Abstract
Thyroid status was characterized in very preterm infants (gestational age≤32 wk; n = 61) from birth through d 14, and in infants who died within 16 d after delivery (n = 10), where it was also correlated with metabolism of iodothyronines in peripheral tissues (brain, liver, kidney, skeletal muscle, and adipose tissue). At 3 d of life, mean plasma levels of thyroxine, triiodothyronine, and TSH started to decrease, being lower in the critically ill compared with healthy premature neonates. Activities of the three iodothyronine deiodinases enzymes (type I, II, and III, respectively) were detected in all postmortem tissue samples, except for absence of the type II activity in kidney. All activities were the highest in liver and differed in other tissues. Lack of correlation between the type I activity in liver(and kidney), and plasma levels of thyroid hormones suggested that the thyroid was the primary source of circulating triiodothyronine. On the other hand, namely in brain, correlations between activity of the deiodinases and plasma hormone levels were found which suggested a complex control by thyroid hormones of their own metabolism. High activity of type III in liver, adipose tissue, and skeletal muscle demonstrated a role of these tissues in thyroid hormones degradation. Results support the view that peripheral tissues of very preterm infants are engaged in local generation of triiodothyronine, and inactivation of thyroid hormones, but do not represent a major source of circulating triiodothyronine.
Similar content being viewed by others
Main
Outcome of preterm infants may be affected by the degree of hypothyroidism during the early postnatal period. All preterm infants show transient hypothyroxinemia during the first 6-8 wk of extrauterine life, with low values of serum T4 and T3, and usually normal TSH levels (except for an attenuation of the postnatal TSH surge), compared with term-born infants. Several studies(1–3) indicated even lower plasma T4 and T3 levels in premature infants with severe respiratory disease and other nonthyroidal systemic diseases than in well being premature infants (euthyroid sick syndrome; low T3 syndrome). A causal link may exist between duration and severity of neonatal hypothyroidism and impaired neurodevelopmental outcome at ages between 18 mo to 9 y(3–5). Intraamniotic T4(6) or maternal administration of TSH, when combined with glucocorticoids(7, 8), may accelerate fetal lung maturation and reduce the incidence of respiratory distress syndrome in preterm newborns(6, 7).
The effect of thyroid hormones depends on intracellular levels of T3, which has by far the highest hormone activity among all the iodothyronines. Metabolism of iodothyronines in peripheral tissues is an important site of T3 production in adults, because at least 80% of circulating T3 (and nearly all rT3) production can be accounted for by peripheral generation from T4(9, 10). However, in neonates the relative contribution of peripheral tissues and the thyroid gland, respectively, to total T3 production is as yet not well defined (see “Discussion”).
Three important enzymes are involved in deiodination of iodothyronines in microsomes. 5′D-I is present mainly in liver, kidney, thyroid gland, and pituitary(11). It catalyses both the outer-ring deiodination of T4 to T3 and inner-ring deiodination of T4 to rT3, as well as conversion of triiodothyronines to diiodothyronines. Activity with the respective substrate is tissue-dependent in human(12), and both in rat and human, is affected by substrate sulfation(13). Activity of 5′D-I is completely inhibited by nanomolar concentrations of gold thioglucose and by micromolar concentrations of PTU. Because of a high activity for the outer-ring deiodination in the liver, this tissue is regarded as an important source of circulating T3, whereas the inner-ring deiodination of T4 is an inactivating pathway, both in rat and human(9, 14). 5′D-II is present in human brown fat, placenta, brain, anterior pituitary, heart, and skeletal muscle(12, 15–17). It catalyzes the outer-ring deiodination of T4 and rT3 to T3 and 3.3′-diiodothyronine, respectively. Unlike 5′D-I, it is relatively insensitive to PTU and gold thioglucose, and has a much higher affinity for substrate (Kmin nanomolar range). It is essential for local generation of T3 in tissues(9), namely brain(15, 18) and brown fat(17) during neonatal development. In adult rats, brown fat 5′D-II is an important source of circulating T3(19); however, 5′D-II may be important for maintaining circulating T3 concentrations even in healthy humans(15). Type III 5-deiodinase (5D-III) catalyzes inner-ring deiodination of T4 and T3 to inactive metabolites, rT3 and 3,3′-diiodothyronine, respectively(9, 20, 21). Like 5′D-II, it is not sensitive to PTU, and it has a very lowKm for both substrates. It is highly expressed in placenta where it regulates the circulating fetal thyroid hormone concentration through gestation(20, 21), and also in several fetal and neonatal tissues that are thus protected against premature exposure to adult levels of thyroid hormones(22).
