Abstract
There are at least two isoenzymes of 3-hydroxy-3-methylglutaryl (HMG)-CoA synthase (EC 4.1.3.5) located in the mitochondrial matrix and the cytoplasm of hepatocytes, respectively. The mitochondrial enzyme is necessary for the synthesis of ketone bodies, which are important fuels during fasting. We report a child with a deficiency of this isoenzyme. He presented at 16 mo with hypoglycemia. There was no rise in ketone bodies during fasting or after a long chain fat load but there was a small rise after a leucine load. Measurement of β-oxidation flux in fibroblasts was normal. Using antibodies specific for mitochondrial HMG-CoA synthase, no immunoreactive material could be detected on Western blotting. Total HMG-CoA synthase activity in liver homogenate was only slightly lower than in control samples. Presumably, as there was no mitochondrial HMG-CoA synthase enzyme protein, this activity arose from the cytoplasmic or other (e.g. peroxisomal) isoenzymes. With avoidance of fasting, our patient has had no problems since presentation and is developing normally at 4 y of age.
Similar content being viewed by others
Main
Ketone bodies (3-hydroxybutyrate and acetoacetate) are important fuels during fasting, both for the brain and for peripheral tissues. They are synthesized in the liver using acetyl-CoA produced by β-oxidation of fatty acids, although they can also be derived from certain amino acids, such as leucine. Two acetyl-CoA molecules condense to form acetoacetyl-CoA, which reacts with water and a third molecule of acetyl-CoA to form HMG-CoA. This is cleaved to acetyl-CoA and acetoacetate, which can be reduced to 3-hydroxybutyrate. Mitochondrial HMG-CoA synthase (EC 4.1.3.5) is a key enzyme in the control of ketogenesis, regulated by succinylation(1) and by developmental, hormonal, and dietary factors(2).
Pathways involving HMG-CoA also occur in the cytoplasm and peroxisomes. Cholesterol synthesis requires HMG-CoA in the cytoplasm, where it is formed by cytoplasmic HMG-CoA synthase. The mitochondrial and cytoplasmic isoenzymes are immunologically distinct(3), the amino acid sequences showing 66% homology(4,5). Cholesterol synthesis also occurs in peroxisomes and several peroxisomal enzymes involved in HMG-CoA metabolism have been demonstrated, including, in rats, a peroxisomal HMG-CoA synthase(6).
Many cases of mitochondrial HMG-CoA lyase deficiency have been described(7), but there has only been one report of mitochondrial HMG-CoA synthase deficiency(8). We report a child with complete absence of immunoreactive mitochondrial HMG-CoA synthase who presented similarly, with hypoketotic hypoglycemia, encephalopathy, and hepatomegaly. The scarcity of other reports may in part result from the lack of specific urinary metabolites and the need for hepatic tissue for diagnosis.
METHODS
Case report. The patient is the first child of healthy nonconsanguineous parents. Pregnancy was uncomplicated and he remained well until he had an episode of diarrhea and vomiting at the age of 16 mo. He was admitted unconscious with a blood glucose of 1.0 mmol/L and marked hepatomegaly (palpable 7 cm below the costal margin). He rapidly regained consciousness with i.v. glucose, and the hepatomegaly diminished although it did not completely resolve. Urine collected 2 d after presentation contained large amounts of dicarboxylic acids (C6, C8, C10, C12, C8:1, and C10:1) and 3-hydroxydicarboxylic acids (C8, C10, C12, C10:1, C12:1, C14:1, C12:2, and C14:2). Ethylmalonate, glutarate, and 3-hydroxybutyrate were not markedly raised, and there were no glycine conjugates. Blood spot acylcarnitine analysis by tandem mass spectrometry showed no abnormality. Plasma transaminase levels were raised initially (peak alanine transaminase 590 IU/L, normal <45) with normal clotting and plasma creatine kinase values. Ultrasound examination of the liver showed increased echogenicity.
After his initial presentation, the patient was managed with a low fat diet and strict avoidance of fasting for longer than 12 h. During intercurrent illnesses he was given regular drinks of glucose polymer solution to maintain a high carbohydrate intake(9). On four occasions, poor feeding has necessitated admission to hospital for i.v. glucose infusions. There have been no further episodes of encephalopathy. At the age of 4 y, clinical examination is entirely normal, apart from borderline hepatomegaly. Growth and development and echocardiography are also normal.
