hepatocytes in primary culture display a reciprocal relationship between degree of differentiation and proliferative activity. Also considered was the hypothesis that the hepatocyte growth arrest that occurs in the term rat fetus is related to the acquisition of differentiated hepatic functions. To test these hypotheses, we examined the cell-by-cell relationship between proliferation and differentiation in primary cultures of late gestation fetal rat hepatocytes. The degree of differentiation was assessed using several markers. α-fetoprotein (AFP) is generally considered to be a marker for proliferative hepatoblasts. Its expression wanes as term approaches. Three markers of enzymic differentiation, glucokinase (GK), phospho enol pyruvate carboxykinase (PEPCK) and carbamoyl phosphate synthetase (CPS) were examined. All are induced before term(1,2). The hepatocyte cell-cell adhesion molecule, C-CAM, was also studied. Its genes undergo transcriptional activation as term approaches, resulting in a marked increase in expression between E15 and E20(9). Using these markers, our results showed an unexpected lack of correlation between induction of a differentiated hepatocyte phenotype and proliferative capacity under defined culture conditions.
METHODS
Fetal and newborn rat hepatocytes were isolated and cultured under defined conditions as described previously(7,8). Culture medium was supplemented MEM that contained hydrocortisone but no insulin. Prior studies(7) showed that the cultures consist of approximately 90% hepatocytes with the remaining cell population comprising a mixture of nonparenchymal cell types. This hepatocyte predominance persists for up to 72 h in culture under the conditions used for these experiments(7). Chamber slides were coated with rat tail collagen and plated at a density of 150 000 cells per 4.2 cm2. Where indicated, rat transforming growth factor α (TGFα; Sigma Chemical Co., St. Louis, MO), human hepatocyte growth factor (HGF; R&D Systems, Inc., Minneapolis, MN), or porcine sodium insulin (Elanco Products, Indianapolis, IN) were added to culture media at a final concentrations of 17, 3, and 17 nM, respectively.
Phenotypic characterization of cultured hepatocytes was carried out using double-label immunofluorescence. Cells were fixed before staining with 100% methanol (-20°C, 10 min), except for detection of C-CAM. For these experiments, primary antibody was applied before fixation for 30 min at 4°C. Analysis of the differentiation status of cells used the following primary antibodies: Sheep anti-rat AFP IgG (Nordic Immunologic Laboratories, Capistrano Beach, CA); sheep anti-GST-rat glucokinase IgG (provided by Dr. Mark A. Magnuson, Vanderbilt University, Nashville, TN); sheep anti-rat PEPCK IgG (provided by Dr. Daryl K. Granner, Vanderbilt University, Nashville, TN); rabbit anti-human CPS IgG (provided by Dr. Gerry Wagenaar, University of Amsterdam, Netherlands); and mouse anti-rat C-CAM IgG2b, MAb 5.4(9). Detection used species and, where appropriate, isotype-specific secondary antibodies conjugated to fluorescein. Negative controls were performed by omission of primary antibody. Two methods were used to identify proliferating hepatocytes; indirect immunostaining for proliferating cell nuclear antigen (PCNA), and detection of 5-bromo-2′-deoxy-uridine (BrdU) incorporation into DNA. Both were accomplished as described previously(8), except that isotype specific secondary antibodies were conjugated to biotin (Boehringer Mannheim, Indianapolis, IN) and detected using streptavidin-Texas red (Vector Laboratories, Burlingame, CA).
Double-label immunofluorescence of fetal liver was carried out using formalin-fixed, paraffin-embedded tissue. After the sections (5 µm) were deparaffinized and rehydrated, staining was performed using the same methods as for cultured hepatocytes.
Data were acquired using either direct photomicroscopy or image acquisition. The latter used a SIT66 camera (Dage-MPIMTI, Michigan City, IN) and Perceptics Frame Grabber with Biovision software (Perceptics Corp., Knoxville, TN). Cells were designated by number and their proliferative status determined by image analysis. The status of staining for differentiation markers was determined visually using the labeled images that showed fluorescein staining only. Thus, the analysis was done with the observer unaware of the proliferation status of individual cells.
