Abstract
Retrograde differentiation (or dedifferentiation) has recently been proposed as a pathogenetic mechanism involved also in various renal diseases. Here we studied whether evidence of these mechanisms can be found in the kidneys of patients with congenital nephrotic syndrome of the Finnish type(CNF). These patients show isolated massive proteinuria but no primary symptoms from any other organ systems. For the analysis we used antibody markers of early (fibronectin, stem cell factor, Wilms' tumor gene product, cytokeratin) and later (laminin, midgestation and kidney, heparin binding growth-associated molecule) stages of nephron differentiation as well as for apoptosis (acridine orange staining), rescue from apoptosis (anti-Bcl-2 antibodies) and cell proliferation (antibodies to proliferating cell nuclear antigen). In the peritubular spaces atypically organized areas were found which appeared positive with markers of low stages of differentiation, but neither abnormal cell proliferation nor activation of the apoptotic pathway could be detected. As morphologic signs of abnormal tissue organization, we found clusters of tightly compacted and large glomeruli corresponding to the size of two to three normal glomeruli. However, all individual glomerular cell compartments (mesangial, endothelial, visceral epithelial cells) appeared balanced in relative cell numbers. Together these results may indicate abnormal early mesenchymoepithelial tissue interaction leading to excessive and poorly organized formation of glomeruli. This could be causally related also to the serious functional immaturity of CNF kidneys presented as isolated proteinuria.
Similar content being viewed by others
Main
Formation of the permanent mammalian kidney involves a complex interaction between the cells of the metanephric mesenchyme and the epithelial cells of the branching ureteric buds. This bidirectional inductive interaction proceeding centrifugally from deeper tissue compartments toward the surface leads to the formation of a closely fixed amount of functioning glomeruli and secretory nephrons(1–4). After induction, further development involves cell growth and spatial arrangements guided in part by finely tuned removal of excess cells by apoptosis and a scheduled appearance of morphologic and functional features ultimately yielding the distinct nephron segments. Changes in the organization of the metanephric mesenchyme and the subsequent epithelial cells as well as local extracellular matrices including the appearance pattern of the individual matrix molecules during nephrogenesis have been extensively characterized(1, 5, 6). Recent identification of several new genes acting coordinately during induction(7–12), together with deregulation experiments of these genes in mice are revealing the complex interplay of the early acting genes in the morphogenetic process. Interestingly, new understanding of the developmental processes has simultaneously revealed new mechanisms of tissue injury and pathogenetic mechanisms of diseases(2). Thus, a regenerative pathway of, e.g. liver cells after tissue damage has been documented in detail(13, 14), and similar mechanisms seem to be involved in various kidney diseases. Wizgall et al.(15) showed a typical expression pattern of distinct genes in the postischemic kidney along the nephron epithelium, evidencing of dedifferentiation pathway, and similar mechanisms have been proposed also for other renal diseases(16–18), although not yet for those limited to the glomerulus.
CNF is a rare autosomal recessive kidney disease of the newborn characterized by massive, treatment-resistant proteinuria(19, 20). Previously, CNF led to death within early months, but the current treatment with early nephrectomy followed by renal transplantation cures all symptoms(21). As no symptoms are found in other organ systems of the treated CNF patients, even after several years of follow-up, in spite of its rarity, CNF is a unique model disease of pure proteinuria and can be used to test various hypotheses of the still poorly known glomerular filtration mechanisms.
CNF kidneys may show areas of irregular tissue organization(19, 20). We have also detected cortical kidney areas with abnormal clustering and remarkably enlarged glomeruli in CNF kidneys as well as areas of atypically organized peritubular compartments (H. Holthöfer and A. Haltia, unpublished observations). Here we used newly identified markers of various differentiation stages of the nephron to characterize the structural abnormalities found especially in the CNF glomeruli in detail. Together with our earlier results evidencing of aberrant differentiation of CNF kidneys(22), the findings suggest abnormal early mesenchymal-epithelial interaction, which may be associated with a generalized functional immaturity, reflected also by proteinuria.