Clinical studies. A closely monitored 18-h fast was performed 2 mo after presentation, following an established protocol(10). Two months later, the ketone body responses after long chain fat and leucine loading were measured. Long chain fat loading was performed according to the protocol used by Saudubray and colleagues(11). After an overnight fast of 11.5 h, sunflower oil(1.5 g/kg) was given via a nasogastric tube. Blood samples were obtained before administration of the oil and subsequently after 30 min, and 1, 2, 3, and 4 h, for measurement of glucose, FFA, acetoacetate, 3-hydroxybutyrate, and lactate concentrations. Leucine loading (200 mg/kg) was performed after an 11-h overnight fast, and specimens were obtained as above; adult control data were obtained using an identical procedure except that subjects were fasted for longer (28 h).
Measurement of β-oxidation flux. β-Oxidation flux was measured in fibroblasts using [9,10-3H]oleic,[9,10-3H]palmitic, and [9,10-3H]myristic acids as previously described(12).
Measurement of total HMG-CoA synthase activity. A percutaneous liver biopsy was obtained under general anesthetic at the age of 2.5 y. Total HMG-CoA synthase activity was assayed (n = 3) in a homogenate of the sample, as described previously(13). The sample was kept on ice while the homogenate was prepared, and the assays were completed within 1.25 h of obtaining the sample. Pediatric control samples were obtained immediately postmorterm from term neonates dying of acute asphyxia and from residual tissue after cut-down liver transplantation. None of the control subjects had evidence of metabolic disease or liver disease. For control subjects, assays (n = 4) were performed on each of four homogenate preparations. For our patient and two control subjects, homogenates were prepared from fresh tissue; in other cases, tissue was frozen before preparing the homogenate.
Detection of mitochondrial HMG-CoA synthase by immunoblotting. Aliquots of human liver homogenate were separated by SDS-PAGE (9.5% wt/vol acrylamide), and the separated proteins were transferred to nitrocellulose membrane (Biometra Ltd., Maidstone, Kent, UK) using the "Hoeffer Double System." Blots were incubated with polyclonal rabbit anti-(ox mitochondrial HMG-CoA synthase) antiserum(2), washed, incubated with secondary antibody (goat anti-rabbit-peroxidase conjugate), and developed.
RESULTS
Diagnostic fast. After 18 h, the patient became drowsy with a blood glucose of 2.3 mmol/L. Plasma insulin concentrations were undetectable with an appropriately raised cortisol level (1036 nmol/L) and normal blood lactate and pyruvate concentrations (1.26 and 0.06 mmol/L, respectively). There was a marked rise in plasma FFA levels with grossly impaired ketone body production (Table 1). Figure 1 shows the relationship between fasting FFA and 3-hydroxybutyrate concentrations for our patient, compared with the relationship in normal subjects and patients with β-oxidation defects. Total and free plasma acylcarnitine concentrations were normal after 12 h of fasting, but after 18 h, the free carnitine level had fallen to 12 mmol/L (normal 22-50). Urine organic acids showed the same pattern as at presentation, and blood acylcarnitine analysis was normal.
Relationship between the plasma FFA concentration and the blood 3-hydroxybutyrate concentration at the end of an 18-h fast in our patient (filled triangle), compared with children known to have β-oxidation defects (open circles). The lines show the 95% predictive intervals for normal children. Reproduced from Morris et al.(10), with permission of BMJ Publishing Group.
Long chain fat and leucine loading tests. Loading with long chain fat did not lead to any rise in blood ketone body concentrations in our patient, although plasma FFA levels rose from 1.67 mmol/L immediately before ingestion to 2.90 mmol/L 4 h later. The ketone body response is shown in Figure 2A, compared with results for four pediatric control subjects(11). After a leucine load, there was a small but unequivocal rise in blood 3-hydroxybutyrate concentrations in our patient (peak value 0.45 mmol/L). The rise after leucine ingestion was similar to that seen in adult controls, as shown in Figure 2B, but fasting induced higher baseline and final 3-hydroxybutyrate concentrations in the control subjects.
Blood ketone body concentrations in our patient (open squares) and controls (filled squares, circles, triangles, and diamonds) (A) after a long chain fat load and (B) after a leucine load. Control data in(A) relate to children aged 4-40 mo and are derived from the literature(11). Control data in (B) are for adult subjects.
Liver histology. Light microscopy was normal but electron microscopy showed marked proliferation and dilatation of the smooth endoplasmic reticulum, which filled the cytoplasm of hepatocytes. Some of the mitochondria had an abnormally dense matrix, whereas others in the same cell were of normal structure. Only rare lipid droplets were found. Peroxisomes were of normal size and did not appear in excess.
