Abstract
The existence of specific immunity against pediatric tumors is not well studied. We report here the CD3+CD4-CD8+ cytotoxic T lymphocyte (CTL) line established from tumor-infiltrating lymphocytes (TIL) of a 3-mo-old child with Wilms' tumor. This CD3+CD4-CD8+ CTL line showed cytotoxicity against the HLA-A2402+ tumor cells including the autologous tumor, adenocarcinomas from various organs (colon, stomach, lung, and ovary), and an esophageal squamous cell carcinoma. No other cell lines examined, including HLA-A2402- tumors, K562 cells, or HLA-A2402+ normal cells were lysed by this CTL line. This CTL line recognized an HLA-A2402- ovarian adenocarcinoma transfected with HLA-A2402 cDNA. These results suggest the existence of HLA-A2402-restricted and tumor-specific CTL at the tumor site of a child with Wilms' tumor.
Similar content being viewed by others
Main
HLA-class I-restricted tumor-specific CTL are reported in TIL of adult patients with metastatic melanomas(1, 2). These CTL recognize tumor rejection antigens in an HLA-class I-restricted manner(3–8), and some of them are currently under clinical trials as cancer vaccines(9). HLA-class I-restricted and tumor-specific CTL have also been reported in TIL of adult patients with ovarian or esophageal cancer(10–12). However, there is no available information, from a search of the literature, on HLA-class I-restricted and tumor-specific CTL at tumor sites of pediatric cancers. T cell-mediated specific immunity against tumors plays a central role in the rejection of tumors. Therefore, it is important to know whether HLA-class I-restricted and tumor-specific CTL exist at the tumor site of pediatric cancers to obtain a better understanding of tumor immunity in children whose immunity is genetically thought to be immature. Wilms' tumor is usually sensitive to chemotherapy, but the recurrent or advanced stage tumor is associated with a poor prognosis. We have established a CD3+CD4-CD8+ CTL line from TIL of a 3-mo-old child with Wilms' tumor. This CTL line possessed HLA-A2402-restricted and tumor-specific cytotoxicity, indicating that there may exist a T cell-mediated specific immunity at the tumor site of children with Wilms' tumor.
METHODS
Subject and tumor cell lines. The subject was a 3-mo-old female infant with Wilm's tumor at clinical stage I. A sample of the tumor was obtained at the time of surgery in our hospital, and the pathologic diagnosis was made of nephroblastoma (classical Wilms' tumor) at the Department of Pathology of the Kurume University. Autologous tumor cells were cultured in vitro with RPMI 1640-medium (Life Technologies, Inc., Grand Island, NY) plus 10% FCS (Whittaker, Walkersville, MA) for more than 4 wk and were used for CTL assay (cells of primary culture). Most of the HLA class I-identified allogenic tumor cell lines used in this study were as reported elsewhere(12). The other allogenic tumor cell lines were as follows. KOC-2S, KOC-3S, KOC-5C, and KOC-7C ovarian cancers were kindly provided by Dr. Kataoka, Kurume University. The other cell lines listed in Table 2 were either established in our laboratory or obtained from the Japanese Cancer Research Resources Bank (JCRB, Tokyo, Japan) or the American Type Culture Collection (ATCC, Rockville, MD). Genotypes of HLA-A and -B alleles of this patient and of most of the tumor cell lines in Table 2 were determined by polymerase chain reaction(PCR), and the sequence-specific oligonucleotide probe hybridization method, as reported previously(13), or nucleotide sequencing with TA cloning system (Invitrogen, San Diego, CA).
HLA typing. The genotyping of HLA-A allele of this patient was determined by sequence-specific oligonucleotide probe hybridization method as reported previously(13) using TIL as a cell source, and it was A2402 and A3302. That of the HLA-B allele was determined by nucleotide sequencing with the TA cloning system. Briefly, mRNA was isolated using the Quick Prep® Micro mRNA purification kit (Pharmacia Biotech Inc., Uppsala, Sweden) from expanded TIL. cDNA was made using the SuperScript (Life Technologies, Inc.) plasmid system. HLA-B allele cDNA was amplified using sense primer ABC-5 (5′-GAATCTCCCCAGACGCCGA) and antisense primer B3P(5′-AAACACAGGTCAGCATGGGAAC). Amplified HLA-B allele cDNA was cloned by the TA cloning system, and the sequences were analyzed by an A.L.F. DNA Sequencer (Pharmacia). The sequences of the alleles were identical to the reported sequences of B4403, and the other allele was identical to that of B4601(14). Based on these results, the genotype of the patient's HLA-A and -B was determined as A2402/A3302 and B4403/B4601, respectively.