Very little is known about the metabolism of iodothyronines in tissues of human newborns, especially during the critical early postnatal period. In this study we present our observations on postnatal development of plasma thyroid hormone levels in normal and critically ill preterm neonates. Activities of all three types of iodothyronine deiodinase were followed in autopsic samples from brain, liver, kidney, muscle, and adipose tissue depots, to better characterize the relationships between peripheral thyroid hormone metabolism and thyroid status in critically ill preterm newborns.
METHODS
Subjects. This study was approved by the Committees of Medical Ethics at all the collaborating institutions. Only liveborn premature newborns, with gestational age ≤32 wk (n = 61) were enrolled (seeTable 1). Infants born from mothers who suffered from endocrinologic disorders, or abused drugs, were not eligible for the study. Tissues of 13 out of 16 infants who died (Table 3) could be analyzed (see below). As controls for serum levels of thyroid hormones and TSH levels. 10 term newborn infants were also included (gestational age of 37-42 wk, birth weight 2700-3800 g, Apgar score at 5 min higher than 8, spontaneously delivered from healthy mothers). Informed consent was obtained from one or both parents.
Hormone levels. Plasma concentrations of total T3, total T4, and total rT3 were estimated by competitive RIA (T3 and T4 using RIA kits from Immunotech, Prague, Czech Republic; rT3 using a kit from Serrono Diagnostics. Biodata S.p.A., Rome, Italy). Plasma TSH concentrations were measured by microparticle enzyme immunoassay(IMx Ultrasensitive hTSH kit, Abbott Laboratories, Abbott Park, IL). When there was limited plasma volume, priority was given to assay the hormone levels in the following order: T3, T4, TSH, and rT3. Blood samples were collected from cord (d 0) and from arterial or venous blood in neonates, between 20-28h after delivery (d 1), and in 3-, 7-, and 14-d-old neonates. In the control group of term newborns, blood samples were collected only up to d 7.
Collection of tissues. Two samples (50-250 mg wet weight) were collected from the following tissues (see Table 4): brain(cortex in the vicinity of the major fontanel); liver; kidney; skeletal muscle(musculus quadriceps); and adipose tissue depots known to contain multilocular brown adipocytes in human newborns(23), located s.c. between scapulae (interscapular fat), in posterior cervical region (cervical fat), and around kidneys (perirenal and suprailiac fat). Each sample was frozen and stored in liquid nitrogen. Enzyme activities were estimated (see below) in two to five replicates in each tissue sample (or fat depot); the mean value for each tissue (depot) was taken as representative data.
Assay of iodothyronine deiodinase activities. Outer-ring(5′D-I and 5′D-II) and inner-ring (5D-III) deiodinating activities were determined in microsomal fractions prepared(24) from the frozen tissue samples, and suspended in a medium containing 100 mM sodium phosphate, 1 mM EDTA, pH 7.0 (5′D-I and 5′D-II) or pH 7.6(5D-III), and protease inhibitors: antipain, aprotinin, leupeptin, and pepstatin A (each at 1 μg/mL). Assays were performed in the same medium(above), except for the absence of the protease inhibitors, with 1.5-300 μg of microsomal protein (depending on the type of tissue and the enzyme measured) in a final volume of 40 μL. During the incubation (for 30 min at 37°C), nonradioactive and 50000 cpm of the corresponding radioactive substrate were also present (giving the final chemical concentrations: 50 nM rT3, 2 nM T4, and 2 nM T3, in the case of 5′D-I, 5′D-II, and 5D-III, respectively), together with: 10 mM DTT(5′D-I); 20 mM DTT and 10 mM PTU (5′D-II); and 40 mM DTT and 10 mM PTU (5D-III), respectively. Less than 30% of substrate was consumed during the incubation. The reaction was stopped on ice by adding 10 μL of a solution containing 10 μM T3 and 10 μM T4 in concentrated ammonium hydroxide, followed by 30 μL of methanol, and centrifugation (2 min at 10 000 × g). Aliquots (8 μL) of supernatant were analyzed by chromatography on a silica gel plate (20 × 20 cm; Merck no. 11798) using an optimized solvent system (chloroform/methanol/concentrated ammonium hydroxide; 40/20/3). The quantities of separated radiolabeled compounds(rT3, T4, T3, 3,3′-T2, 3′-T1, and I-: in order of the increasing mobilities of the individual compounds during the separation) were evaluated using the Phosphorlmager SF(Molecular Dynamics), relative to the total radioactivity applied in each sample (5000 cpm). Separated products of the reactions containing ≥0.5% from the total radioactivity applied (corresponding to ≥25 cpm) could be detected. Activities were expressed as picomoles of rT3, femtomoles of T4, and femtomoles of T3 converted/h/mg of microsomal protein in the case of 5′D-I, 5′D-II, and 5D-III, respectively (seeFig. 1 andTable 4). Further details of these assays will be published elsewhere (S. Pavelka, P. Brauner, J. Kopecký, manuscript in preparation).
