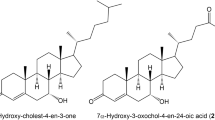
Abstract
Unusual bile acids, such as unsaturated ketonic and 7β-hydroxylated bile acids, have been detected in urine early in life. To elucidate the normal profiles of usual and unusual urinary bile acids in the neonatal and pediatric periods, we measured the concentrations of 28 kinds in urine from normal newborns, infants, and children by gas chromatography-mass spectrometry. The mean total bile acid/Cr ratio in 7-d-old infants was significantly higher than in subjects of other age groups (birth, 2-4 mo, 5-7 mo, 11-12 mo, 2-3 y, 9-14 y, and adult) (p < 0.05). Relatively large amounts of unusual bile acids were detected during infancy, especially during the period up to 1 mo of age. At that time, 1β,3α,7α,12α-tetrahydroxy-5β-cholan-24-oic, 7α,12α-dihydroxy-3-oxo-5β-chol-1-en-24-oic, and 7α,12α-dihydroxy-3-oxo-4-cholen-24-oic acids were predominant among the unusual urinary bile acids present. Moreover, the levels of 3α,7β,12α-trihydroxy-5β-cholan-24-oic acid increased significantly after 2-4 mo of age. These results indicate that bile acid synthesis and metabolism in the liver of developing infants are significantly different from that occurring in the liver of adults. Significant amounts of urinary isomerized 7β-hydroxylated bile acids were detected after late infancy, probably because of changes in the intestinal bacterial flora response to a change in nutrition. We describe, for the first time, evidence of the epimerization of the 7α-hydroxyl group of cholic acid, which may be unique to human development.
Similar content being viewed by others
Main
The newly recognized inborn errors of bile acid metabolism, 3β-hydroxy-Δ5-C27-steroid dehydrogenase/isomerase deficiency(1) and 3-oxo-Δ4-steroid 5β-reductase deficiency(2), involve defects in the transformation of the nucleus of the steroid skeleton and are both associated with familial neonatal cholestasis that progresses to severe liver disease. Therefore, 3β-hydroxy-Δ5- and 3-oxo-Δ4-bile acids, the levels of which increase in individuals with either of these two newly identified inborn errors of bile acid metabolism, have lately attracted considerable attention. Also, the levels of 1β- and 6α-hydroxylated and unsaturated bile acids in urine increase more during the course of cholestatic liver disease than had previously been thought(3–6). These unusual bile acids, especially 1β-hydroxylated bile acids, have been detected in the urine of healthy newborn infants together with usual bile acids such as cholic and chenodeoxycholic acids(7,8). This observation suggests that similarities may exist between altered metabolic states in healthy newborn infants and cholestatic liver disease(4). Moreover, unusual bile acids, such as 2β-, 4β-, and 19-hydroxylated bile acids, have been identified in human biologic fluids(9–12). Interestingly, trace amounts of these unusual bile acids have been detected in the urine of healthy newborn infants(13,14).
Although pediatric hepatologists are well aware of the occurrence of these unusual bile acids in urine, most pediatric physicians know little about this matter. It is important to know the normal levels of usual and unusual bile acids excreted in urine in the neonatal and infantile periods to understand the pathogenesis of inborn errors of bile acid synthesis and neonatal cholestatic syndromes. Therefore, we examined the developmental pattern of urinary excretion of bile acids in normal neonates, infants, and adults by GC-MS with SIM.
The objectives of this study were as follows: a) to elucidate the normal levels of usual and unusual bile acids excreted in urine in the neonatal, infantile, and pediatric periods and b) to elucidate the development pattern of urinary excretion of cholic, 1β,3α,7α,12α-tetrahydroxy-5β-cholan-24-oic, 7α,12α-dihydroxy-3-oxo-5β-chol-1-en-24-oic, 7α,12α-dihydroxy-3-oxo-4-cholen-24-oic, and 3α,7β,12α-trihydroxy-5β-cholan-24-oic acids.
METHODS
Subjects. Twenty-eight healthy neonates (16 males and 12 females), 18 healthy infants (10 males and eight females), 13 healthy children (eight males and five females), and seven healthy adults (four males and three females) were selected as subjects. None of the subjects had a history or showed signs of hepatobiliary or gastrointestinal disease. The infants were fed only breast milk until approximately 6 mo of age. Informed consent was obtained from the adults and from the parents of the newborn infants and children.
Sample collection. Randomly-timed urine samples were collected from each of the subjects and stored at -25°C until analysis. Concentrations of individual bile acids in the urine from each subject were corrected for Cr concentration and expressed as µmol/mmol of Cr.