Generation of CTL. The sample containing tumor cells and TIL was finely minced with scissors and then cultured with RPMI 1640 medium supplemented with 10% FCS and 100 U/mL IL-2 (a generous gift from Shionogi Pharmaceutical Co., Osaka, Japan) at 37 °C in a 5% CO2 incubator for up to 12 wk. The surface phenotype of the generated CTL was analyzed by the three-color immunofluorescence technique with FITC-conjugated anti-CD3, phycoerythrin-conjugated anti-CD4, and PerCP-conjugated anti-CD8 MAb(Nichirei, Tokyo), and a FACScan (Becton Dickinson, San Jose, CA) as reported previously(12). Anti-CD3 (OKT3; ATCC), -CD4 (Nichirei),-CD8 (Nichirei), HLA-class I (W6/32, ATCC), -CD11a (YH384) and HLA-DR MAb(H-DR1) were used for the inhibition experiments of CTL activity as reported elsewhere(12).
Functional assays. A 6-h 51Cr-release assay was performed to measure cytotoxicity of CTL as reported elsewhere(12). The percent spontaneous release was less than 30%(variation from 7 to 30%), and that of a 4-h assay was similar, but slightly lower (<25%). In contrast, the percent specific lysis at 6 h was always higher than that at 4 h as reported previously(15–17). Therefore, the 6-h assay was used in this study. The values represent the mean of the triplicate determinants in each assay, and the SD of the triplicate determinants was usually less than 5%. For IFN-γ production, 2 × 104 tumor cells were cultured for 16 h in a well of a 96-well flat bottom culture plates followed by addition of 2 × 104 CTL. After an 18-h culture, the supernatants were collected, and the IFN-γ concentration of the supernatants was measured by an ELISA.
Transfection. HLA-A2402 cDNA was isolated from an KE-4 esophageal squamous cell carcinoma cell line as reported elsewhere(12). Briefly, mRNA was prepared from KE-4 cells (1× 107) by a Quick Prep mRNA purification kit (Phamacia), and first strand cDNA was synthesized using a SuperScript preamplification kit(Life Technologies, Inc., Gaithersburg, MD). Full-length HLA-A cDNA was amplified by PCR with HLA-A locus-specific oligonucleotides (sense primer, 5′-CCGAGATGGCCGTCATG and antisense primer, 5′-TGTCTCACACTTTACAAGCTGTGAGAG). Amplified cDNA was ligated with pCR3(Invitrogen), cloned, and sequenced. An ovarian cancer cell line KOC-7C was transfected with cloned HLA-A 2402 cDNA and Lipofectin (Life Technologies, Inc.). Briefly, 5 × 105 cells in 2 mL of Opti-MEM (Life Technologies, Inc.) were transfected with 2 μg of the plasmid DNA and 20μg/mL Lipofectin. After an 48-h culture, the KOC-7C cells were harvested and used for experiments.
RESULTS
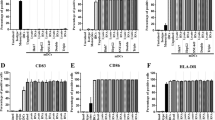
A minced sample from 1 g of tumor was cultured with 100 U/mL IL-2 for up to 12 wk. Activated lymphocytes began to be observed microscopically around the tumor cells after 7-10 d of culture. Lymphocytes began to proliferate actively after 14-21 d of culture and became eligible for the kinetic study of the three-color phenotypic analyses (Fig. 1 and Table 1) and the serial cytotoxicity assays (Fig 2A). A large number (15 × 104) of cells per sample were counted by the three-color analysis at d 0 (fresh sample), 23, 37, 48, 73, and 88. Representative results of the gating patterns and histograms at d 0, 37, and 73 are shown in Fig. 1. The majority of the cells from the sample of d 0 were microscopically tumor cells, whereas those from the samples of d 37 and 73 were T cells. The gating for the sample of d 0 was set to the two different regions (R1 and R2) based on the different size and granularity. There were 0.5% of CD3+ cells, <0.01% of CD3+CD4+CD8- cells, and 0.38% CD3+CD4-CD8+ cells in region 1, where relatively larger cells were present, and were 0.06% of CD3+ cells, <0.01% of CD3+CD4+CD8- cells, and 0.04% CD3+CD4-CD8+ cells in region 2, where relatively smaller cells were present. It is of note that there were 32 and 88% of CD3-CD4+CD8- cells in regions 1 and 2, respectively. These CD3-CD4+CD8- cells are considered to be Wilms' tumor cells, because some non-T cell tumors possess CD4 antigen(23, 24).