RESULTS
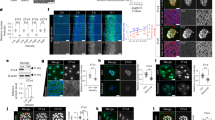
Freshly isolated E19 hepatocytes, fixed after a 2-h attachment period to a collagen substratum, showed nearly uniform positive staining for both AFP and PCNA (not shown). However, after 48 h under defined conditions in the presence of hydrocortisone, 20 to 40% of cells were AFP negative. Cultured E21 (term) hepatocytes showed a much higher proportion of AFP negative cells by 48 h in culture (approximately 80%). Co-analysis for PCNA staining in E19 hepatocytes cultured for 48 h (Fig. 1a) showed no apparent correlation between AFP and PCNA staining. Correlation of AFP immunoreactivity with BrdU incorporation (Figs. 1b and 2) showed that AFP positive cells were no more likely to traverse S-phase during the first 48 h in culture than were AFP negative cells. Similarly, the limited population of proliferative E21 hepatocytes was no more likely to be AFP positive than the nonproliferative cells (Fig. 2).
Immunofluorescent microscopy of cultured fetal rat hepatocytes for markers of differentiation (FITC, yellow/green staining) and proliferation (Texas red). For the latter, cells were stained for PCNA (a) or were stained for BrdU incorporation after 48 h exposure to the nucleotide analogue (b-f). (a) E19 hepatocytes cultured for 24 h and stained for AFP and PCNA. (b) E19 hepatocytes stained for AFP and BrdU. (c) E19 hepatocytes stained for GK and BrdU. (d) E21 hepatocytes stained for PEPCK and BrdU. (e) E19 hepatocytes stained for CPS and BrdU. (f) E19 hepatocytes stained for C-CAM and BrdU.
DNA synthesis by AFP positive vs AFP negative fetal hepatocytes isolated on E19 or E21. After a 2-h attachment period, cells were exposed to BrdU for 48 h. At the end of that time, they were fixed and stained for BrdU and AFP. Data represent nuclear labeling indices for the total cell population (solid bars), AFP-negative cells (open bars) and AFP-positive cells (stippled bars). Similar results were obtained in a second experiment.
We had observed previously that E19 fetal hepatocytes proliferating in mitogen-free defined medium showed TGFα-and and HGF-mediated potentiation of DNA synthesis, which peaked after 40 h of exposure(7). The mitogenic activity of insulin in these cultures was much weaker and had a more marked effect at a shorter duration of exposure. These findings suggested a corollary hypothesis that differentiated cells (AFP negative) would preferentially show a mitogenic response to TGFα, HGF, or insulin, as would be the case for mature adult rat hepatocytes. To test this hypothesis, E19 hepatocytes were exposed continuously to the three mitogens starting after the usual 2-h initial attachment period. BrdU was added from 40 to 48 h in culture. Cells were fixed and stained for BrdU and AFP. Results (Fig. 3) showed that AFP positive cells were no less sensitive to the mitogenic effects of TGFα or HGF than AFP positive cells. As expected, based on past studies, insulin did not potentiate the DNA synthesis of either subset of hepatocytes.
DNA synthesis by AFP positive vs AFP negative fetal hepatocytes isolated on E19 and exposed to hepatocyte mitogens. Cells were cultured for 48 h in MEM without mitogens (open bars), TGFα (stippled bars), HGF (cross-hatched bars) or insulin (solid bars). BrdU was added to the cultures for the final 6 h of the 48 h incubation period. Cells were then fixed and stained for AFP and BrdU. Data represent nuclear labeling indices for the total cell population, AFP negative cells and AFP positive cells. A replicate experiment showed similar results.
Three markers of enzymic differentiation, GK, PEPCK, and CPS, were used to correlate functional differentiation with proliferation (Fig. 1,c-e). For GK and CPS, E19 hepatocytes were maintained in culture for 48 h in the presence of BrdU. For PEPCK, minimal expression was seen on E19; therefore, E21 hepatocytes were studied. As was the case for AFP, dual immunofluorescent staining for these enzymic differentiation markers and the proliferation markers did not show a reciprocal relationship.