METHODS
CNF patients and tissue samples. Diagnosis of CNF (n= 6) was based on the typical clinical picture (large placenta, massive proteinuria at birth), and exclusion of other types of congenital nephroses and later by the typical pathology at nephrectomy(20, 21). Before nephrectomy, treatment of the patients included daily albumin infusions, aggressive nutrition, and as the weight of 8-9 kg was achieved (at an age of 9-18 mo), nephrectomy of both kidneys was performed(21). The kidneys at nephrectomy were immediately perfused with Ringer's buffer solution (Orion Pharmaceuticals, Espoo, Finland) via the renal arteries and processed for samples for immunohistochemistry (see below).
Cadaver kidneys (ages 3, 12, and 22 y) unsuitable for transplantation due to vascular anatomic reasons (Department of Surgery, University of Helsinki) were used as controls, and processed as the CNF samples.
After removal, the kidneys were cut into two halves along the longitudinal axis across the renal pelvis to get the gross anatomic appearance of the corticomedullary relationship. For immunohistochemistry, samples of cortical tissue as well as samples from the corticomedullary areas were cut with a scalpel into small cubes in a drop of 3.5% paraformaldehyde fixative, and frozen in isopentane cooled with liquid nitrogen until used. Slices were cut routinely at 3-4 μm with a cryostat.
Staining procedure. For immunofluorescence microscopy(23) the tissue sections were washed with PBS (pH 7.2) and incubated with the appropriate dilutions of the primary antibodies overnight at 4 °C. Then the sections were washed thoroughly in PBS and incubated for 1 h with the respective FITC-coupled anti-rabbit (R) or anti-mouse (M) IgG second antibodies (Jackson Laboratories, Westgrowe, Pa), washed, and mounted in a nonfading mounting medium (Mowiol; Calbiochem Corp., La Jolla, Ca).
Antibodies and lectins used. The following antibodies were used to characterize the tissue samples: rabbit (R) antifibronectin (diluted at 1:100 in PBS; Serotec, Oxford, UK), R anti-laminin (1:50; Bethesda Research Laboratories, Gaithersburg, MD), mouse (M) anti-cytokeratin (Pkk1, 1:50; Labsystems, Helsinki, Finland), M anti-human monocytes/macrophages (M 718, 1:10; Dakopatts, Glostrup, Denmark), M anti-human leukocyte common antigen(M701, 1:20; Dakopatts) M anti-stem cell factor (SCF, used at 1:50; R&D Systems, Minneapolis, Mn), M anti-Bcl-2 (1:20; Upstate Biologicals), R anti-Wilms' tumor gene protein product [clone HC17 raised against the amino-terminal 173 amino acids of WT-1, used at 1:250; kindly provided by Dr. Frank Rauscher, The Wistar Institute, Philadelphia; production of the antibody has been described by Amin et al.(24)], M anti-PCNA (Boehringer Mannheim, Mannheim, Germany), R anti-HB-GAM, used at 1:20; kindly provided by Dr. Heikki Rauvala, Institute of Biotechnology, University of Helsinki), R anti-MK [kindly provided by Dr. Takashi Muramatsu, University of Nagoya(25, 26)], and FITC-Triticum vulgaris-lectin (WGA; Vector Laboratories, Burlingame, Ca). To detect cells with compacted nuclei typical of apoptosis, the staining method described by Abrams et al.(27) was used, employing 5 μg/mL acridine orange (Sigma Chemical Co., St. Louis, MO).
An Olympus Ox50 microscope equipped with an appropriate filter system for FITC fluorescence was used for microscopy.
RESULTS
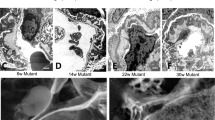
At macroscopic examination, the longitudinal cutting surface of CNF kidneys regularly revealed a cortical thickness of 3-6 mm and also areas of poor demarcation between the cortical and medullary compartments with cortical tissue extending to the medulla (Fig. 1).