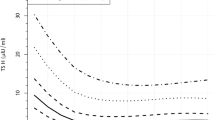
Deiodinase activities in liver, kidney, and interscapular adipose tissue of premature newborns as a function of postnatal age. For mean values, see Table 4.
Reagents. High specific activity l-[3′.5′-125I]T4 (55 MBq/μg) and l-[3′-125I]T3 (114 MBq/μg) were obtained from Amersham Corp. and l-[125I]rT3 (35 MBq/μg) from DuPont NEN. Immediately before deiodination assays, the radioactive hormones were purified by paper electrophoresis(24). All the other reagents were from Sigma Chemical Co.
Statistical analysis. Mean ± SEM values are presented. Statistical comparisons were made using Fischer's exact test, linear regression, and nonparametric Kruskal-Wallis one-way ANOVA with post hoc comparisons. All tests were judged to be significant at p< 0.05.
RESULTS
Patient population and outcome. The main focus of the present study was on very low birth weight infants (69% of neonates ≤1000 g) with a high rate of mortality (26% infants died within 6 mo, and 20% within 1 mo after delivery). Mean gestational age and birth weight of children who survived were higher compared with children who died (Table 1). However, only the difference in birth weight was statistically significant, apparently due to a higher incidence of multiple gestation in children who died (see below). The presence of various further factors was compared between the two groups of children, with the following incidences found (expressed as percent in survivors versus percent in children who died; statistically significant differences are denoted by p values): birth weight ≤1000 g (60 versus 94%; p ≤ 0.05); multiple gestation (29 versus 50%); vaginal (i.e. not cesarean delivery; 34 versus 26%; p ≤ 0.05); intrauterine asphyxia (Apgar score ≤5 at 5 min; and/or pH of cord blood≤7.1; 25 versus 27%); corticoids administered to the mother before delivery (75 versus 58%); tocolysis (38 versus 38%); premature rupture of membranes (27 versus 38%); hypotrophy[defined according to Lubchenco et al.(25); 33 versus 19%]; respiratory distress syndrome (82 versus 94%); chronic lung disease (33 versus 19%); intracranial hemorrhage(53 versus 44%); patent ductus arteriosus (22 versus 44%); corticoids (52 versus 88%; p ≤ 0.05), or catecholamines(67 versus 94%) administered to children; oral nutrition(i.e. not i.v. fluids only, any time during the first 2 wk; 36versus 0%; p ≤ 0.05); artificial respiration (any time during the first 2 wk; 77 versus 100%; p ≤ 0.05); and high frequency oscillatory ventilation (i.e. not conventional ventilation or normal respiration; 23 versus 22%). All the premature neonates had been given antibiotics.