Materials and reagents. Cholic acid, chenodeoxycholic acid, deoxycholic acid, lithocholic acid, hyocholic acid, and ursodeoxycholic acid were obtained from Sigma Chemical Co. (St. Louis, MO). The other bile acids (Table 1) were synthesized as described previously(9,12,15–18).
Derivatization of bile acids for GC-MS analysis. Each bile acid (2 µg) or mixture of bile acids was dissolved in distilled water (1.0 mL). An equal volume of pyridinium acetate buffer (1.5 M, pH 5) containing 1.0 M O-methylhydroxylamine hydrochloride was added to the solution, and the mixture was kept at 37°C for 2 h. The bile acids were extracted from the solution with a Bond Elut C18 cartridge (3 mL; Varian, Harbor City, CA). The cartridge was washed with water (5 mL), and the bile acids were eluted with ethanol (5 mL). After removal of the solvents by evaporation, the residue was dissolved in 1 mL of 90% aqueous ethanol. The solution was applied to a piperidinohydroxypropyl dextran gel (Shimadzu Co., Kyoto, Japan) column (30 × 6 mm inner diameter) equilibrated with 90% aqueous ethanol. The column was washed with 90% ethanol (4 mL) to remove neutral compounds, and the bile acids were eluted with 0.1 M acetic acid in 90% ethanol (5 mL). After evaporation, the purified bile acid methoximes were derivatized by treatment with diazomethane at room temperature for 10 min to form the methyl esters. After the removal of excess reagent, the dimethylethylsilyl ether was obtained by heating the residue with 30 µL of dimethylethylsilylimidazole (Tokyo Kasei, Japan) at 60°C for 40 min. The resulting preparation was applied to a silica gel column (30 × 6 mm inner diameter) and eluted with n-hexane/ethyl acetate (3:1 by volume). The derivatized bile acids were recovered in the first 5 mL of effluent, and the solvent was evaporated to dryness under reduced pressure. The residue was dissolved in n-hexane (50 µL), and an aliquot (1 µL) was injected into a splitless injection port of the CG-MS system.
Analysis of bile acids in urine. In the standard procedure, samples of human biologic fluids were routinely prepared for CG-MS analysis as described in a preceding paper(19) and as follows. An internal standard (3α,7α-dihydroxy-24-nor-5β-cholan-23-oic acid, 2 µg) was added to 1 mL of each urine sample. The 3-oxo bile acids were derivatized to methoximes, and the conjugated bile acids were extracted from the solution with a Bond Elut C18 cartridge as described above. After the solvents were removed by evaporation, the residue was subjected to enzymatic hydrolysis and solvolysis by treatment with 30 U of choloylglycine hydrolase (Sigma Chemical Co., St. Louis, MO) and 150 U of sulfatase Type H-1 from Helix pomatia (Sigma Chemical Co., St. Louis, MO) in 200 µL of 0.05 M sodium acetate buffer (pH 5.6), 200 µL of 0.6 mM DTT, 200 µL of 0.05 M EDTA, and 100 µL of distilled water at 37°C for 12 h. The resulting unconjugated bile acids were extracted again with a Bond Elut C18 cartridge. The cartridge was washed with 5 mL of distilled water and eluted with 5 mL of 90% ethanol. The unconjugated bile acids were extracted with piperidinohydroxypropyl dextran gel, eluted with 5 mL of 0.1 M acetic acid in 90% ethanol, and converted to the Me-DMES derivatives for GC-MS analysis.
By this method, the recovery of bile acids and their conjugates from urine, relative to the internal standard, ranged from 94.2 to 105.9% of the amount added to the samples. The mean recovery rate of sulfates of lithocholic and 3β-hydroxy-5-cholen-24-oic acids was 98.4 (n = 5) and 94.2% (n = 4), respectively.
GC-MS. GC-MS was performed on a JEOL JMS-AM 150 instrument (JEOL Co., Tokyo, Japan) by use of a gas chromatographic column DB-1 (30 m × 0.2 mm inner diameter, fused silica capillary column bonded with methylsilicon; J & W Scientific, Folsom, CA) with the column temperature programmed from 170 to 230°C at 10°C/min and from 230 to 310°C at 3°C/min. Helium was used as the carrier gas with a flow rate of 45 cm/s. The mass spectra were recorded at an ionization energy of 70 eV with an ion source temperature of 290°C. The film thickness of the stationary phase was 0.1 µm.
We showed a chromatogram obtained by SIM of the characteristic fragments of the Me-DMES-methoxime derivatives of the bile acids in the standard sample(14).
Identification and quantification of individual bile acids. The GC-MS data for individual bile acids are summarized in Table 1, including the relative retention times with respect to the internal standard and their base peak ions.