Surface phenotypes of TIL. The surface phenotype of the generated CTL and fresh sample were serially analyzed (d 0, 23, 37, 48, 73, and 88 of culture) by a three-color immunofluorescence technique with FITC-conjugated anti-CD3, phycoerythrin-conjugated anti-CD4 and PerCP-conjugated anti-CD8 MAb, and a FACScan. Representative results at d 0, 37, and 73 are shown. The summarized results are given in Table 1.
(A) Cytotoxicity of the CTL from a child with Wilms' tumor. IL-2-activated TIL from a child with Wilms' tumor was serially tested for cytotoxicity against autologous tumor cells (primary culture) HCT116 and MKN28 tumor cells at E/T ratio 10 in a 6-h 51Cr-release assay. (B) Cytotoxicity of the CTL line against autologous tumor cells and MKN28 and HCT116 cell lines at three different E/T ratios (2.5, 5, and 10) was also analyzed in a 6-h 51Cr-release assay.
Percentages of CD3+ T cells increased by the prolonged incubation, and reached the maximal level at d 88 (96%) (Table 1). The percentage of CD3+CD4+CD8- T cells also increased up to 56% at d 37 by prolonged incubation, but dropped transiently down to 30% at d 48 and 15% at d 73, followed by the rapid reincrease up to 88% at d 88. In contrast, the percentage of CD3+CD4-CD8+ T cells increased slowly until d 37 (13%), rapidly increased thereafter, and reached the highest level at d 73 (60%) followed by a rapid decrease.
Modest levels (10-20%) of autologous tumor cell lysis were observed in IL-2-activated TIL at d 23, 28, and 35 of culture at an E/T cell ratio of 10(Fig. 2A). The highest level (43%) of autologous tumor cell lysis was observed at 42 d. The higher cytotoxicity (36%) was also observed at d 59 followed by a gradual decrease. These TIL did not lyse HLA-A allele-mismatched allogenic HCT116 (HLA-A0101/A0201) or MKN28 (HLA-A3101/-) tumor cells at either d 42 or 59. The levels of cytotoxicity were dependent on increased numbers of effector cells (Fig. 2B). These results suggest that IL-2-activated TIL showed autologous tumor-specific cytotoxicity at 42-59 d of culture. Subsequently, these TIL were used for the following studies as the CTL line.
This CTL line was tested for HLA restriction and tumor specificity with a panel of HLA-class I identified tumor cell lines and normal cells. The experiments were repeated at least twice, and the representative results are shown in Table 2. The CTL line (HLA-A2402/3302, B4403/4601) showed significant levels (>10%) of lysis against the autologous tumor cells and seven HLA-A2402+ allogenic tumor cells, including six adenocarcinomas and one squamous cell carcinoma. None of the other cell lines tested, including nine HLA-2402- tumors, 15 HLA-2402+ tumors, HLA-A2402+ normal cells (EBV-transformed B cell lines), or natural killer-sensitive K562 cells, were lysed by the CTL line. The following tumor cells were also resistant to the lysis: three HLA-A3302+ tumor cells (RMUG-S, HAK-1B, and KUR-11), HLA-B44+ TOC-2 tumor cells, and HLA-B46+ MCAS tumor cells in which one of the HLA-class I alleles matched that of the CTL line. The cytotoxicity against HLA-A2402+ SW620 tumor cells was inhibited by anti-class I but not anti-class II (DR) MAb. Namely, the percent lysis at an E/T cell ratio of 10 in the absence of MAb or presence of anti-class I or class II MAb was 28, 16, or 25%, respectively. Anti-CD3 or -CD8 MAb, but not anti-CD4 MAb, also inhibited the cytotoxicity (data not shown).