Plasma thyroid hormones. In cord blood, higher mean levels of TSH were found in premature compared with mature newborns(Table 2), reflecting apparently the gradual decline in fetal TSH, which follows the mid-gestation increase(1, 26). In contrast, both mean T3 and T4 levels were significantly lower in premature versus term-born infants(1). In newborns who died, the values in cord blood were lower compared with survivors, namely in the case of T3, which could account for a difference in birth weight between the two groups of premature newborns (Table 1; see above). No differences in rT3 levels in cord blood in premature versus mature infants were observed (Table 2). In mature newborns, the typical postnatal surge of TSH(1, 2) was accompanied by elevation of both T3 and T4 levels. In the case of T3, the increase was biphasic(1), with the first maximum at d 1, nadir at d 3, and the second maximum at d 7 (the last time point analyzed in the control group of mature newborns). In premature newborns, changes of plasma T3, T4, and TSH during the early postnatal life reflected the outcome of infants. In survivors, no significant changes of plasma T3 and T4 levels during the first 2 wk of life were observed (Table 2). In infants who died, both T3 and T4 concentrations reached maximum levels at d 1, but the levels were not significantly different from those in premature infants who survived. However, this maximum was followed by a significant decline in concentrations of both hormones, resulting into 2-3-fold lower mean plasma concentrations (between d 7 and 14; most of these children died between d 3 and 16; see Table 3) versus survivors. These differences remained significant when children who died were compared with their body weight-matched counterparts among survivors (except for T4 levels on d 7; not shown). Also TSH levels between d 7 and 14 tended to be lower in children who died compared with survivors. The levels of rT3 were similarly high in both premature and mature infants until d 1, whereas, beginning from d 3, lower rT3 values were observed in pretermversus term infants (and no differences related to infant mortality were detected; Table 2).
Iodothyronine deiodinase activities in tissues. Activities of the three enzymes (5′D-I, 5′D-II, and 5D-III) involved in tissue metabolism of T4 were measured in autopsic samples of brain, liver, kidney, skeletal muscle, and adipose tissue. Tissues of 13 out of 16 infants who died could be dissected within 6 h after death (Table 3) and used for the analysis. In general, the activities in children(n = 3) who died between 90 and 140 d after delivery were much lower compared with the larger group of infants (n = 10) surviving from 7 h up 16 d (Fig. 1). On the other hand, no correlation between postnatal age and the enzyme activities was found in different tissues in the group of infants who died by 16 d of age (except for 5′D-II in the interscapular fat; r = -0.76; p = 0.05; seeFig. 1). Therefore, the activities of 5′D-I, 5′D-II, and 5D-III in different tissues were compared further only in the 10 preterm newborns who died within 16 d after birth(Table 4).
All three types of iodothyronine deiodinase activities were detected in all the tissues studied, except for absence of 5′D-II in kidney. Importantly, because the activity of each of the three types of iodothyronine deiodinase was measured using a single substrate concentration and under different experimental conditions, optimized for each type of enzyme (see“Methods”), the activity of each type of deiodinase could be compared among various tissues, but not relative to another type of iodothyronine deiodinase in the same tissue. Mean activity of each of the enzymes was always the highest in liver, with a variable pattern of expression in other tissues, depending on the type of enzyme (Table 4).
Lack of correlation between activity of iodothyronine deiodinases in tissues and gestational age. Activities of 5′D-I, 5′D-II, and 5D-III were compared in tissues (Table 4) of infants who differed in gestational age (range 23-32 wk; see Table 3). However, linear regression analysis (not shown) has not revealed any significant correlation between enzyme activity and the gestational age, except for 5D-III in brain (r = 0.69; p = 0.03) and liver(r = -0.64; p = 0.04). These observations suggested that, in general, the length of survival (see above) and clinical conditions were much more important factors than was the gestational age.
Correlations between tissue metabolism and plasma levels of thyroid hormones. In the 10 cases of children who died within 16 d after delivery, possible correlations between tissue activities of iodothyronine deiodinases (Table 4) and plasma thyroid hormone levels before death were sought. Linear regression analysis of the data were performed using the T3/T4 ratio (Table 5) and T3 or T4 concentration alone (not shown), respectively, as one of the variables. Statistically significant correlations have been found mostly in the case of the T3/T4 ratio, positive correlation with 5′D-I activities in brain and fat, and 5D-III in kidney, respectively(Table 5). Concentration of T4 correlated positively with 5D-III activity in brain (r = 0.66; p = 0.05), whereas the concentration of T3 correlated negatively with 5D-III activity in liver (r = -0.64; p = 0.05). No other correlation was found.