Statistical analysis. All data are reported as mean ± SD in the Table and as mean ± SE in the Figure. One-way ANOVA was used to determine significance of the differences between groups. Groups were compared using Scheffé's post hoc test. A level of p < 0.05 was accepted as statistically significant.
RESULTS
Urinary concentrations of usual and unusual bile acids (Table 1).
Total bile acids. In the case of newborn infants, the mean total bile acid/Cr ratio rapidly increased between birth (5.62 µmol/mmol Cr) and 7 d after birth (41.94 µmol/mmol Cr), and it gradually decreased thereafter.
The differences in mean total bile acid/Cr ratio between 7-d-old newborn infants and the other groups (at birth, 2-4 mo, 5-7 mo, 11-12 mo, 2-3 y, 9-14 y, and adult) were statistically significant (p < 0.05).
Usual bile acids. The primary bile acids (cholic and chenodeoxycholic) were predominant among usual bile acids in adults during the present study. The mean cholic acid/Cr ratio and chenodeoxycholic acid/Cr ratio increased between birth and 1 mo and between birth and 2-4 mo after birth, respectively, and gradually decreased thereafter. Only trace amounts of secondary bile acids (deoxycholic and lithocholic) were detected in the urine.
Polyhydroxylated bile acids. Relatively large amounts of tetrahydroxylated bile acids, such as 1β,3α,7α,12α-tetrahydroxy-(4.68 µmol/mmol Cr), 2β,3α,7α,12α-tetrahydroxy- (2.60 µmol/mmol Cr), 3α,4β,7α,12α-tetrahydroxy- (2.54 µmol/mmol Cr), and 3α,6α,7α,12α-tetrahydroxy-5β-cholan-24-oic acids (1.24 µmol/mmol Cr) were found in the urine during the present study, especially 7 d after birth. A relatively large amount of hyocholic acid was also detected at 7 d after birth (1.47 µmol/mmol Cr). These polyhydroxylated bile acids were predominant among the urinary bile acids between 7 d and 11-12 mo after birth.
The mean polyhydroxylated bile acid/Cr ratio rapidly increased during the first week of life. However, at 11-12 mo after birth, the mean polyhydroxylated bile acid/Cr ratio declined to values approaching those seen in healthy adults.
Isomerized bile acids. The mean 3β-hydroxy bile acids (3β,7α,12α-trihydroxy- and 3β,4β,7α,12α-tetrahydroxy-5β-cholan-24-oic)/Cr ratio increased between birth and 7 d postpartum, and it gradually decreased thereafter. The mean isomerized 7β-hydroxy bile acids (3α,7β,12α-trihydroxy-5β-cholan-24-oic and ursodeoxycholic)/Cr ratio increased after 2-4 mo of age. Only trace amounts of the allo (5α) bile acids (allocholic and allochenodeoxycholic) were detected.
3β-Hydroxy-Δ5-bile acids. We detected small amounts of 3β,12α-dihydroxy- and 3β-hydroxy-5-cholen-24-oic acids during infancy.
Unsaturated ketonic bile acids. Large amounts of unsaturated ketonic bile acids except for 3-oxo-4,6-choladien-24-oic acid were detected during the first week of life. The unsaturated ketonic bile acids, 3-oxo-Δ1-bile acid (7α,12α-dihydroxy-3-oxo-5β-chol-1-en-24-oic), and 3-oxo-Δ4-bile acids (7α,12α-dihydroxy-3-oxo-4-cholen-24-oic, 12α-hydroxy-3-oxo-4,6-choladien-24-oic, and 7α-hydroxy-3-oxo-4-cholen-24-oic) rapidly increased between birth and 7 d after birth and sharply deceased thereafter.
The mean 7α,12α-dihydroxy-3-oxo-5β-chol-1-en-24-oic acid/Cr ratio showed the greatest increase in the 7-d period after birth (15.93 µmol/mmol Cr, p < 0.05 versus at birth, 1 mo, 2-4 mo, 5-7 mo, 11-12 mo, 2-3 y, 9-14 y, and adult). Also, the mean 7α,12α-dihydroxy-3-oxo-4-cholen-24-oic acid/Cr ratio increased between birth and 7 d after birth (6.34 µmol/mmol Cr). These two bile acids were predominant among the urinary bile acids in the first week of life.
