Abstract
We determined the concentrations of copper, the activities of ceruloplasmin and peptidylglycine α-amidating monooxygenase (PAM), and the stimulation index of PAM by the in vitro addition of copper in plasma samples obtained from three male patients with occipital horns and a milder Menkes disease phenotype, having severe copper deficiency due to the defect in copper transport. We found a decreased plasma ceruloplasmin activity and an increased copper stimulation index of plasma PAM in these patients compared with healthy control subjects. The combination of these two determinations may provide a means for the assessment of copper nutriture in humans using blood samples obtained in a single microhematocrit tube. Further investigation is warranted to evaluate whether these noninvasive measurements can be used for the diagnosis of mild copper deficiency in humans with sufficient specificity and sensitivity.
Similar content being viewed by others
Main
It has been recognized for many years that copper is a structural component of many enzymes; however, its essentiality in humans was not firmly established until the discovery of Menkes disease, an X-linked recessive disorder of copper transport, in the early 1970s(1, 2). Many of the features of Menkes disease parallel those of copper deficiency and can be explained by changes in cuproenzymes including hypopigmentation (tyrosinase) and connective tissue alterations (lysyl oxidase). Although severe dietary copper deficiency is considered to be rare in the general population, the diagnosis of a mild (or subclinical) copper deficiency may be important, because such a mild deficiency may lead to undesirable clinical consequences(1, 3–5). Clinical copper deficiency has been recognized more frequently in infants than in adults. Predisposing events leading to copper deficiency include premature birth, total-parenteral nutrition, diets low in copper, and excessive zinc intake(1). Signs of nutritional copper deficiency include neutropenia, hypochromic anemia, and bone abnormalities. Several biochemical measurements using various components of blood, such as plasma copper and ceruloplasmin (EC 1.16.3.1), erythrocyte SOD (EC 1.15.1.1), and platelet cytochrome c oxidase (EC 1.9.3.1), have been used to estimate copper status in humans(1, 3, 6). However, most of these measurements are considered to be of limited diagnostic value of copper nutriture, particularly in mild copper deficiency, due to the lack of sensitivity and specificity. For example, when infection exists, plasma ceruloplasmin concentrations are increased as an acute-phase reactant(3). Plasma copper will parallel plasma ceruloplasmin changes. Erythrocyte SOD should not be responsible to acute changes in copper status because of the prolonged life time of red cells. The assay of cytochrome c oxidase is somewhat difficult to standardize and must be performed on fresh cells. Therefore, it has been recognized that the development of a reliable biochemical method to assess copper nutriture in humans is necessary(3).
Recently, Prohaska(7) reported that the determination of PAM (EC 1.14.17.3) activity in serum and in heart tissue, and the stimulation of PAM by the in vitro addition of copper (CuSI) are sensitive indicators of copper intake in the rat. PAM is a cuproenzyme that catalyzes the conversion of neuropeptides containing glycine at the COOH terminus to the corresponding α-amide(8). We hypothesized that these determinations are also suitable for the assessment of copper nutriture in humans, and tested this hypothesis by determining PAM activity and the CuSI of PAM in plasma samples obtained from one family of three male patients with occipital horns and Menkes disease variant(10, 11), and comparing these to the values obtained from healthy male control subjects. A Menkes disease phenotype with occipital horns is characterized by a less severe degree of neurodegeneration, growth retardation, and abnormalities of connective tissues compared with the classic Menkes disease(9). Details of the clinical background of the three subjects with the Menkes disease variant are provided elsewhere(11). They are characterized by failure to thrive, psychomotor retardation, childhood-onset seizures, pili torti, skin laxity, bladder diverticula, chronic diarrhea, tortuous vessels, and occipital exostoses. The defect in copper transport in these patients may represent a unique and ideal model of severe copper deficiency in humans. We report our findings of plasma copper, ceruloplasmin, PAM, and the CuSI of PAM measured in these patients and discuss the possible usefulness of these determinations for the diagnosis of mild copper deficiency in humans.
METHODS
Patients and control subjects. Three male patients (5-39 y) from one family were identified as having a Menkes disease variant with occipital horns which results from a mutation in the Menkes disease gene(MNK). MNK encodes a protein similar to copper-transporting P-type ATPases(9). Detailed clinical signs, radiographic as well as biochemical findings, and the identification of genetic abnormalities have been reported previously(10, 11). Healthy male control subjects (age 21-32 y) were selected from the volunteers who participated in a study to estimate copper requirements, which was performed at the metabolic unit of the Western Human Nutrition Research Center in San Francisco(12). This study was approved by the Bionetics Institutional Review Board and the Human Studies Review Committee of the Agricultural Research Service, U.S. Department of Agriculture, and informed consent was provided for each subject.