DISCUSSION
This study was focused on the characterization of thyroid hormone metabolism in tissues of preterm newborns, particularly in relation to their plasma thyroid hormone levels. Accordingly, the main focus was on critically ill very preterm neonates, with a high rate of mortality, who faced disturbances in the yet immature hypothalamic-pituitary-thyroid axis(1, 3). As far as we know, this is the first study, in which a systematic analysis of iodothyronines metabolism in tissues of human newborns was performed by enzyme activity measurements.
Our data on plasma thyroid hormone levels (Table 2) are in agreement with previously published studies(1–3). Lower T3 levels in cord blood of premature infants who died, compared with survivors, probably reflects significantly lower birth weight in the former group of infants(3). One day after delivery, T3, T4, and TSH levels were similarly high, both in children who survived or those who died. However, lower mean plasma levels of T3 and T4 were found between 3 and 14 d of age in children who died shortly thereafter(Table 3). An association between neonatal mortality and low serum T3 early after delivery has already been described(3) and could account for a higher incidence of prematurity and respiratory disease in the cases with fatal outcome(3). Weight loss or malnutrition may also be involved(27, 28). Because T4(29), and TSH were also found to decline in parallel with T3 levels in children who died (Table 2), it is likely that rather than relative decrease of TSH secretion and/or lower T3 and T4 release from thyroid gland, defective tissue metabolism of thyroid hormones was primarily involved (see below). Similar conclusions could be drawn from some previous studies on the euthyroid sick syndrome in premature newborns(1–3). Accordingly, no correlation of plasma levels of rT3 (which is produced mainly in peripheral tissues and not in the thyroid gland)(9) and mortality could be detected. However, in the absence of tissues from healthy control infants, any definitive conclusion about the effect of the health status on the enzymes' activities are difficult to be made. Also some changes due to the gestational age (see below) could be masked by the differences in the clinical conditions and the length of survival (see Table 3 and Fig. 1).
In humans, in analogy with other precocial species (in which thyroid system matures before birth), such as lamb(30), or pig(31), parturition may induce abrupt changes in tissue metabolism of iodothyronines. However, appropriate experimental data are difficult to obtain. In aborted fetuses, activity of 5′D-II in cerebral cortex increased through 11-22 wk of gestation (period of neuroblastogenesis) and decreased thereafter(16). Our previous analysis on newborns with gestational age of 25-40 wk, indicated that the activity of 5′D-II in interscapular brown adipose tissue exhibited a major increase around 30 wk of gestation(17). Induction of the enzyme activity coincided with the appearance of the mitochondrial marker of the thermogenic function of brown fat, the uncoupling protein. In the present study performed on younger, very preterm infants (Table 1), activity of 5′D-II in interscapular brown fat was lower and tended to decrease during the first 16 d of life (see Fig. 1). This finding may reflect a decline of adaptive thermogenesis restricted to interscapular fat, the thermogenesis stimulated by cold shock(24), and other stress factors during birth. No other gestational age-dependent changes (occurring between 23 and 32 wk of gestation) of tissue metabolism of iodothyronines could be noticed, except for 5D-III in brain and liver. Although the positive correlation found in brain remains unexplained, the negative correlation in liver is in agreement with the physiologic function of this enzyme (see below). Our recent findings in the two cases of term-born neonates (who died within 4 h after birth, with transposition of the great vessels and osteogenesis imperfecta, respectively, as the major diagnosis) support the differential effect of the gestational age on the 5D-III activity in brain and liver. Moreover, about a 2-3-fold increase of 5′D-I activity in brain, liver, and kidney (compared with the very premature newborns; see Table 4) was also noticed (not shown).
Detection of activities of the three types of iodothyronine deiodinase in various tissues (brain, liver, kidney, adipose tissue, and skeletal muscle) was mostly in agreement with the recent characterization of differential expression of the corresponding genes(11, 15, 20, 21). In brain, detection of 5′D-I activity contrasted with a lack of such an activity in adult humans(12). This 5′D-I activity positively correlated with the T3/T4 ratio in plasma, suggesting control by thyroid hormone levels. Detection of a 5′D-II activity in the brain reflects the importance of this enzyme for local T3 production(9, 15, 18). The presence of 5D-III in brain, and a positive correlation between the 5D-III activity and plasma T4 levels, may be related with a protective role of the enzyme against excessive levels of thyroid hormones(21, 22). Data strongly suggest a complex control by thyroid hormones of their own metabolism in a developing brain.