Urinary excretion pattern and mean percentage of bile acids (Fig. 1)
Developmental pattern of urinary excretion of bile acids; mean percentage in relation to total bile acids (TBA). TBA (closed circle); %, percentage of each bile acid in relation to urinary TBA; CA (open square), cholic acid; CA-Δ4-3-one (closed square), 7α,12α-dihydroxy-3-oxo-4-cholen-24-oic acid; CA-1β-ol (open triangle), 1β,3α,7α,12α-tetrahydroxy-5β-cholan-24-oic acid; CA-Δ1-3-one (closed triangle), 7α,12α-dihydroxy-3-oxo-5β-chol-1-en-24-oic acid; DCA (open diamond), deoxycholic acid; UCA (closed diamond), 3α,7β,12α-trihydroxy-5β-cholan-24-oic acid. a, p < 0.05 vs 0 d, 2-4 mo; p < 0.01 vs 5-7 mo, 11-12 mo, 2-3 y, 9-14 y, adult. b, p < 0.05 vs 3-4 d; p < 0.01 vs 1 mo, 2-4 mo, 5-7 mo, 11-12 mo, 2-3 y, 9-14 y, adult. c, p < 0.05 vs 2-4 mo, 5-7 mo, 11-12 mo, 2-3 y, 9-14 y; p < 0.01 vs adult. d, p < 0.01 vs 1 mo; p < 0.001 vs 2-4 mo, 5-7 mo, 11-12 mo, 2-3 y, 9-14 y, adult. e, p < 0.05 vs 1 mo; p < 0.01 vs 2-4 mo, 5-7 mo, 11-12 mo, 2-3 y, 9-14 y, adult. f, p < 0.05 vs 2-3 y, 9-14 y, adult.
We examined the developmental pattern of urinary excretion of cholic, 1β,3α,7α,12α-tetrahydroxy-5β-cholan-24-oic, 7α,12α-dihydroxy-3-oxo-5β-chol-1-en-24-oic, 7α,12α-dihydroxy-3-oxo-4-cholen-24-oic, and 3α,7β,12α-trihydroxy-5β-cholan-24-oic acids and the relative abundance of each as a mean percentage of total bile acids.
The mean percentage of 7α,12α-dihydroxy-3-oxo-4-cholen-24-oic acid gradually decreased from 23.8% at birth to 1.7% at 1 mo after birth, and it was not detected during the period from 2-4 to 11-12 mo of age. Trace amounts of 7α,12α-dihydroxy-3-oxo-4-cholen-24-oic acid (1.4-3.4%) were detected thereafter. In contrast, the mean percentage of cholic acid increased between 7 d after birth (8.4%) and 1 mo after birth (20.7%), and thereafter the levels in urine remained between 9.5 and 17.5% during the present study.
The mean percentage of 7α,12α-dihydroxy-3-oxo-5β-chol-1-en-24-oic acid increased from 23.2% at birth to 29.5% at 3-4 d after birth and sharply decreased to 0.3% at 2-4 mo after birth. Thereafter, 7α,12α-dihydroxy-3-oxo-5β-chol-1-en-24-oic acid was scarcely detected (0-0.6%). The mean percentage of 1β,3α,7α,12α-tetrahydroxy-5β-cholan-24-oic acid gradually increased from 5.1 to 25.2% during the first month of life and gradually deceased until 2-3 y of age (2.2%).
The mean percentage of 3α,7β,12α-trihydroxy-5β-cholan-24-oic acid significantly increased after 2-4 mo of age, from 1.4% at 2-4 mo after birth to 31.0% at 11-12 mo; thereafter, the levels in urine remained between 20.1 and 29.7% during the present study.
DISCUSSION
This investigation of the urinary excretion of bile acids revealed that the mean total bile acid/Cr ratio rapidly increased in the first week after birth, then decreased gradually. However, the high total bile acid/Cr ratio continued until 1 y of age. The mean total bile acid/Cr ratio at 1 y of age was about 3 times greater than in adults (Table 1). The serum concentration of total bile acids in healthy infants significantly exceeds that in children >1 y of age, a condition called physiologic cholestasis(20–22). The high mean total bile acid/Cr ratio in the urine may be attributable to either an enhanced stimulation of the enterohepatic circulation of bile acids or an impaired hepatic clearance or excretion(8). Polyhydroxylated and unsaturated ketonic bile acids may have a high urinary clearance in early infancy.
For polyhydroxylated bile acids such as 1β-, 2β-, 4β-, 6β-, and 19-hydroxylated, the mean bile acid/Cr ratio reached a peak in the urine between 7 d and 1 mo of age and gradually decreased thereafter (Table 1). The formation of these bile acids is probably linked to mechanisms for bile salt excretion in infants with physiologic cholestasis. Unless they are metabolized into more polar compounds, bile acids have a pronounced hepatotoxic effect in neonates(23,24). Cholic and chenodeoxycholic acids are transformed by hydroxylation into 1β-hydroxylated bile acids(3,7,23). The process of 6α-hydroxylation is probably also important in detoxification(6). Moreover, in the early neonatal period, unsaturated ketonic bile acids, especially 7α,12α-dihydroxy-3-oxo-5β-chol-1-en-24-oic acid, may also be subject to this process of detoxification by nuclear hydroxylation(14,25,26).