Blood sampling and biochemical determinations. Nonfasting blood samples were obtained from the patients using trace mineral-free evacuated tubes with heparin (Vacutainer, Becton Dikinson, Rutherford, NJ) at their regular clinical visit to the Children's Hospital of Alabama. Blood samples were kept under refrigeration immediately after sampling until plasma samples were separated by centrifugation(13). Aliquots of plasma samples were kept at -70°C, and an aliquot from each patient was shipped from Birmingham to Duluth on dry ice for the determination of the activities of plasma PAM and ceruloplasmin.
The measurements of copper and zinc concentrations in plasma of the patients were performed by flame-atomicabsorption spectrophotometry (model 2380, Perkin Elmer, Norwark, CT) as described previously(13). The dilutions of the plasma samples were made using distilled-deionized water for copper analysis and 3.6% trichloroacetic acid solution for zinc. The interassay coefficient of variation of these analyses is approximately 4%(13). Handling of the blood samples as well as the determination of plasma copper and zinc concentrations obtained from the control subjects used methods similar to those described above(12).
Plasma ceruloplasmin activities were measured after the oxidation ofo-dianisidine at 30°C(14). PAM activities were measured by incubating trinitrophenyl-labeled substrate with 10 μL of plasma in the presence of 2 mmol/L of N-ethylmaleimide for 2 h at 37°C. The amount of the product was quantified by measuring the absorbance at 350 nm after its separation from the substrate using reverse-phase HPLC(15). We had established that product formation was linear up to 3 h, and up to 20 μL of plasma. When the CuSI of plasma PAM was measured, 2 μmol/L Cu2+ was added as cupric sulfate to optimize the Cu2+ stimulation of human plasma PAM activity. The CuSI of PAM was calculated by determining the ratio of the activity with added Cu2+ to the activity without added Cu2+(7). Basal Cu2+ in PAM assay was 0.25 μmol/L.
Comparisons between patients and controls were made by an unpaired two-tailed t test, α = 0.05, using statistical software(Statview 4.5, Abacus Concept, Berkeley, CA). Variance equality was evaluated by the F test.
RESULTS
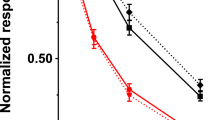
The concentrations of plasma copper and zinc, the activities of plasma PAM and ceruloplasmin, and the CuSI of PAM by the in vitro addition of copper in patients with occipital horns and Menkes disease variant and control subjects are summarized in Table 1. The concentrations of plasma copper and zinc and the activity of ceruloplasmin in patients were significantly lower than those in the controls (p < 0.05), whereas basal PAM activities were not different between patients and the controls (p > 0.05). Mean PAM activity in the presence of 2μmol/L Cu2+ was significantly higher in the patients [3.64 ± 0.48 (SD) nmol/h/mL] than in the control subjects (2.43 ± 0.81 nmol/h/mL) (p < 0.05). The CuSI values of plasma PAM in patients were approximately two times higher than those in the controls (p< 0.05) (Table 1). The relationship between plasma ceruloplasmin activity and the CuSI of plasma PAM in both patients and control subjects is shown in Figure 1. A clear separation is noted between these two indices in patients, which clustered at the upper left, and in the controls, which clustered at the lower right of the figure.
DISCUSSION
We determined the activities of plasma ceruloplasmin and the CuSI of plasma PAM activities by the in vitro addition of copper in three male patients with a milder form of Menkes disease including occipital horns, a defect in copper transport resembling a severe copper deficiency, and compared these values to those in healthy male adults. As expected, plasma ceruloplasmin activities were significantly lower in the patients with the syndrome than the reported values of healthy adult males which range between 62 and 140 U/L(14). The CuSI values of plasma PAM activity were significantly higher in patients than in the control subjects. The higher CuSI among patients with Menkes disease variant may indicate higher levels of PAM apoenzyme in severe copper deficiency than under copper-sufficient conditions. Further research is necessary to validate this point in humans because experiments using the copper-deficient rat as a model did not detect differences in stimulated serum PAM activity when excess Cu2+ was provided in the assay(7).