A transfection experiment was performed to confirm the HLA-A2402 restriction. An ovarian cancer cell line (KOC-7C; HLA-A0201/3101, B51/52) which was resistant to the lysis by this CTL line, was transfected with HLA-A2402 cDNA. The transfectant (KOC-7CA24), but not the parental KOC-7C cells, expressed HLA-A24 molecules on the cell surface (Fig. 3A). The CTL line produced significant levels of IFN-γ by recognition of HLA-A2402+ SW620 tumor cells (as a positive control) and the transfectant (KOC-7CA24), but not of the parental KOC-7C tumor cells (Fig. 3B).
Transfection experiments. (A) Expression of HLA-A2402 on the cell surface of SW620 cells (HLA-A0201/2402), the parental KOC-7C cells (HLA-A0201/3101), and those transfected with HLA-A2402 cDNA(KOC-7CA24) was analyzed using a FACScan with anti-HLA-A24 MAb (A11.1 M; ATCC). (B) Levels of IFN-γ produced by the CTL line in response to SW620, KOC-7C, and KOC-7CA24 tumor cells at an E/T ratio of 1.
DISCUSSION
Cytotoxicity against a panel of different types of HLA-A- and B-allele-identified cancer and normal cells indicated that the CTL line from IL-2-activated TIL of Wilms' tumor showed HLA-A2402-restricted and tumor-specific cytotoxicity. The restriction was confirmed by a transfection study with HLA-A2402 cDNA. These results suggest the existence of T cell-mediated specific immunity at the tumor site of a 3-mo-old child whose immunity is ontogenically thought to be immature. There are few reports on HLA-class I-restricted CTL activity against pediatric tumors(18, 19). Lymphokine-activated killer cell activity was reported in IL-2-activated TIL of Wilms' tumor(16). CTL activity was induced by stimulation of autologous tumor cells in peripheral blood T cells of a patient with neuroblastoma(18). Reports on CTL activity against Wilms' tumor were not found in a search of the literature.
Six HLA-A2402+ adenocarcinomas from four different organs (stomach, colon, lung, and ovary) as well as one HLA-A2402+ squamous cell carcinoma from esophagus were lysed by this CTL line. The result suggests that the tumor antigen(s) recognized by this CTL line is expressed not only on the autologous Wilms' tumor but also abundantly on adenocarcinomas from various organs. Although the tumor antigen(s) recognized by this CTL line has not yet been identified, it might be one of the tumor-specific shared antigens such as MAGE, BAGE, or GAGE that were expressed on various types of cancers(3, 20, 21). Alternatively, ubiquitous antigens such as HER-2/neu(10, 11) are also possible candidates for the antigen(s). Wilms' tumor is histopathologically classified as a nephroblastoma that consists of immature multipotent cells, and can differentiate into mature renal cells including both epithelial and mesenchymal cells. Therefore, it is also conceivable that the tumor antigen(s) is a kind of shared tissue antigen(s) expressed on a wide spectrum of tumors with different origins. Further study is necessary to identify the tumor antigen(s).
The data of this study suggest that HLA-A2402+/3302+ KUR-11 cells do not express an antigen on the HLA-A2402 allele recognized by the established CTL line. In contrast, such an antigen would be expressed on HLA-A2402+ SSTW-9 and the other A2402+ tumors that are susceptible to lysis. There are no HLA-A3302+ tumors susceptible to lysis by the established CTL line, suggesting that this CTL line does not recognize antigens presented on the HLA-A3302 allele.
We reported previously that a large number of CD3+ T cells infiltrated into the majority of adult tumors(17, 22). In contrast, the number of CD3+ T cells in this tumor was very few, only 0.5%. Most of them possessed a CD4-CD8+ killer cell phenotype. Therefore, the longer incubation period was needed for their proliferation and also subsequent CTL activity to the autologous tumor cells. CD3+CD4+CD8- T cells, a helper T cell phenotype, were not countable in the sample of d 0, but increased up to 56% at d 37 of culture.