With respect to ketonic bile acids, high concentrations of 3-oxo-Δ4-bile acids in serum and urine have been associated with a lack or reduced levels of 3-oxo-Δ4-steroid 5β-reductase activity, the enzyme that catalyzes the conversion of 3-oxo-Δ4-cholestene sterol intermediates to 3-oxo-5β-products in the normal pathway for primary bile acid synthesis(2). The mean percentage of urinary 7α,12α-dihydroxy-3-oxo-4-cholen-24-oic acid in relation to total bile acids was higher in the first week after birth than at any other age (Fig 1). This reflects the normal development of bile acid metabolism, including the initial immaturity with respect to expression of liver enzymes such as 3-oxo-Δ4-steroid 5β-reductase. Apparently, after the maturity of the expression of 3-oxo-Δ4-steroid 5β-reductase, the mean percentage of urinary cholic acid in relation to total bile acids increases between 7 d and 1 mo after birth (Fig. 1). After 1 y of age, we detected a low mean percentage of urinary 7α,12α-dihydroxy-3-oxo-4-cholen-24-oic acid in the urine, probably associated with the action of the intestinal bacterial flora due to food supplements(27).
During the first 7 d after birth, 7α,12α-dihydroxy-3-oxo-5β-chol-1-en-24-oic acid was the predominant bile acid in the urine of neonates (Fig. 1). Thereafter, however, the mean percentage of this unsaturated ketonic bile acid decreased, whereas that of 1β,3α,7α,12α-tetrahydroxy-5β-cholan-24-oic acid increased (Fig. 1). These findings suggest that the former may be produced by dehydration of a precursor in the pathway for synthesis of the latter. The former unsaturated ketonic bile acid, however, may not be synthesized in humans. We have detected trace amounts of 7α,12α-dihydroxy-3-oxo-5β-chol-1-en-24-oic acid during late gestation (30-41 wk), which is <1% of urinary total bile acids (as described previously)(13). Further, we have found that >95% of the 1β,7α,12α-trihydroxy-3-oxo-5β-cholan-24-oic acid in the urine was converted into 7α,12α-dihydroxy-3-oxo-5β-chol-1-en-24-oic acid. However, it is unclear whether it was generated spontaneously or during the sample workup. Therefore, we suggest that 1β,7α,12α-trihydroxy-3-oxo-5β-cholan-24-oic acid is acid by dehydration of the β-ketol in urine. Alternatively, 1β,3α,7α,12α-tetrahydroxy-5β-cholan-24-oic acid may be synthesized from 1β,7α,12α-trihydroxy-3-oxo-5β-cholan-24-oic acid by reduction of the 3-oxo group, after 7α,12α-dihydroxy-3-oxo-5β-cholan-24-oic acid has been converted into its corresponding 1β-hydroxylated bile acid(13). 1β,3α,7α,12α-tetrahydroxy-5β-cholan-24-oic acid may be synthesized from cholic acid by hydroxylation at C-1, as the mean percentage of 1β,3α,7α,12α-tetrahydroxy-5β-cholan-24-oic acid in relation to total bile acids in the urine was increased at the same time as that of cholic acid (Fig. 1).