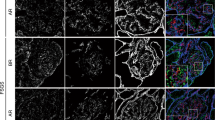
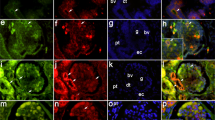
Immuno- and lectin histochemical stainings of CNF kidneys revealed normal appearing tubular profiles with morphologically distinguishable epithelial cells of the proximal tubules (cells with apical brush border), loops of Henle, distal tubules (number of tubular profiles within kidney cortex, no brush border), and collecting ducts (variability in cell size and location of nucleus, bifurcation of ducts) as in the controls. When compared with normal kidney cortex (Fig. 2a) characteristic areas of tubular dilatations in CNF as reported earlier(19) were seen (Fig. 2b). In all CNF kidneys studied, tubules were focally surrounded by areas of poorly organized cells (Figs. 2b and 3b). Staining for human lymphocytes and monocyte/macrophages only partially colocalized with these areas (data not shown), which were faintly positive for anti-fibronectin (Fig. 2b), anti-stem cell factor (Fig. 3b; Fig. 3a shows reactivity of normal kidney cortex for stem cell factor) and also for anti-cytokeratin antibodies, whereas no laminin-specific staining was found in these areas (Fig. 4). These areas contained only occasional cells staining with the anti-Bcl-2 (Fig. 5) and anti-PCNA antibodies (Fig. 6) or cells with acridine orange reactive compacted nuclei (Fig. 7). Together these results indicate of no aberrations in the apoptotic or cell proliferation pathways in this compartment. No reactivity with antibodies specific for WT-1, MK or HB-GAM (Fig. 8) could be seen.
Several glomeruli were found in each cortical section in all kidney samples, but their arrangement differed characteristically from that of the control kidneys: densely clustered glomeruli separated by narrow Bowman's capsules were seen (Fig. 9) in all the CNF samples especially at the corticomedullary zone. In addition, large glomeruli suggesting fusion of two to three individual glomeruli were found (Fig. 10). These “fused” glomeruli were typically surrounded by a continuous laminin-containing Bowman's capsule. The cellular composition within these fused glomeruli appeared balanced, with no prominence of podocyte, mesangial or endothelial elements as decided by morphology and reactivity for WT-1 antibodies and WGA lectin (Fig. 11). In these CNF glomeruli, immunoreactivity for WT-1 protein (Fig. 12b; Fig. 12a shows reactivity of normal glomeruli for WT-1) appeared exclusively in the visceral epithelial cells in a pattern as in normal kidneys; the WT-1 positive cells with nuclear but also cytoplasmic reactivity were seen facing the urinary space. No signs of cell proliferation as demonstrated by PCNA positive cells were seen in the visceral epithelial areas of the CNF glomeruli although in a few glomeruli PCNA positive cells were detected in the more central mesangial zone. No cells with aberrations in the apoptotic pathway (positivity for Bcl-2, compacted nuclei positive for acridine orange) could be seen within CNF or control kidney glomeruli. In addition, the functionally important anionic charge of CNF glomeruli appeared normal as decided by the comparable intensity and distribution of WGA lectin binding sites within the epithelial cells and glomerular basement membranes of the “fused” glomeruli (Fig. 11), compared with those of the controls.
DISCUSSION
Here we wanted to apply newly established markers for early and later stages of nephron differentiation to analyze whether evidence of either arrest of differentiation or dedifferentiation could be found in the massively proteinuric kidneys of congenital nephrotic syndrome patients. The results revealed enlarged single glomeruli but also areas of tightly compacted clusters of glomeruli of normal size. These results are in agreement with the results of Tryggvason and Kouvalainen(28), who could show an increase in the total number of glomeruli in CNF kidneys. The number of functional nephrons and glomeruli is determined by the number of branches of the ingrowing ureteric buds. These then interact with the metanephric mesenchyme to reach the final number of glomeruli(1, 4, 29). Thus, the finding of clustered glomeruli may suggest a failure in the coordinated mesenchymoepithelial interaction during nephrogenesis in CNF. The terminally differentiated podocytes(30) or other intraglomerular cells did not, however, show signs of continued cell proliferation or excess cell death by apoptosis in these glomeruli. Furthermore, the peritubular cellular areas expressing markers of early differentiation (fibronectin, stem cell factor, cytokeratin) further support the conclusions of arrested differentiation in this disease. The results with Bcl-2 and PCNA antibodies as well as acridine orange staining suggest that excess rescue from apoptosis or increased cellular proliferation, mechanisms normally controlling the ultimate cell number of the mature kidney(31) may not be perturbed in CNF kidneys. Thus, the proteinuria of CNF could be due to a developmental arrest at a stage characterized by a nonfunctional glomerular filtration barrier.