It is of note that Wilms' tumor cells used in this study seemed positive for CD4 antigen on their surface. CD4 antigen is expressed on a subset of T cells (helper T cells) and also some antigen-presenting cells (monocyte and macrophages)(23, 24). Leukemia cells derived from these cells are positive for CD4 antigen(23, 24). There is no available information on CD4 antigen on Wilms' tumor from a search of the literature. This issue shall be tested on the other Wilms' tumor cells.
Wilms' tumor is a subdominant tumor among pediatric malignant solid tumors. The present study showed evidence for the presence of T cell-mediated specific immunity at tumor sites of Wilms' tumor in a 3-mo-old patient. These results will be important for a better understanding of host-tumor interaction in children with Wilms' tumor.
Abbreviations
- TIL:
-
tumor-infiltrating lymphocytes
- CTL:
-
cytotoxic T lymphocytes
- IFN-γ:
-
interferon-γ
- PCR:
-
polymerase chain reaction
- E/T:
-
effector to target
References
Itoh K, Plastoucas CD, Blach CM 1988 Autologous tumor-specific cytotoxic T lymphocytes in the infiltrate of human metastatic melanomas. J Exp Med 168: 1419–1441.
Itoh K, Salmeron MA, Morita T, Seito D, Mansfield PF, Ross MI, Balch CM, Augustus LB 1992 Distribution of autologous tumor-specific cytotoxic T lymphocytes in human metastatic melanoma. Int J Cancer 52: 52–59.
van der Bruggen P, Traversari C, Chomez P, Lurquin C, de Plaen E, van der Eynde B, Knuth A, Boon T 1991 A gene encoding an antigen recognized by cytolytic T lymphocytes on human melanoma. Science 254: 1643–1647.
Kawakami Y, Eliyahu S, Delgado CH, Robbins PF, Rivoltini L, Topalian SL, Miki T, Rosenberg SA 1994 Cloning of the gene coding for a shared human melanoma antigen recognized by autologous T cells infiltrating into tumor. Proc Natl Acad Sci USA 91: 3515–3519.
Robbins PF, Gamil ME, Young FL, Topalian SL, Rivoltini L, Sakaguchi K, Appella E, Kawakami Y, Rosenberg SA 1995 Cloning of a new gene encoding an antigen recognized by melanoma-specific HLA-A24-restricted tumor-infiltrating lymphocytes. J Immunol 154: 5944–5950.
Coulie PG, Brichard V, Van pel A, Wolfel T, Schneider J, Traverasari C, Mattei S, De Plaen E, Lurquin C, Szikora TP 1994 A new gene coding for a differentiation antigen recognized by autologous cytotoxic T lymphocytes on HLA-A2 melanoma. J Exp Med 180: 35–42.
Kawakami Y, Eliyahu S, Jennings C, Sakaguchi K, Kang X, Southwood S, Robbins PF, Sette A, Appella E, Rosenberg SA 1995 Recognition of multiple epitopes in human melanoma antigen gp 100 by tumor-infiltrating T lymphocytes associated with in vivo tumor regression. J Immunol 154: 3961–3968.
Kang X, Kawakami Y, el Gamil M, Wang R, Sakaguchi K, Yannelli JR, Appella E, Rosenberg SA, Robbins PF 1995 Identification of a tyrosinase epitope recognized by HLA-A24-restricted tumor-infiltrating lymphocytes. J Immunol 155: 1343–1348.
Marchand M, Weynants P, Rankin E, Arienti F, Belli F, Parmiani G, Cascinelli N, Bourlond A, Vanwijck R, Hunbelt Y, Canon JL, Laurent C, Neyaert JM, Plagne R, Deraemaeker R, Knuth A, Jager F, Brasseur F, Herman J, Coulie PG, Boon T 1995 Tumor regression responses in melanoma patients treated with a peptide encoded by gene MAGE-3. Int J Cancer 63: 883–885.
Peoples GE, Goedegebuure PS, Smith R, Linehan DC, Yoshino I, Eberlein TJ 1994 Breast and ovarian cancer-specific cytotoxic T lymphocytes recognize the same HER2/neu-derived peptide. Immunology 92: 432–436.