In spite of the fact that liver 5′D-I is a major source of circulating T3 in adults(9, 10), no correlation was found between 5′D-I activity in liver (or kidney) of premature newborns and T3/T4 ratio in plasma. Still, 5′D-I activity in liver was the highest relative to the other tissues(Table 4), with the kidney only second to the liver. In another study on premature infants with similar age as here (under 30 wk of gestation), plasma T3 concentrations did not increase after T4 administration, suggesting insufficient production of T3 by peripheral 5′-deiodination(2). Also in agreement with some animal studies(32), these data may indicate that in(preterm) newborns, it is not the release of T3 from the liver, but rather from the thyroid (and other tissues) that could be the most important source of circulating T3 (see also above). The relatively high activity of 5′D-I in the liver of the newborns may be explained by the involvement in deiodination of rT3. In fact, rT3 is a much more preferable substrate for 5′D-I, and T4 is converted into T3 with relatively poor efficiency(9).
The finding of the highest activity of 5D-III in liver, relative to other tissues, indicates a prominent role of the liver in thyroid hormone inactivation (and rT3 production) in preterm newborns. Negative correlation between the enzyme activity and both gestational age and plasma T3 levels is in agreement with this notion. In a recent report(21), no mRNA for 5D-III was detected in human liver, but the age of the donor was not specified. Also detection of the 5′D-II activity in liver is not in agreement with studies at the level of gene expression(15), and may represent an artefact in the activity measurement (due to the high activity of 5′D-I, not inhibited completely by PTU). However, very high concentration of the inhibitor was used in the assay, and under similar conditions, no 5′D-II activity was detected in kidney (Table 4).
In skeletal muscle and adipose tissue, all three types of iodothyronine deiodinase activities were detected. Whereas in muscle both 5′D-I and 5′D-II activities were the lowest relative to the other tissues, 5D-III activity was relatively high (Table 4). Expression of the 5′D-II gene in human skeletal muscle was described recently(15), and its significance for maintaining of tissue T3 and circulating T3 levels was discussed. In adipose tissue, all three activities were relatively high, especially for 5′D-II and 5D-III. The 5′D-II activity found in all the fat depots clearly indicates the presence of brown adipocytes(15, 17, 23). Our previous study suggested that a cold-induced activation of 5′D-II in brown fat may contribute to the sharp transient increase of serum T3 in full-term human newborns(17). The high activity of 5D-III in muscle, and namely in fat (Table 4), indicates that these tissues, together with liver (see above), are significantly involved in thyroid hormone inactivation in premature newborns.
In conclusion, available evidence suggests than in very preterm newborns the thyroid is of primary importance for T3 production, especially in critically ill infants. Further studies in this direction are required. On the other hand, peripheral tissues, namely liver, adipose tissue, and skeletal muscle are likely to be highly engaged in degradation of thyroid hormones. The peripheral production of T3 may not only be lower in (premature) newborns than in adults, but it may also be localized in different tissues(e.g. brown fat 5′D-II). In any case, in contrast to adults, iodothyronine metabolism in peripheral tissues of preterm newborns seems to be dominated by thyroid hormone inactivation and T3 production for local use inside tissues. Regarding the possible link between thyroid status of preterm newborns and their outcome, new clinical studies should be initiated, to define indications and optimum protocol(6–8) for thyroid hormones supplementation in preterm children. In this respect, advanced animal studies(33) are of great importance.
Abbreviations
- T3:
-
3,5,3′-triiodothyronine
- T4:
-
thyroxine
- rT3:
-
3,3′,5′-triiodothyronine (reverse triiodothyronine)
- 5′D-I:
-
Type I iodothyronine 5′-deiodinase
- 5′D-II:
-
type II iodothyronine 5′deiodinase
- 5D-III:
-
type III 5-deiodinase
- PTU:
-
6-n-propyl-2-thiouracil
References
Fisher DA, Klein AH 1981; Thyroid development and disorders of thyroid function in the newborn. N Engl J Med 304: 702–711.
van Wassenaer AG. Kok JH, Endert E, Vulsma T, de Vijlder JJM 1993; Thyroxine administration to infants of less than 30 wk gestational age does not increase plasma triiodothyronine concentrations. Acta Endocrinol 129: 139–146.