Previous studies have shown that the rat liver adhesion molecule, C-CAM, is expressed by E20 fetal hepatocytes in vivo(9). E19 hepatocytes were cultured under defined conditions for 48 h in the presence of BrdU. Double immunofluorescent stains for BrdU and C-CAM showed considerable heterogeneity in expression of the latter. Examination of the double immunofluorescent stains indicated that this marker of differentiation was not predictive for progression through S-phase (Fig. 1f). This was confirmed by image analysis and cell counting (Fig. 4), which showed that BrdU nuclear labeling indices in both E19 and E21 cultured hepatocytes were similar for C-CAM positive and C-CAM negative populations.
DNA synthesis by C-CAM positive vs negative fetal hepatocytes isolated on E19 or E21. After a 2-h attachment period, cells were exposed to BrdU for 48 h. At the end of that time, they were fixed and stained for BrdU and C-CAM. Data represent nuclear labeling indices for the C-CAM positive cells (stippled bars) and C-CAM negative cells (open bars). These results were confirmed in a second experiment.
Given the result that in vitro fetal hepatocyte proliferation was unrelated to differentiation status, we sought to obtain parallel in vivo data. The approach used was to analyze formalin-fixed liver by double immunofluorescent staining. We were able to establish fixation conditions that allowed double staining for three of the differentiation markers, AFP, CPS, and PEPCK, along with PCNA. Results of these studies supported a lack of correlation between staining for PCNA and differentiation markers that was similar to that seen in the primary hepatocyte cultures, although limitations in interpretation of the findings must be taken into account.
AFP and CPS analyses were performed using E19 liver. As expected, based on staining of freshly isolated hepatocytes, E19 liver showed extensive positive staining for AFP. A small number of PCNA positive, AFP negative cells were present (Fig. 5a, arrows). The nuclear size and morphology of these cells were most consistent with their being hepatocytes. However, we could not rule out the possibility that they represented a proliferating, nonparenchymal cell population. In contrast, CPS positive cells, which were clearly visible in E19 liver (Fig. 5b), represent differentiating hepatocytes. Double immunofluorescent staining demonstrated numerous cells positive for both CPS and PCNA. PEPCK staining could not be detected before birth. We therefore stained postnatal liver at a developmental age (3 d postnatal) coincident with the wave of hepatocyte proliferation that occurs 2 to 4 d after birth(7,8). Dual staining for PEPCK and PCNA demonstrated occasional cells positive for both (Fig. 5c, arrows). These in vivo results were interpreted as consistent with the lack of association between differentiation and growth arrest in primary cultures of fetal hepatocytes.
Immunofluorescent microscopy of developing liver for markers of differentiation (FITC, yellow/green staining) and proliferation (Texas red). (a) E19 liver stained for AFP and PCNA. Arrows indicate cells that were AFP-negative and PCNA-positive. (b) E19 liver stained for CPS and PCNA. (c) Liver from a 3-d-old rat pup stained for PEPCK and PCNA. Arrows indicate cells positive for both PEPCK and PCNA.
DISCUSSION
The present studies were based on our prior observations(7,8,10,11) indicating that fetal hepatocytes are programmed to proliferate unless subjected to growth inhibition. As noted above, fetal hepatocytes in vivo undergo a period of temporary quiescence at term(7,8). The growth inhibition is relieved when the cells from E21 fetuses are isolated and cultured. This results in synchronous entry into the cell cycle by a high proportion, but not all, of the cultured hepatocytes. Similarly, within a population of asynchronously proliferative E19 hepatocytes, there is a subpopulation of cells that are quiescent(8).
A second observation upon which our experiments are based relates to the ability of fetal rat hepatocytes to differentiate in vitro when cultured in the presence of hydrocortisone. This has been seen with regard to loss of AFP expression(12) (confirmed in the present studies), and induction of enzymes of intermediary metabolism(13–15). However, these published studies did not include analyses that would test for a relationship between in vitro differentiation and proliferation. Of note, only two published studies have examined this issue. The first used newborn rat hepatocytes isolated on postpartum d 1(16). In these studies, Nakamura et al. used tryptophan 2, 3-dioxy-genase as a marker for "terminal" hepatocyte differentiation. In contrast to our findings, the authors observed a clear reciprocal relationship between expression of this enzyme and proliferation. More recently, Roncero et al.(17) showed that phorbol esters down-regulate AFP gene expression in cultured fetal rat hepatocytes without affecting growth. These results are consistent with our own.