β-Oxidation flux in fibroblasts. Measurements ofβ-oxidation flux in fibroblasts were within normal limits for all substrates (Table 2).
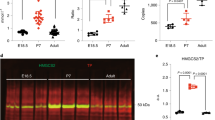
Immunoblotting of mitochondrial HMG-CoA synthase. Probing with antibody specific for the mitochondrial isoenzyme showed no immunoreactive material of the expected molecular weight in the liver homogenate from our patient (Fig. 3).
Immunoblots of liver homogenate from our patient and control subjects, incubated with anti-mitochondrial HMG-CoA synthase antiserum. Samples from our patient were loaded in lanes 5 and 8. Control samples were obtained postmortem from a 19-wk fetus (lanes 1 and 6), a 22-wk fetus (lanes 2 and 7), a 36-wk gestation neonate (lane 3), and a 17-mo-old infant after cut-down liver transplantation (lanes 4, 9, and 10). Loading: 86 µg of protein in lanes 1-5; 100µg of protein in lanes 8 and 9; 200 µg of protein in lanes 6, 7, and 10. Size markers (in KD) are shown on the right.
Total HMG-CoA synthase activity in liver homogenate. The assay results for our patient and controls are shown in Figure 4. For control subjects, each value is the mean of the four homogenate preparations; in all cases, the SD was less than 17%. Activity has been plotted against age as there is evidence (in other species) for changes, particularly during the fetal-neonatal transition. The activity in our patient was 50% of the median control value but, as the control data were widely scattered, the patient's activity was only marginally lower than the minimum control value.
DISCUSSION
The clinical studies in this boy indicate a severe defect of ketone body synthesis from fat, both during fasting and after a long chain fat load. The low levels of ketone bodies detected in our patient could be derived from protein catabolism, as suggested by the rise after a leucine load. Defects ofβ-oxidation, the commonest cause of impaired ketogenesis, were excluded by the normal β-oxidation flux in fibroblasts. Tissue-specific isoenzymes have been reported only for carnitine palmitoyl-transferase I and, for this, the same isoenzymes are active in liver and fibroblasts(14). The technique used to measure β-oxidation flux does not exclude defects in enzymes specific for short chain substrates but these are not associated with impaired ketogenesis(15). Secondary defects of ketone body production occur in some other metabolic diseases(16–18), but the impairment is less severe, and different clinical and laboratory features would be expected. A primary defect in the pathway of ketone body synthesis was therefore suspected. The pattern of urinary organic acids and blood acylcarnitines were not consistent with HMG-CoA lyase deficiency. By contrast, no specific abnormalities would be expected with defects of mitochondrial HMG-CoA synthase.
The absence of immunoreactive mitochondrial HMG-CoA synthase in liver homogenate from our patient confirmed the diagnosis. Many mutant enzymes have reduced stability, with reduced staining on Western blots. Mitochondrial HMG-CoA synthase contains several regions rich in proline, glutamate, serine, and threonine ("PEST sequences"), which are generally associated with a short half-life(4). Mutations may not, therefore, need to cause gross structural alterations to render the enzyme very unstable.
The observed level of HMG-CoA synthase activity in liver homogenate came as a surprise. As there was insufficient material to separate organelles, the isoenzyme responsible for this activity could not be determined. Potentially, the activity detected could all have arisen from the cytoplasmic isoenzyme. Previous studies have suggested that the cytoplasmic isoenzyme accounts for less than 10% of the total activity under normal circumstances(13,19), but levels might be raised in the absence of the mitochondrial isoenzyme. An alternative explanation would be the presence of a peroxisomal isoenzyme.
Studies measuring HMG-CoA synthase activity in subcellular fractions have used separation techniques that would include peroxisomes in the mitochondrial fraction(19). Peroxisomes are known to be involved in cholesterol metabolism, and rat liver peroxisomes have been shown to contain an HMG-CoA synthase isoenzyme(6). Although this has not yet been demonstrated in man, human peroxisomes do contain several other enzymes of HMG-CoA metabolism, including HMG-CoA lyase(20).