The mean percentage of 3α,7β,12α-trihydroxy-5β-cholan-24-oic acid significantly increased after 2-4 mo of age, whereas that of 1β,3α,7α,12α-tetrahydroxy-5β-cholan-24-oic acid gradually decreased at the same time (Fig. 1). The urinary levels of isomerized 7β-hydroxy bile acids were low in neonates during the period of breast milk feeding. These observations suggest that the large amounts of isomerized 7β-hydroxy bile acids may be related to the use of food supplements(28,29). The 7β-epimers of cholic and chenodeoxycholic acids are frequently detected in the feces and bile of humans. We detected large amounts of isomerized 7β-hydroxy bile acids in the urine in the present study, especially 3α,7β,12α-trihydroxy-5β-cholan-24-oic acid. In the case of the epimerization of the 7α-hydroxyl group, the types of anaerobes displaying such activity in vitro include Clostridium absonum(30), lecithinase-lipase-negative Clostridia(31), Eubacterium aerofaciens(32), and an unidentified Gram-positive anaerobe(33). Eubacterium aerofaciens and the Gram-positive anaerobe possess only the 7β-HSDH and, therefore, must be cocultured with an organism synthesizing 7α-HSDH to epimerize the 7α-hydroxyl group(32,33). The enzymes 7α- and 7β-HSDH in C. absonum are inducible by chenodeoxycholic and deoxycholic acids. Thus a two-enzyme pathway consisting of a) 7α-HSDH (oxidative direction) and b) 7β-HSDH (reductive direction) is, in effect, induced (i.e. chenodeoxycholic acid ←(a)→ 7-ketolithocholic acid ←(b)→ ursodeoxycholic acid). These enzymes are also inducible by 12- and 7-ketolithocholic acids but repressed by the end product ursodeoxycholic acid(30). Moreover, a C. limosum soil isolate has been shown to be capable of producing 3α,7β,12α-trihydroxy-5β-cholan-24-oic and ursodeoxycholic acids from the corresponding primary bile acids(34). The epimerization of the axial 7 α-hydroxyl group may represent a detoxification process for these intestinal bacteria, as ursodeoxycholic acid is less hydrophobic (less toxic) than chenodeoxycholic acid(35). Therefore, the epimerization of the 7 α-hydroxyl group may result from oxidation of the primary bile acid by bacterial 7 α-HSDH followed by either hepatic or bacterial reduction of the 7-oxo-intermediate to the 7β-hydroxy bile acid(36–38). Isomerized 7β-hydroxy bile acids, especially 3α,7β,12α-trihydroxy-5β-cholan-24-oic acid, may be important in the urine of pediatric and adolescent patients.
In conclusion, the metabolism of bile acids in the infant changes significantly during the first year of life. The formation of unsaturated ketonic and polyhydroxylated bile acids probably represents a mechanism for the excretion of bile salts. The formation of isomerized 7β-hydroxylated bile acids from primary bile acids probably occurs because of the human intestinal bacterial flora, and, quantitatively, this is an important pathway in bile acid metabolism after late infancy. We described, for the first time, evidence for the existence of the epimerization of the 7α-hydroxyl group of cholic acid, which may be unique to human development.
A further study is underway to determine the profile of the conjugated forms of each bile acid during the pediatric period. According to present knowledge, the main conjugated bile acids are taurine- or glycine-conjugated forms, especially taurine-conjugated 1β-hydroxylated bile acids, until 1 mo of age. Thereafter, sulfate-conjugated bile acids, mainly sulfate and glycine double conjugated forms of chenodeoxycholoc acid, are detected after approximately 3 mo of age.
Abbreviations
- GC-MS:
-
gas chromatography-mass spectrometry
- SIM:
-
selected ion monitoring
- Me-DMES:
-
methyl ester-dimethylethylsilyl ether
- HSDH:
-
hydroxysteroid dehydrogenase
References
Clayton PT, Leonard JV, Lawson AM, Setchell KDR, Andersson S, Egestad B, Sjövall J 1987 Familial giant cell hepatitis associated with synthesis of 3β,7α-dihydroxy- and 3β,7α,12α-trihydroxy-5-cholenoic acids. J Clin Invest 79: 1031–1038.
Setchell KDR, Suchy FJ, Weish MB, Zimmer-Nechemias L, Heubi J, Balistreri WF 1988 Δ4-3-Oxosteroid 5β-reductase deficiency described in identical twins with neonatal hepatitis: a new inborn error in bile acid synthesis. J Clin Invest 82: 2148–2157.
Almé B, Bremmelgaard A, Sjövall J, Thomassen P 1977 Analysis of metabolic profiles of bile acids in urine using a lipophilic anion exchanger and computerized gas-liquid chromatography-mass spectrometry. J Lipid Res 18: 339–362.
Shoda J, Mahara R, Osuga T, Tohma M, Ohnishi S, Miyazaki H, Tanaka N, Matsuzaki Y 1988 Similarity of unusual bile acids in human umbilical cord blood and amniotic fluid from newborns and in sera and urine from adult patients with cholestatic liver diseases. J Lipid Res 29: 847–858.
Shoda J, Osuga T, Matsuura K, Mahara R, Tohma M, Tanaka N, Matsuzaki Y, Miyazaki H 1989 Concurrent occurrence of 3β,12α-dihydroxy-5-cholenoic acid associated with 3β-hydroxy-5-cholenoic acid and their preferential urinary excretion in liver disease. J Lipid Res 30: 1233–1242.
Shoda J, Tanaka N, Ousuga T, Matsuura K, Miyazaki H 1990 Altered bile acid metabolism in liver disease. concurrent occurrence of C-1 and C-6 hydroxylated bile acid metabolites and their preferential excretion into urine. J Lipid Res 31: 249–259.