The relationship between hepatocyte differentiation and proliferation has been intensively studied in adult rats and, in particular, in the model of liver regeneration after partial hepatectomy in the rat. Adult rat hepatocytes manipulated through changes in culture conditions can show an inverse relationship between the expression of genes associated with growth versus liver-specific functions(18). Subsequent studies on the CCAAT enhance-binding proteins, C/EBPα and C/EBPβ, at first supported the concept of a reciprocal relationship between proliferation and maintenance of hepatic-specific functions in cultured hepatocytes(19,20) and in hepatocytes proliferating in vivo after partial hepatectomy(20). Buck et al.(21), studying the expression and function of C/EBPβ, concluded that this transcriptional activator, known to support liver-specific functions, acted as an inhibitor of hepatoma cell proliferation. Thus, C/EBPα and C/EBPβ were considered to be involved in a reciprocal relationship between hepatocyte differentiation and proliferation.
More recent studies of C/EBPβ null mice(22) indicate that absence of this transcription factor attenuates hepatocyte proliferation after partial hepatectomy. Furthermore, these animals also showed exaggerated hypoglycemia after partial hepatectomy, suggesting a role for C/EBPβ in simultaneously maintaining liver-specific functions while supporting proliferation. These findings may relate to the mechanisms that account for the observation that hepatocytes in the regenerating liver do not show the morphologic or functional characteristics of "dedifferentiated" hepatocytes(6).
The overall interpretation of our data is that cell cycle arrest does not coincide with the differentiation of fetal rat hepatocytes. This interpretation should be restricted to the animal model tested, because significant differences in growth regulation between fetal hepatocytes from different species are likely. Our main conclusion differs from an obvious, alternative hypothesis; that late gestation and early postnatal liver differentiation reflects the gradual accumulation of quiescent, differentiated hepatocytes. Rather, our data support the presence of simultaneously proliferating and differentiating hepatocytes in the term rat. This is consistent with the behavior of hepatocytes induced to proliferate by partial hepatectomy. Finally, our observations indicate independent signaling pathways for control of proliferation and differentiation in developing fetal and neonatal hepatocytes.
Abbreviations
- E:
-
embryonic day
- AFP:
-
α-fetoprotein
- GK:
-
glucokinase
- PEPCK:
-
phosphoenolpyruvate carboxykinase
- CPS:
-
carbamoyl phosphate synthase
- C-CAM:
-
cell-cell adhesion molecule
- PCNA:
-
proliferating cell nuclear antigen
- BrdU:
-
5-bromo-2′deoxy-uridine
- TGFα:
-
transforming growth factor α
- HGF:
-
hepatocyte growth factor
- C/EBP:
-
CCAAT enhancer-binding protein
References
Dawkins MJ 1966 Biochemical aspects of developing function in newborn mammalian liver. Br Med Bull 22: 27–33
Greengard O 1969 Enzymic differentiation in the mammalian liver. Science 163: 891–895
Greene LA, Tischler AS 1976 Establishment of a noradrenergic clonal line of rat adrenal pheochromocytoma cells which respond to nerve growth factor. Proc Natl Acad Sci USA 73: 2424–2428
Green H, Kehinde O 1975 An established preadipose cell line and its differentiation in culture. II: Factors affecting the adipose conversion. Cell 5: 19–27
Weintraub H 1993 The MyoD family and myogenesis: redundancy, networks and thresholds. Cell 75: 1241–1244
Fausto N, Webber EM 1994 Liver regeneration. In: Arias AMBJ, Fausto N, Jacoby WB, Schlacter D, Shafrits DA (eds) The Liver: Biology and Pathobiology. Raven, New York, pp 1059–1084
Curran TRJ, Bahner RIJ, Oh W, Gruppuso PA 1993 Mitogen-independent DNA synthesis by fetal rat hepatocytes in primary culture. Exp Cell Res 209: 53–57