Although the findings presented here may be valid only in a severe degree of copper deficiency, such as the unique cases of inborn error of copper transport, the data presented here are consistent with the previous observation by Prohaska(7), who reported that the mean CuSI of serum PAM in marginally copper-deficient rats was more than five times higher than that in copper-sufficient controls. The higher activity of PAM, when incubated with exogenous Cu2+, might be due to the activation of apo-PAM or inhibition of endogenous inhibitors(8). Dopamine-β-monooxygenase, another cuproenzyme, exchanges copper rapidly(16). The similarity in the catalytic domain of PAM to this enzyme makes it likely that PAM exchanges copper rapidly as well(17).
This property of rapid activation is not shared by all apocuproproteins. For example, DiSilvestro(18), in 1989, reported that the activities of SOD in intact erythrocytes obtained from copper-deficient rats increased by 63% after a 4-h incubation with 3 μM copper. However, such an increase was not observed in erythrocyte lysates, indicating that the activation of SOD requires an unknown cell component which was destroyed during hemolysis. A similar observation was reported in the intact aortic cells obtained from copper-deficient chicks after incubation in culture medium supplemented with copper(19). Rossi et al.(20) did present evidence for the activation of apo-SOD in the liver of copper-deficient rats by the in vitro addition of Cu2+, where millimolar Cu2+ levels were required for the activation with 3-h incubation followed by exhaustive dialysis. The activation of apo-ceruloplasmin and apo-cytochrome c oxidase by the in vitro addition of Cu2+ in tissues obtained from copper-deficient rats does not occur to any appreciable degree (J. R. Prohaska, unpublished results).
To our knowledge, however, data presented here are the first documentation of a method using the in vitro copper stimulation of a cuproenzyme in human samples. Our findings may be analogous to those of Prasad et al.(21), who suggested that the in vitro zinc stimulation of plasma thymulin, a thymic hormone, is a sensitive indicator of zinc nutriture in humans. With regard to the diagnosis of mild zinc deficiency, a similar attempt has been made by Tamura et al.(22), who evaluated the stimulation of the activity of angiotensin-converting enzyme, a zinc-dependent enzyme, by the in vitro addition of zinc to plasma samples obtained from human subjects who had inadequate zinc nutriture. However, stimulation of enzyme activity was not observed in these samples, although Reeves and O'Dell(23), Dahlheim et al.(24), and Tamura et al.(25) reported that the activity of angiotensin-converting enzyme was stimulated by the in vitro addition of zinc to plasma obtained from severely zinc-deficient rats, but not to plasma from zinc-sufficient controls.
In summary, we found that plasma ceruloplasmin activity was decreased and the CuSI of plasma PAM was increased in patients with severe copper deficiency due to Menkes disease variant with occipital horns. It is likely that the combination of these two determinations along with plasma copper concentrations provides a means to assess copper nutriture more accurately than the conventional indices being used in humans. Further investigation is warranted to evaluate whether these noninvasive measurements can be used for the diagnosis of mild copper deficiency in humans with sufficient specificity and sensitivity.
Abbreviations
- PAM:
-
peptidylglycineα-amidating monooxygenase
- SOD:
-
superoxide dismutase
- CuSI:
-
copper stimulation index
References
Danks DM 1988; Copper deficiency in humans. Annu Rev Nutr 8: 235–257.
Danks DM 1995; The Metabolic and Molecular Basis of Inherited Disease. McGraw-Hill, New York. 2211–2235
Milne DB 1994; Assessment of copper nutritional status. Clin Chem 40: 1479–1484.
Kelley DS, Daudu PA, Taylor PC, Mackey BE, Turnlund JR 1995; Effects of low-copper diets on human immune response. Am J Clin Nutr 62: 412–416.
Klevey LM, Medeiros DM 1996; Deliberations and evaluations of the approaches, endpoints and paradigms for dietary recommendations about copper. J Nutr 126: 2419S–2426S.
Milne DB, Nielsen FH 1996; Effects of a diet low in copper on copper-status indicators in postmenopausal women. Am J Clin Nutr 63: 358–364.
Prohaska JR 1997; Responses of rat cuproenzymes to variable dietary copper. J Nutr Biochem 8: 316–321.
Eipper BA, Stoffers DA, Mains RE 1992; The biosynthesis of neuropeptides: peptideamidation. Annu Rev Neurosci 15: 57–85.