Tohma M, Mahara R, Takeshita H, Krosawa T, Ikegawa S, Nittono H 1985 Synthesis of the 1β-hydroxylated bile acids and identification of 1β,3α,7α-trihydroxy- and 1β,3α,12α-tetrahydroxy-5β-cholan-24-oic acids in human meconium. Chem Pharm Bull 33: 3071–3073.
Kimura A, Yamakawa R, Ushijima K, Fujisawa T, Kuriya N, Kato H, Inokuchi T, Mahara R, Kurosawa T, Tohma M 1994 Fetal bile acid metabolism during infancy: analysis of 1β-hydroxylated bile acids in urine, meconium, and feces. Hepatology 20: 819–824.
Mahara R, Kurosawa T, Tohma M 1989 Profile analysis of bile acids in human fetal and neonatal periods by gas chromatography-mass spectrometry. Proc Jap Soc Biomed Mass Spectrom 14: 67–74.
Setchell KDR, Dumaswala R, Colombo C, Ronchi M 1988 Hepatic bile acid metabolism during early development revealed from the analysis of human fetal gallbladder bile. J Biol Chem 263: 16637–16644.
Dumaswala R, Setchell KDR, Zimmer-Nechemias L, Iida J, Goto J, Nambara T 1989 Identification of 3α,4β,7α-trihydroxy-5β-cholanoic acid in human bile: reflection of a new pathway in bile acid metabolism in humans. J Lipid Res 30: 847–856.
Kurosawa T, Nomura Y, Mahara R, Yoshimura T, Kimura A, Ikegawa S, Tohma M 1995 Synthesis of 19-hydroxylated bile acids and identification of 3α,7α,12α,19-tetrahydroxy-5β-cholan-24-oic acid in human neonatal urine. Chem Pharm Bull 43: 1551–1557.
Murai T, Mahara R, Kurosawa T, Kimura A, Tohma M 1997 Determination of fetal bile acids in biological fluids from neonates by gas chromatography-negative ion chemical ionization mass spectrometry. J Chromatogr B 691: 13–22.
Kimura A, Suzuki M, Murai T, Inoue T, Kato H, Hori D, Nomura Y, Yoshimura T, Kurosawa T, Tohma M 1997 Perinatal bile acid metabolism: analysis of urinary bile acids in pregnant women and newborns. J Lipid Res 38: 1954–1962.
Tohma M, Mahara R, Takeshita H, Kurosawa T, Ikegawa S 1986 Synthesis of the 1β-hydroxylated bile acids, unusual bile acids in human biological fluids. Chem Pharm Bull 34: 2890–2899.
Tohma M, Mahara R, Takeshita H, Kurosawa T 1986 A convenient synthesis of 3β,12α-, 3β,7α-, and 3β,7β-dihydroxy-5-cholen-24-oic acids: unusual bile acids in human biological fluids. Steroids 48: 331–338.
Kurosawa T, Mahara R, Nittono H, Tohma 1989 Synthesis of 6-hydroxylated bile acids and identification of 3α,6α,7α,12α-tetrahydroxy-5β-cholan-24-oic acid in human meconium and neonatal urine. Chem Pharm Bull 37: 557–559.
Yoshimura T, Mahara R, Kurosawa T, Ikegawa S, Tohma M 1993 An efficient synthesis of 4β- and 6α-hydroxylated bile acids. Steroids 58: 52–58.
Suzuki M, Murai T, Yoshimura T, Kimura A, Kurosawa T, Tohma M 1997 Determination of 3-oxo-Δ4- and 3-oxo-Δ 4:6-bile acids and related compounds in biological fluids of infants with cholestasis by gas chromatography-mass spectrometry. J Chromatogr B 693: 11–21.
Lester R 1980 Physiological cholestasis. Gastroenterology 78: 864–865.
Suchy FJ, Balistreri WF, Heubi JE, Searcy JE, Levin RS 1981 Physiologic cholestasis: elevation of the primary serum bile acid concentration in normal infants. Gastroenterology 80: 1037–1041.
Balistreri WF, Heubi JE, Suchy FJ 1983 Immaturity of the enterohepatic circulation in early life: factors predisposing to "physiologic" maldigestion and cholestasis. J Pediatr Gastroenterol Nutr 2: 346–354.
Kimura A, Mahara R, Tohma M, Ushijima K, Yuge K, Ono E, Yamashita F 1989 Unusual 1β-hydroxylated bile acids in children with a paucity in interlobular bile ducts. Clin Chim Acta 185: 215–218.