Gruppuso PA, Awad M, Bienieki TC, Boylan JM, Fernando S, Faris RA 1997 Modulation of mitogen-independent hepatocyte proliferation during the perinatal period in the rat. In Vitro Cell Dev Biol Anim 33: 562–568
Thompson NL, Panzica MA, Hull G, Lin SH, Curran TR, Gruppuso PA, Baum O, Reutter W, Hixson DC 1993 Spatiotemporal expression of two cell-cell adhesion molecule 105 isoforms during liver development. Cell Growth Diff 4: 257–268
Gruppuso PA, Curran TR, Bahner RIJ 1992 Fetal Growth Factors. In: Herrera E, Knopp RH (eds) Perinatal Biochemistry. CRC Press, Boca Raton, pp 193–212
Gruppuso PA, Boylan JM, Bienieki TC, Curran Jr TR 1994 Evidence for a direct hepatotrophic role for insulin in the fetal rat: implications for the impaired hepatic growth seen in fetal growth retardation. Endocrinology 134: 769–775
Milward EA, Yeoh GC 1990 Expression of alpha-fetoprotein by differentiating fetal rat hepatocytes in vitro. Eur J Cell Biol 52: 185–192
Scott RJ, English V, Noguchi T, Tanaka T, Yeoh GC 1988 Pyruvate kinase isoenzyme transitions in cultures of fetal rat hepatocytes. Cell Differ Dev 25: 109–118
Shelly LL, Tynan W, Schmid W, Schutz G, Yeoh GC 1989 Hepatocyte differentiation in vitro: initiation of tyrosine aminotransferase expression in cultured fetal rat hepatocytes. J Cell Biol 109: 3403–3410
Yeoh GC, Edkins E, Mackenzi K, Fuller S, Mercer JF, Dahl HH 1988 The development of phenylalanine hydroxylase in rat liver; in vivo, and in vitro studies utilizing fetal hepatocyte cultures. Differentiation 38: 42–48
Nakamura T, Nagao M, Ichihara A 1987 In vitro induction of terminal differentiation of neonatal rat hepatocytes by direct contact with adult rat hepatocytes. Exp Cell Res 169: 1–14
Roncero C, Ventura JJ, Sanchez A, Bois-Joyeux B, Mesa ML, Thomassin H, Danan JL, Benito M, Fabregat I 1998 Phorbol esters down-regulate alpha-fetoprotein gene expression without affecting growth in fetal hepatocytes in primary culture. Biochim Biophys Acta 1402: 151–158
Ben-Ze'ev A, Robinson GS, Bucher NL, Farmer SR 1988 Cell-cell and cell-matrix interactions differentially regulate the expression of hepatic and cytoskeletal genes in primary cultures of rat hepatocytes. Proc Natl Acad Sci USA 85: 2161–2165
Rana B, Mischoulon D, Xie Y, Bucher NL, Farmer SR 1994 Cell-extracellular matrix interactions can regulate the switch between growth and differentiation in rat hepatocytes: reciprocal expression of C/EBPα and immediate-early response transcription factors. Mol Cell Biol 14: 5858–5869
Mischoulon D, Rana B, Bucher NL, Farmer SR 1992 Growth-dependent inhibition of CCAAT enhancer-binding protein (C/EBPα) gene expression during hepatocyte proliferation in the regenerating liver and in culture. Mol Cell Biol 12: 2553–2560
Buck M, Turler H, Chojkier M 1994 LAP (NF-IL-6), a tissue-specific transcriptional activator, is an inhibitor of hepatoma cell proliferation. EMBO J 13: 851–860
Greenbaum LE, Li W, Cressman DE, Peng Y, Ciliberto G, Poli V, Taub R 1998 CCAAT enhancer-binding protein β is required for normal hepatocyte proliferation in mice after partial hepatectomy. J Clin Invest 102: 996–1007
Acknowledgements
The authors thank Drs. Magnuson, Granner, and Wagenaar for generously providing the antibodies used in these studies, and Patrick Verdier in the Central Research Facilities at Rhode Island Hospital for his skilled assistance with image acquisition and analysis.
Author information
Authors and Affiliations
Additional information
Supported by National Institutes of Health Grants HD24455 (to P.A.G.), HD11343 (to P.A.G.), and CA66005 (to R. A. F.), and by the Rhode Island Hospital Pediatric Research Endowment Funds.
Rights and permissions
About this article
Cite this article
Gruppuso, P., Bienieki, T. & Faris, R. The Relationship between Differentiation and Proliferation in Late Gestation Fetal Rat Hepatocytes. Pediatr Res 46, 14–19 (1999). https://doi.org/10.1203/00006450-199907000-00003
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1203/00006450-199907000-00003