Vulpe C, Levinson B, Whitney S Packman S Gitschier J 1993; Isolation of a candidate gene for Menkes disease and evidence that it encodes a copper-transporting ATPase. Nat Genet 3: 7–13
Kaler SG, Gallo LK, Proud VK, Percy AK. Mark Y, Segal NA, Goldstein DS, Holmes CS, Gahl WA 1994; Occipital horn syndrome and a mild Menkes phenotype associated with splice site mutations at the MNK locus. Nat Genet 8: 195–202
Proud VK, Mussell HG, Kaler SG, Young DW Percy AK 1996; Distinctive Menkes disease variant with occipital horns: Delineation of natural history and clinical phenotype. Am J Med Genet 65: 44–51.
Turnlund JR, Scott KC, Pfeiffer GL, Jang AM, Keen CL, Sakanashi TM 1997; Copper status of young men consuming a low copper diet. Am J Clin Nutr 65: 72–78.
Tamura T, Johnston KE, Freeberg LE, Perkins LL, Goldenberg RL 1994; Refrigeration of blood samples prior to separation is essential for the accurate determination of plasma or serum zinc concentrations. Biol Trace Elem Res 41: 165–173.
Lehman HP, Schosinsky KH, Beeler MF 1974; Standardization of serum ceruloplasmin concentrations in international enzyme units with o-dianisidine dihydrochloride as substrate. Clin Chem 20: 1564–1567.
Prohaska JR, Bailey WR, Lear PM 1995; Copper deficiency alters rat peptidylglycine α-amidating monooxygenase activity. J. Nutr 125: 1447–1454.
Skotland T, Ljones T 1979; The enzyme-bound copper of dopamine β-monooxygenase. Reaction with copper chelators, preparation of the apoprotein, and kinetics of the reconstitution by added copper. Eur J Biochem 94: 145–151.
Southern C, Kruse LI 1989; Sequence similarity between dopamine β-hydroxylase and peptide α-amidating enzyme: evidence for a conserved catalytic domain. FEBS Lett 255: 116–120.
DiSilvestro RA 1989; Copper activation of superoxide dismutase in rat erythrocytes. Arch Biochem Biophys 274: 298–303.
Dameron CT, Harris ED 1987; Regulation of aortic CuZn-superoxide dismutase with copper. Ceruloplasmin and albumin re-activate and transfer copper to the enzyme in culture. Biochem J 248: 669–675.
Rossi L, Ciriolo MR, Marchese E, De Martino A, Giorgi M, Rotilio G 1994; Differential decrease of copper content and of copper binding to superoxide dismutase in liver, heart and brain of copper-deficient rats. Biochem Biophys Res Commun 203: 1028–1034.
Prasad AS, Meftah S. Abdalla J, Kaplan J, Brewer GJ, Bach JF, Dardenne M 1988; Serum thymulin in human zinc deficiency. J Clin Invest 82: 1202–1210.
Tamura T, Goldenberg RL, Johnston KE Freeberg LE Dubard MB Thomas EA 1996; In vitro zinc stimulation of angiotensin-converting enzyme activities in human plasma. J Nutr Biochem 7: 55–59
Reeves PG, O'Dell BL 1985; An experimental study of the effect of zinc on the activity of angiotensin converting enzyme in serum. Clin Chem 31: 581–584.
Dahlheim H, White CL, Rothemund J, von Lutterotti N JacobI CM Rosenthal J 1989; Effect of zinc depletion on angiotensin I-converting enzyme in arterial walls and plasma of the rat. Miner Electrolyte Metab 15: 125–129
Tamura T, Freeberg LE, Johnston KE, Keen CL 1994; In vitro zinc stimulation of angiotensin-converting enzyme activities in various tissues of zinc-deficient rats. Nutr Res 14: 919–928.
Author information
Authors and Affiliations
Additional information
Supported by Grant 96-35200-3138 from NRI Competitive Grants Program/USDA and National Institutes of Health Grant HD32901.
Rights and permissions
About this article
Cite this article
Prohaska, J., Tamura, T., Percy, A. et al. In Vitro Copper Stimulation of Plasma Peptidylglycineα-Amidating Monooxygenase in Menkes Disease Variant with Occipital Horns. Pediatr Res 42, 862–865 (1997). https://doi.org/10.1203/00006450-199712000-00023
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1203/00006450-199712000-00023
This article is cited by
-
Perinatal Copper Deficiency Alters Rat Cerebellar Purkinje Cell Size and Distribution
The Cerebellum (2010)
-
Copper-transporting ATPases ATP7A and ATP7B: cousins, not twins
Journal of Bioenergetics and Biomembranes (2007)