Nakagawa M, Setchell KDR 1990 Bile acid metabolism in early life: studies of amniotic fluid. J Lipid Res 31: 1089–1098.
Wahlén E, Egestad B, Strandvik B, Sjövall J 1989 Ketonic bile acids in urine of infants during the neonatal period. J Lipid Res 30: 1847–1857.
Strandvik B, Wahlen E, Wikstrom S-A 1994 The urinary bile acid excretion in healthy premature and full-term infants during the neonatal period. Scand J Clin Lab Invest 54: 1–10.
Björkhem I, Einarsson K, Melone P, Hylemon P 1989 Mechanism of intestinal formation of deoxycholic acid from cholic acid in humans: evidence for a 3-oxo-Δ4-steroid intermediate. J Lipid Res 30: 1033–1039.
Wahlén E, Stradvik B 1993 Effects of different formula feeds on the developmental pattern of urinary bile acid excretion in infants. J Pediatr Gastroenterol Nutr 18: 9–19.
Jönsson G, Midtvedt AC, Norman A, Midtvedt T 1995 Intestinal microbial bile acid transformation in healthy infants. J Pediatr Gastroenterol Nutr 20: 394–402.
Macdonald IA, Sutherland JD 1983 Further studies on the bile salt induction of 7α-and 7β-hydroxysteroid dehydrogenases in Clostridium absonum.. Biochim Biophys Acta 750: 397–403.
Edenharder R, Knaflic T 1981 Epimerization of chenodeoxycholic acid to ursodeoxycholic acid by human intestinal lecithinase-lipase-negative Clostridia.. J Lipid Res 22: 652–658.
Hirano S, Masuda N 1982 Characterization of NADP-dependent 7β-hydroxysteroid dehydrogenases from Peptostreptococcus productus and Eubacterium aerofaciens. Appl Environ Microbiol 43: 1057–1063.
Hirano S, Masuda N 1981 Epimerization of the 7-hydroxy group of bile acids by combination of two kinds of microorganisms with 7α- and 7β-hydroxysteroid dehydrogenase activity, respectively. J Lipid Res 22: 1060–1068.
Sutherland JD, Holdeman LV, Williams CN, Macdonald IA 1984 Formation of urso-and ursodeoxy-cholic acids from primary bile acids by Clostridium limosum soil isolate. J Lipid Res 25: 1084–1089.
Armstrong MJ, Carey MC 1982 The hydrophobic-hydrophilic balance of bile salts. Inverse correlation between reverse-phase high performance liquid chromatographic mobilities and micellar cholesterol-solubilizing capacities. J Lipid Res 23: 70–80.
Gustafsson BE, Norman A, Sjövall J 1960 Influence of E. coli infection on turnover and metabolism of cholic acid in germ-free rats. Arch Biochem Biophys 91: 83–100.
Fromm H, Carlson EL, Hofmann AF, Farivar S, Amin P 1980 Metabolism in man of 7-keto lithocholic acid, precursor of cheno- and urso-deoxycholic acids. Am J Physiol 239:G161–G166.
Macdonald IA, Bokkenheuser VD, Winter J, McLernon AM, Mosbach EH 1983 Degradation of steroids in the human gut. J Lipid Res 24: 675–700.
Author information
Authors and Affiliations
Additional information
Supported in part by Grant-in-Aid 07670924 for Scientific Research from the Ministry of Education, Science, and Culture of Japan.
Rights and permissions
About this article
Cite this article
Kimura, A., Mahara, R., Inoue, T. et al. Profile of Urinary Bile Acids in Infants and Children: Developmental Pattern of Excretion of Unsaturated Ketonic Bile Acids and 7 β-Hydroxylated Bile Acids. Pediatr Res 45, 603–609 (1999). https://doi.org/10.1203/00006450-199904010-00022
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1203/00006450-199904010-00022
This article is cited by
-
Perinatal exposure to UDCA prevents neonatal cholestasis in Cyp2c70-/- mice with human-like bile acids
Pediatric Research (2023)
-
One-pot biosynthesis of 7β-hydroxyandrost-4-ene-3,17-dione from phytosterols by cofactor regeneration system in engineered mycolicibacterium neoaurum
Microbial Cell Factories (2022)
-
Bile Acid Synthesis Disorders in Japan: Long-Term Outcome and Chenodeoxycholic Acid Treatment
Digestive Diseases and Sciences (2021)
-
Urinary and serum oxysterols in children: developmental pattern and potential biomarker for pediatric liver disease
Scientific Reports (2020)
-
A combination of mutations in AKR1D1 and SKIV2L in a family with severe infantile liver disease
Orphanet Journal of Rare Diseases (2013)