The frequency of CNF in Finland has been estimated to be 8 per 100 000 newborn with only sporadic cases outside Finland or without Finnish descendence. However, it is evident that the recently recognized gene locus of CNF on the long arm of chromosome 19(32) is shared with cases outside Finland(33, 34) but the CNF gene has not been identified as yet. In support to our interpretation of a developmental arrest in CNF the treatment-resistant proteinuria in this disease starts already in utero, as evidenced by the exceedingly high content of α-fetoprotein in amniotic fluid and in maternal circulation(35). The typical morphologic finding in CNF glomeruli is the flattening of podocytes with retraction of the foot processes(20); whether this is secondary to the heavy proteinuria or reflects immaturity of podocytes is not known.
The MAb for WT-1 (clone HC17, against the aminoterminal 173 amino acids) showed remarkable and unexpected cytoplasmic reactivity in podocytes, in addition to the nuclear staining, although WT-1 has been considered a strictly nuclear protein(11). One possibility for this might be that a specific splice variant of WT-1 is detected. However, cytoplasmic reactivity of WT-1 has been repeatedly observed though not systematically reported [see e.g. Amin et al.(24) and Menssen et al.(36)]. Furthermore, the protein itself, before transport to the nucleus is produced in the cytoplasm and could thus explain its cytoplasmic detection in podocytes. A disease-specific feature of WT-1 expression does not appear probable, because the control kidneys used also showed variable cytoplasmic reactivity in addition to the nuclear one. A phenomenon due to a fixation artifact does not appear true, either, as additional fixation experiments showed similar reactivity.
The possibility that abnormal early inductive events may lead to a distinct functional abnormality of glomeruli is intriguing but well supported by the findings of gene deregulation studies in mice. The early developmental gene Pax-2 overexpression in mice results in proteinuria and morphologic changes typical for congenital nephrosis(8). Our results have shown that Pax-2-specific mRNA as well as the corresponding protein product is intact in CNF kidneys(37). Furthermore, Kestiläet al.(38) have excluded any gene defects in the human homologue of Pax-2 in CNF. Mice lacking the functional S-laminin gene similarly produce a phenotype closely resembling that of human congenital nephroses(39). However, the recently recognized disease locus of CNF excludes also S-laminin to be defective in this disease(32). We have earlier shown that the cell type-specific glomerular gangliosides found transiently during early stages of glomerulogenesis(23) remain atypically expressed in the CNF kidney glomeruli(22), supporting the possibility of developmental arrest in CNF glomeruli.
In conclusion, morphologic changes suggesting abnormal mesenchymal-epithelial tissue interaction was found in the CNF cortical kidney and this was also supported by the markers of nephron differentiation stages used. However, the markers used did not appear optimal to characterize the glomerular functional disturbance in CNF kidneys, proteinuria, which could be the consequence of such a proposed developmental arrest.