Lucas A. Rennie J, Baker BA, Morley R 1988; Low plasma triiodothyronine concentrations and outcome in preterm infants. Arch Dis Child 63: 1201–1206.
Meijer WJ, Verloove-Vanhorick SP, Brand R, van den Brande JL 1992; Transient hypothyroxinemia associated with developmental delay in very preterm infants. Arch Dis Child 67: 944–947.
Den Ouden AL, Kok JH, Verkerk PH, Brand R, Verloove-Vanhorick SP 1996; The relation between neonatal thyroxine levels and neurodevelopmental outcome at age 5 and 9 years in a national cohort of very preterm and/or very low birth weight infants. Pediatr Res 39: 142–145.
Romaguera J, Ramirez M, Adamsons K 1993; Intra-amniotic thyroxine to accelerate fetal maturation. Semin Perinatol 17: 260–266.
Moya FR, Gross I 1993; Combined hormonal therapy for the prevention of respiratory distress syndrome and its consequences. Semin Perinatol 17: 267–274.
Ballard PL, Ballard RA, Creasy RK, Padbury J, Polk DH, Bracken M, Moya FR, Gross I 1992; Plasma thyroid hormones and prolactin in premature infants and their mothers after prenatal treatment with thyrotropin-releasing hormone. Pediatr Res 32: 673–678.
Larsen R, Ingbar SH 1992; The thyroid gland. In: Wilson JD, Foster DW (eds) Williams Textbook of Endocrinology. WB Saunders, Philadelphia. pp 357–487
Pilo A, Iervasi G, Vitek F, Ferdeghini M, Cazzuola F, Bianchi R 1990; Thyroidal and peripheral production of 3,5,3′-triiodothyronine in humans by multicompartmental analysis. Am J Physiol 1990:E715–E726.
Berry MJ, Banu L, Larsen PR 1991; Type I iodothyronine deiodinase is a selenocysteine-containing enzyme. Nature 349: 438–440.
Campos-Barros A, Hoell T, Musa A, Sampaolo S, Stoltenburg G, Pinna G. Eravci M, Meinhold H, Baumgartner A 1996; Phenolic and tyrosyl ring iodothyronine deiodination and thyroid hormone concentrations in the human central nervous system. J Clin Endocrinol Metab 81: 2179–2185.
Visser TJ 1996; Role of sulfate in thyroid hormone sulfation. Eur J Endocrinol 134: 12–14.
Visser TJ, Kaptein E, Terpstra OT, Krenning EP 1988; Deiodination of thyroid hormone by human liver. J Clin Endocrinol Metab 67: 17–24.
Croteau W, Davey JC, Galton VA, St-Germain DL 1996; Cloning of the mammalian type II iodothyronine deiodinase. A selenoprotein differentially expressed and regulated in human and rat brain and other tissues. J Clin Invest 98: 405–417.
Karmarkar MG, Prabarkaran D, Godbole MM 1993; 5′-Monodeiodinase activity in developing human cerebral cortex. Am J Clin Nutr Suppl 57: 291S–294S.
Houstek J, Vizek K, Pavelka S, Kopecky J, Krjcova E. Hermanska E, Cermakova S 1993; Type II iodothyronine 5′-deiodinase and uncoupling protein in brown adipose tissue of human newborns. J Clin Endocrinol Metab 77: 382–387.
Calvo R, Obregon MJ, Ruiz de Ona C, Escobar del Rey F. Morreale de Escobar G 1990; Congenital hypothyroidism, as studied in rats. Crucial role of maternal thyroxine but not of 3,5,3′-triiodothyronine in the protection of the fetal brain. J Clin Invest 86: 889–899.
Fernandez JA. Mampel T, Villarroya F, Iglesias R 1987; Direct assessment of brown adipose tissue as a site of systemic tri-iodothyronine production in the rat. Biochem J 243: 281–284.
Croteau W. Whittemore SL, Schneider MJ, St-Germain DL 1995; Cloning and expression of cDNA for a mammalian type III deiodinase. J Biol Chem 270: 16569–16575.