The clinical and biochemical features in our patient closely resemble those seen in the only reported case of mitochondrial HMG-CoA synthase deficiency(8). Both patients presented with hypoglycaemic encephalopathy and had markedly impaired ketogenesis after fasting and long chain fat loading. In the previous case, ketogenesis was also impaired after medium chain triglyceride loading. As this caused the patient to lose consciousness, it was not undertaken in our patient. Urinary excretion of ethylmalonate and 3-ketohexanoate was raised after medium chain triglyceride loading in the previous patient, but he differed from our patient in having normal urinary organic acids after the initial presentation and the diagnostic fast. Blood acylcarnitine analysis was normal in both patients. Hepatomegaly was not noted in the previous patient, but there was mild hepatic steatosis; electron microscopy showed variable sized mitochondria with crystalline inclusions but no abnormality of the endoplasmic reticulum. Total HMG-CoA synthase activity in liver from the previous patient was 5-20% of three control subjects; immunoblotting was not performed. Although mitochondrial HMG-CoA synthase is expressed in rat lymphocytes(21), Thompson et al.(22) could not demonstrate deficiency in lymphocytes, fibroblasts, or transformed lymphoblasts from their patient.
Both patients have responded well to avoidance of fasting and are developing normally. Although ketone bodies are thought to be the preferred substrate for synthesis of myelin cholesterol(23), it would appear that they are not essential. The good prognosis with simple treatment makes mitochondrial HMG-CoA synthase deficiency an important diagnosis to establish. Cases may well have been missed previously due to the lack of specific urinary metabolites; defects in which these are found, such as HMG-CoA lyase deficiency, are more readily diagnosed. Moreover, confirmation of the diagnosis requires a liver biopsy as it has not been possible to demonstrate the defect in more accessible tissues(22). The clinical presentation closely resembles that of β-oxidation defects, and it is likely that some patients have been classified as such; fortunately, treatment of the conditions is very similar.
Abbreviations
- HMG:
-
3-hydroxy-3-methylglutaryl
References
Quant PA, Tubbs PK, Brand MD 1990 Glucagon activates mitochondrial 3-hydroxy-3-methylglutaryl-CoA synthase in vivo by decreasing the extent of succinylation of the enzyme. Eur J Biochem 187: 169–174.
Quant PA, Robin D, Robin P, Ferre P, Brand MD, Girard J 1991 Control of hepatic mitochondrial 3-hydroxy-3-methylglutaryl-coenzyme A synthase during the foetal/neonatal transition, suckling and weaning in the rat. Eur J Biochem 195: 449–454.
Reed WD, Clinkenbeard KD, Lane MD 1975 Molecular and catalytic properties of mitochondrial (ketogenic) 3-hydroxy-3-methylglutaryl coenzyme A synthase of liver. J Biol Chem 250: 3117–3123.
Boukaftane Y, Duncan A, Wang S, Labuda D, Robert M-F, Sarrazin J, Schappert K, Mitchell GA 1994 Human mitochondrial HMG-CoA synthase: liver cDNA and partial genomic cloning, chromosomal mapping to 1p12-p13, and possible role in vertebrate evolution. Genomics 23: 552–559.
Russ AP, Ruzicka V, Maerz V, Appelhans H, Gross W 1992 Amplification and direct sequencing of a cDNA encoding human cytosolic 3-hydroxy-3-methylglutaryl-coenzyme A synthase. Biochim Biophys Acta 1132: 329–331.
Krisans SK, Rusnak N, Keller GA, Edwards PA 1988 Localisation of 3-hydroxy-3-methylglutaryl coenzyme A synthase in rat liver peroxisomes. J Cell Biol 107: 122a
Gibson KM, Breuer J, Nyhan WL 1988 3-Hydroxy-3-methylglutaryl-coenzyme A lyase deficiency: review of 18 reported patients. Eur J Pediatr 143: 180–186.
Thompson GN, Hsu BYL, Pitt J, Treacey E, Stanley CA 1997 Fasting hypoketotic coma in a child with deficiency of mitochondrial 3-hydroxy-3-methylglutaryl-CoA synthase. N Engl J Med 337: 1203–1207.
Dixon MA, Leonard JV 1992 Intercurrent illness in inborn errors of metabolism. Arch Dis Child 67: 1387–1391.
Morris AAM, Thekekara AG, Wilks Z, Clayton PT, Leonard JV, Aynsley-Green A 1996 Evaluation of fasts for investigating hypoglycaemia or suspected metabolic disease. Arch Dis Child 75: 115–119.