Abbreviations
- CNF:
-
congenital nephrotic syndrome of the Finnish type
- HB-GAM:
-
heparin binding growth-associated molecule
- MK:
-
midgestation and kidney
- PCNA:
-
proliferating cell nuclear antigen
- WT:
-
Wilms' tumor
- WGA:
-
wheat germ agglutinin
References
Saxen L 1987 Organogenesis of the Kidney. Cambridge University Press, Cambridge
Bard JBL, Woolf A 1992 Nephrogenesis and the development of renal disease. Nephrol Dial Transplant 7: 563–572
Bacallao R, Fine LGL 1989 Molecular events in the organization of renal tubular epithelium: from nephrogenesis to regeneration. Am J Physiol 26:F913–F924
Ekblom P 1989 Developmentally regulated conversion of mesenchyme to epithelium. FASEB J 3: 2141–2150
Sorokin L, Sonnenberg A, Aumailley M, Timpl R, Ekblom P 1990 Recognition of laminin E8 cell-binding site by an integrin possessing the a6 subunit is essential for epithelial polarization in developing kidney tubules. J Cell Biol 111: 1265–1273
Ekblom M, Klein G, Mugrauer G, Fecker L, Deutzmann R, Timpl R, Ekblom P 1990 Transient and locally restricted expression of laminin A chain mRNA by developing epithelial cells during kidney organogenesis. Cell 60: 337–346
Bard JBL, McConnel JE, Davies JA 1994 Towards a genetic basis for kidney development. Mech Dev 48: 3–11
Dressler GR, Deutsch U, Chowdhury K, Normes HO, Gruss P 1990 Pax-2, a new murine paired-box-containing gene and its expression in the developing excretory system. Development 109: 787–795
Madden SL, Cook DM, Morris JF, Gashler A, Sukhatme VP, Rauscher FJ 1991 Transcriptional repression mediated by the WT-1 Wilms tumor gene product. Science 253: 1550–1553
Sukhatme VP 1992 The EGR transcription factor family: from signal transduction to kidney differentiation. Kidney Int 41: 550–553
Buckler AJ, Pelletier J, Haber DA, Glaser T, Housman DE 1991 Isolation, characterization and expression of the murine Wilms' tumor gene (WT-1) during kidney development. Mol Cell Biol 11: 1707–1712
Igarashi P 1992 Transcription factors and apoptosis in kidney development. Curr Opin Nephrol Hypertens 3: 308–317
Martinez-Hernandez A, Amenta PS 1995 Extracellular matrix in hepatic regeneration. FASEB J 9: 1401–1410
Fausto N, Laird AD, Webber EM 1995 Liver regeneration. Role of growth factors and cytokines in hepatic regeneration. FASEB J 9: 1527–1536
Wizgall R, Brown D, Schwarz C, Bonventre JV 1994 Localization of proliferating cell nuclear antigen, vimentin, c-Fos, and clusterin in the postischemic kidney. Evidence for a heterogenous genetic response among nephron segments, and a large pool of mitotically active and dedifferentiated cells. J Clin Invest 93: 2175–2188
Calvet JP 1993 Injury and development in polycystic kidney disease. Curr Opin Nephrol Hypertens 3: 340–348
Safirstein R 1994 Gene expression in nephrotoxic and ischemic acute renal failure. J Am Soc Nephrol 4: 1387–1395
Gobe GC, Buttyan R, Wyburn KR, Ethridge MR, Smith PJ 1995 Clusterin expression and apoptosis in tissue remodeling associated with renal regeneration. Kidney Int 47: 411–420
Hallman N, Norio R, Kouvalainen K 1967 Main features of the congenital nephrotic syndrome. Acta Pediatr Fenn 172: 75–78
Rapola J 1987 Congenital nephrotic syndrome. Pediatr Nephrol 1: 441–446
Holmberg C, Antikainen M, Rönnholm K, Ala-Houhala M, Jalanko H 1995 Management of congenital nephrotic syndrome of the Finnish type. Pediatr Nephrol 9: 87–93
Holthöfer H, Reivinen J, Miettinen A 1992 Acidic glycolipids of human glomeruli. Evidence of specific changes in congenital nephrosis of the Finnish type. J Am Soc Nephrol 1: 525
Holthöfer H, Reivinen J, Miettinen A 1994 Nephron segment and cell type specific gangliosides in the developing and adult kidney. Kidney Int 45: 123–130