Salvatore D, Low SC, Berry M, Maia AL, Harney JW, Croteau W, St-Germain DL, Larsen PR, Type III iodothyronine deiodinase: cloning, in vitro expression, and functional analysis of the placental selenoenzyme. J Clin Invest 96: 2421–2430
St-Germain DL 1994; Biochemical study of type III iodothyronine deiodinase. In: Wu S-Y, Visser TJ (eds) Thyroid Hormone Metabolism: Molecular Biology and Alternative Pathways. CRC Press, Ann Arbor, MI, 45–66.
Merklin RJ 1974; Growth and distribution of human fetal brown fat expression, and functional analysis of the placental selenoenzyme. Anat Rec 178: 637–645.
Kopecký J, Sigurdson L, Park IR, Himms Hagen J 1986; Thyroxine 5′-deiodinase in hamster and rat brown adipose tissue: effect of cold and diet. Am J Physiol 251:E1–E7.
Lubchenco LO, Hansman C, Dressler N, Boyd E 1963; Intrauterine growth as estimated from life-born birth weight data at 24-32 wk of gestation. Pediatrics 32: 793–800.
Penny R. Spencer CA, Frasier SD, Nicoloff JT 1984; Cord serum thyroid-stimulating hormone and thyroglobulin levels decline with increasing birth weight in newborns. J Clin Endocrinol Metab 59: 979–985.
Cavalieri RR, Rapoport B 1977; Impaired peripheral conversion of thyroxine to triiodothyronine. Annu Rev Med 28: 57–65.
Chopra IJ, Solomon DH, Chopra U, Wu SY, Fisher DA, Nakamura Y 1978; Pathways of metabolism of thyroid hormones. Recent Prog Horm Res 34: 521–567.
Marsh TD, Freeman D, McKeown RE, Bowyer FP 1993; Increased mortality in neonates with low thyroxine values. J Perinatol 13: 201–204.
Wu S-Y, Klein AH, Chopra IJ, Fisher DA 1978; Alterations in tissue thyroxine-5′-monodeiodinating activity in perinatal period. Endocrinology 103: 235–239.
Slebodzinski AB, Brzezinska-Slebodzinska E 1994; The appearance and activity of tissue thyroxine 5′- and 5-monodeiodinase during ontogenesis in the fetal pig. J Endocr 141: 243–249.
Chanoine J-P, Veronikis I, Alex S, Stone S, Fang SL, Leonard JL. Braverman LE 1993; The postnatal serum 3,5,3′-triiodothyronine (T3) surge in the rat is largely independent of extrathyroidal 5′-deiodination of thyroxine to T3 . Endocrinology 133: 2604–2609.
Escobar-Morreale HF, Escobar del Rey F, Obregon MJ, Morreale de Escobar G 1996; Only the combined treatment with thyroxine and triiodothyronine ensures euthyroidism in all tissue of the thyroidectomized rat. Endocrinology 137: 2490–2502.
Acknowledgements
The authors thank Jaroslav Vorlíček for statistical consultation.
Author information
Authors and Affiliations
Additional information
Supported by the Grant Agency of the Ministry of Health of the Czech Republic (Grant Z 629-3), and by the Howard Hughes Medical Institute (Grant 75195-541001).
Deceased
Rights and permissions
About this article
Cite this article
Pavelka, S., Kopecký, P., Bendlová, B. et al. Tissue Metabolism and Plasma Levels of Thyroid Hormones in Critically Ill Very Premature Infants. Pediatr Res 42, 812–818 (1997). https://doi.org/10.1203/00006450-199712000-00016
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1203/00006450-199712000-00016
This article is cited by
-
Effect of levothyroxine supplementation in extremely low birth weight infants with transient hypothyroxinemia of prematurity
Scientific Reports (2022)
-
Initial and delayed thyroid-stimulating hormone elevation in extremely low-birth-weight infants
BMC Pediatrics (2019)
-
Incidence and severity of transient hypothyroxinaemia of prematurity associated with survival without composite morbidities in extremely low birth weight infants
Scientific Reports (2019)
-
Type I iodothyronine 5′-deiodinase mRNA and activity is increased in adipose tissue of obese subjects
International Journal of Obesity (2012)
-
125I-labelled iodothyronines: useful tools for studies of effects of an antidepressant drug fluoxetine in the rat
Journal of Radioanalytical and Nuclear Chemistry (2010)