Demaugre F, Bonnefont JP, Colonna M, Cepanec C, Leroux JP, Saudubray JM 1991 Infantile form of carnitine palmitoyltransferase II deficiency with hepatomuscular symptoms and sudden death. Physiopathological approach to carnitine palmitoyltransferase II deficiencies. J Clin Invest 87: 859–864.
Manning N, Olpin S, Pollitt R, Webley J 1990 A comparison of [9,10-3H]palmitic and [9,10-3H]myristic acids for the detection of defects of fatty acid oxidation in intact cultured fibroblasts. J Inherit Metab Dis 13: 58–68.
Lascelles C, Quant PA 1997 Investigation of human hepatic mitochondrial 3-hydroxy-3-methylglutaryl-coenzyme A synthase in post mortem and biopsy tissue. Clin Chim Acta 260: 85–96.
Tein I, Demaugre F, Bonnefont JP, Saudubray JM 1989 Normal muscle CPT1 and CPT2 activities in hepatic presentation patients with CPT1 deficiency in fibroblasts. Tissue specific isoforms of CPT1?. J Neurol Sci 92: 229–245.
Coates PM, Hale DE, Finocchiaeo G, Tanaka K, Winter SC 1988 Genetic deficiency of short-chain acyl-coenzyme A dehydrogenase in cultured fibroblasts from a patient with muscle carnitine deficiency and severe skeletal muscle weakness. J Clin Invest 81: 171–175.
Binkiewicz A, Senior B 1973 Decreased ketogenesis in Von Gierke's disease (type I glycogenosis). J Pediatr 83: 973–978.
Morris AAM, Deshpande S, Ward-Platt MP, Whitfield AE, Aynsley-Green A, Leonard JV, Pourfarzam M, Bartlett K 1995 Impaired ketogenesis in fructose-1,6-bisphosphatase deficiency. J Inherit Metab Dis 18: 28–32.
Morris AAM, Taanman J-W, Blake J, Cooper JM, Lake BD, Malone M, Love S, Leonard JV, Clayton PT, Schapira AHV 1998 Liver failure associated with mitochondrial DNA depletion. J Hepatol 28: 556–563.
Quant PA, Tubbs PK, Brand MD 1989 Treatment of rats with glucagon or mannoheptulose increases mitochondrial 3-hydroxy-3-methylglutaryl-coenzyme A synthase activity and decreases succinyl-CoA content in liver. Biochem J 262: 159–164.
Ashmarina LI, Rusnak N, Miziorko HM, Mitchell GA 1994 3-Hydroxy-3-methylglutaryl-coenzyme A lyase is present in mouse and human peroxisomes. J Biol Chem 269: 31929–31932.
Curi R, Williams JF, Newsholme EA 1989 Formation of ketone bodies by resting lymphocytes. Int J Biochem 21: 1133–1136.
Thompson G, Pitt J, Treacey E, Stanley C VIth International Congress on Inherited Metabolic Disease Milan 1994 Abstract W28.1
Koper JW, Lopes-Cardoso ML, Van Gold LMG . 1981 Preferential use of ketone bodies for the synthesis of myelin cholesterol in vivo. Biochim Biophys Acta 666: 411–417.
Acknowledgements
The authors thank Drs. D. M. Easton and K. Hussain for referring the patient and Drs. S Krywawych and A. W. Johnson for organic acid and acylcarnitine analysis.
Author information
Authors and Affiliations
Additional information
Support was provided by the Sir Halley Stewart Trust and the Sir Samuel Scott of Yews Trust, C.V.L. holds a Krebs Memorial Scholarship.
Rights and permissions
About this article
Cite this article
Morris, A., Lascelles, C., Olpin, S. et al. Hepatic Mitochondrial 3-Hydroxy-3-Methylglutaryl-Coenzyme A Synthase Deficiency. Pediatr Res 44, 392–396 (1998). https://doi.org/10.1203/00006450-199809000-00021
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1203/00006450-199809000-00021
This article is cited by
-
Severe clinical manifestation of mitochondrial 3-hydroxy-3-methylglutaryl-CoA synthase deficiency associated with two novel mutations: a case report
BMC Pediatrics (2019)
-
Mitochondrial 3‐hydroxy‐3‐methylglutaryl‐CoA synthase deficiency: urinary organic acid profiles and expanded spectrum of mutations
Journal of Inherited Metabolic Disease (2015)
-
Ketone body metabolism and its defects
Journal of Inherited Metabolic Disease (2014)
-
Refining the diagnosis of mitochondrial HMG‐CoA synthase deficiency
Journal of Inherited Metabolic Disease (2006)