Yoshino I, Goedegebuure PS, Peoples GE, Parikh AS, DiMaio JM, Lyerly HK, Gazdar AF, Eberlein TJ 1994 HER2/neu-derived peptides are shared antigens among human non-small cell lung cancer and ovarian cancer. Cancer Res 54: 3387–3390.
Nakao M, Yamana Y, Imai Y, Toh U, Kimura A, Yamana S, Kakegawa T, Itoh K 1995 HLA A2601-restricted CTLs recognize a peptide antigen expressed on squamous cell carcinoma. Cancer Res 55: 4248–4252.
Date Y, Kimura A, Kato H, Sasazuki T 1996 DNA typing of the HLA-A gene: population study and identification of four new alleles in Japanese. Tissue Antigens 47: 93–101.
Arnet KL, Parham P 1995 HLA Class I nucleotide sequences. Tissue Antigens 45: 217–257.
Itoh K, Platsoucas CD, Blach CM 1987 Monocyte- and natural killer cell-mediated spontaneous cytotoxicity against human non-cultured melanoma tumor cells. Cell Immunol 108: 495–500.
Itoh K, Tilden AB, Balch CM 1986 Lysis of human solid tumor cells by lymphokine activated natural killer cells. J Immunol 136: 3910–3915.
Itoh K, Platsoucas CD, Blach CM 1988 Autologous tumor-specific cytotoxic T lymphocytes in the infiltrate of human metastatic melanomas. Activation by interleukin 2 and autologous tumor cells, and the involvement of the T cell receptor. J Exp Med 168: 1419–1441.
Azuma E, Hanada M, Masuda SI, Komada Y, Sakurai M 1990 Autologous tumor-specific cytotoxic T-lymphocytes in a child with neuroblastoma. Biomed Pharmachother 44: 487–493.
Haas GP, Solomon D, Rosenberg SA 1990 Tumor-infiltrating lymphocytes from non renal urological malignancies. Cancer Immunol Immunother 30: 342–350.
Boel P, Wildmann C, Sensi MC, Brasseur R, Renald TC, Coulie P, Boon T, Van der Bruggen P 1955 BAGE: a new gene encoding an antigen recognized on human melanomas by cytolytic T lymphocytes. Immunity 2: 167–175.
Van den Eynde B, Peeters O, De Backer O, Gaugler B, Lucas S, Boon T 1995 A new family of genes coding for an antigen recognized by autologous cytolytic T lymphocytes on a human melanoma. J Exp Med 182: 689–698.
Balch CM, Riley LB, Bae YJ, Salmeron MA, Platsoucas CD, von Eschenbach AC, Itoh K 1990 Patterns of human tumor-infiltrating lymphocytes in 120 human cancers. Arch Surg 125: 200–205.
Dubreuil P, Mannoni P, Olive D, Winker-Lowen B, Mawas C 1986 Expression of T cell-related antigens on cells from the myelo-monocytic lineage. In: Reinherz EL, Haynes BF, Nadler LM Bernstein ID (eds) Leukocyte Typing II. Springer-Verlag, New York, pp 335–342.
Minowada J, Menon M, Guenter D, Gignac S, Misra B, Skowron L 1986 Study on human T leukemia-lymphoma cell lines by the second international workshop monoclonal antibodies of the T cell protocol. In: Reinherz EL, Haynes BF, Nadler LM Bernstein ID (eds) Leukocyte Typing II. Springer-Verlag, New York, pp 317–334.
Author information
Authors and Affiliations
Additional information
Supported in part by a Grant-in-Aid for Scientific Research from the Ministry of Education, Science and Culture of Japan, a grant from the Science Research Promotion Fund of the Japanese Private School Promotion Foundation, and a grant from the YASUDA Medical Research Found of Japan.
Rights and permissions
About this article
Cite this article
Nagai, K., Yamada, A., Eguchi, H. et al. HLA-A2402-Restricted and Tumor-Specific Cytotoxic T Lymphocytes from Tumor-Infiltrating Lymphocytes of a Child with Wilms' Tumor. Pediatr Res 42, 122–127 (1997). https://doi.org/10.1203/00006450-199707000-00019
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1203/00006450-199707000-00019