Amin KM, Litzky LA, Smythe WR, Mooney AM, Morris JM, Mews DJY, Pass HI, Kari C, Rodeck U, Rauscher FJ, Kaiser LR, Albelda SM 1995 Wilms' tumor 1 susceptibility (WT1) gene products are selectively expressed in malignant mesothelioma. Am J Pathol 146: 344–356
Muramatsu H, Muramatsu T 1991 Purification of recombinant midkine and examination of its biological activities: functional comparison of new heparin binding factors. Biochem Biophys Res Commun 177: 652–658
Tsutsui J-I, Uehara K, Kadomatsu K, Matsubara S, Muramatsu T 1991 A new family of heparin binding factors: Strong conservation of midkine (MK) sequences between the human and the mouse. Biohem Biophys Res Commun 176: 792–797
Abrams JM, White K, Fessler LI, Steller H 1993 Programmed cell death during Drosophila embryogenesis. Development 117: 29–43
Tryggvason K, Kouvalainen K 1975 Number of nephrons in normal human kidneys and kidneys with the congenital nephrotic syndrome. Nephron 15: 62–68
Burrow CR, Wilson PD 1994 Renal progenitor cells: problems of definition, isolation and characterization. Exp Nephrol 2: 1–12
Pabst R, Sterzel RB 1983 Cell renewal of glomerular cell types in normal rats. An autoradiographic analysis. Kidney Int 24: 626–631
Herzlinger D, Koseki C, Mikawa T, Al-Awqati Q 1992 Metanephric mesenchyme contains multipotent stem cells whose fate is restricted after induction. Development 114: 565–572
Kestilä M, Männikkö M, Holmberg C, Gyupay G, Weissenbach J, Savolainen E-R, Peltonen L, Tryggvason K 1994 Congenital nephrotic syndrome of the Finnish type maps to the long arm of chromosome 19. Am J Hum Genet 54: 757–764
Jefferson JA, Shanks JH, Maxwell AP, Hill CM, Gill D, Savage JM 1995 Congenital nephrotic syndrome of the Finnish type maps to chromosome 19q in Irish families. J Am Soc Nephrol 6: 722( abstr)
Kashtan C, Männikkö M, Lenkkeri U, Mauch T, Tryggvason K 1995 Mapping of the congenital nephrotic syndrome locus in North American families. J Am Soc Nephrol 3: 723
Aula P, Rapola J, Karjalainen O, Lindgren J, Hartikainen AL, Seppälä M 1978 Prenatal diagnosis of congenital nephrosis in 23 high-risk families. Am J Dis Child 1332: 984–987
Menssen HD, Renkl H-J, Rodeck U, Kari C, Schwartz S, Thiel E 1997 Detection by monoclonal antibodies of the Wilms' tumor (WT1) nuclear protein in patients with acute leukemia. Int J Cancer 70: 518–523
Haltia A, Solin M, Muramatsu T, Jalanko H, Holmberg C, Miettinen A, Holthöfer H 1997 Expression of nine developmental stage specific genes in human kidney and cultured renal cells. Exp Nephrol 5: 457–464
Kestilä M, Männikkö M, Holmberg C, Tryggvason K, Peltonen L 1994 Congenital nephrotic syndrome of the Finnish type is not associated with Pax-2 gene despite the promising transgenic animal model. Genomics 19: 570–572
Noakes PG, Miner JH, Gautam M, Cunningham JM, Sanes JR, Merlie JP 1995 The renal glomerulus of mice lacking S-laminin/laminin β2: nephrosis despite molecular compensation by laminin β1. Nat Genet 10: 400–406
Acknowledgements
The authors thank Riitta Väisänen for expert technical assistance and Profs. Wilhelm Kritz and Dontscho Kerjaschki for helpful discussions.
Author information
Authors and Affiliations
Additional information
Supported by the Paulo Foundation, Päivikki and Sakari Sohlberg Foundation, and Helsinki University Central Hospital.
Rights and permissions
About this article
Cite this article
Haltia, A., Solin, ML., Holmberg, C. et al. Morphologic Changes Suggesting Abnormal Renal Differentiation in Congenital Nephrotic Syndrome. Pediatr Res 43, 410–414 (1998). https://doi.org/10.1203/00006450-199803000-00017
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1203/00006450-199803000-00017
This article is cited by
-
Mechanism of cystogenesis in nephrotic kidneys: a histopathological study
BMC Nephrology (2014)
-
Familial forms of nephrotic syndrome
Pediatric Nephrology (2010)
-
Glomerular structural factors in progression of congenital nephrotic syndrome
Pediatric Nephrology (2003)