Abstract
Tyrosine may be a conditionally indispensable amino acid in the neonate; however, the provision of aromatic amino acids to neonates receiving total parenteral nutrition (TPN) is complicated by the poor solubility of crystalline tyrosine. In the present study, we investigated tyrosine kinetics and requirements during TPN, when tyrosine was supplied as the soluble dipeptide, glycyl-L-tyrosine in a neonatal piglet model. Fifteen 3-d-old male Yorkshire piglets were fitted with extermal jugular and femoral catheters and randomized to one of five tyrosine intakes: 0.11, 0.31, 0.41, 0.51 and 0.71 g·kg-1·d-1. Total parenteral amino acid and energy intakes were 15.0 g·kg-1·d-1 and 1.1 MJ·kg-1·d-1, respectively. Piglets were maintained on TPN for 6 d, with nitrogen balance measured over the final 3 d of the study. On the final study day, tyrosine kinetics were measured during a 4-h primed-constant infusion of L-[114C]tyrosine. Nitrogen retention was 67% at the lowest tyrosine intake and increased significantly (p< 0.05) at intakes of 0.31 g·kg-1·d-1 and above(84, 86, 87, and 88% for intakes of 0.31, 0.41, 0.51, and 0.71 g·kg-1·d-1, respectively). Plasma tyrosine concentrations and tyrosine oxidation (expressed as either a percentage of the dose oxidized or when corrected for flux) were low and similar at the two lowest intakes, but increased significantly at the higher intakes. Two-phase regression analysis of the data (plasma tyrosine, tyrosine oxidation) yielded estimates of a mean tyrosine requirement of 0.31 and 0.35, respectively, with estimated safe intakes (upper 95% confidence limit) of 0.44 and 0.42 g·kg-1·d-1. The present work also indicates that oxidation techniques may be suitable for the estimation of amino acid requirements during TPN in the neonate.
Similar content being viewed by others
Main
Generally, humans do not have a requirement for a dietary source of preformed tyrosine, as the endogenous supply of this amino acid arises from the hydroxylation of phenylalanine within hepatocytes(1), via the enzyme phenylalanine-4-monooxygenase (EC 1.14.16.1). However, a dietary supply of tyrosine was required to optimize growth and nitrogen retention in LBW infants receiving oral formulas(2). Furthermore, some infants receiving TPN solutions in which phenylalanine is present at 8% of the total amino acid content have developed hyperphenylalanemia(3–5), leading to the suggestion that the phenylalanine hydroxylation pathway required for the synthesis of tyrosine may be immature in the LBW infant. Due to the poor solubility of crystalline tyrosine, most parenteral amino acid solutions contain less than 1% of the total amino acid profile as tyrosine. The challenge, therefore, is to develop solutions for those infants requiring TPN which provides sufficient phenylalanine and tyrosine for anabolic processes, but without exceeding the capacity of the catabolic pathways.
Alternative measures for the provision of tyrosine to infants receiving TPN include the use of soluble tyrosine-containing compounds or dipeptides which, upon administration, yield tyrosine in vivo. For example,N- acetyltyrosine has been shown to be an efficiently used source of tyrosine for rats when incorporated into TPN solutions(6, 7); however its biologic effectiveness for humans is equivocal(8–10). Alternatively, tyrosine dipeptides, such as L-alanyl-L-tyrosine and GT, have been shown to be biologically effective as parenteral tyrosine sources in both laboratory animals and humans(7, 9, 11). Recent research by us(11), using a neonatal piglet model representative of the premature human infant(12), demonstarted the ability of GT to supply tyrosine in vivo to neonates receiving TPN. However, the inclusion of GT at a level consistent with the tyrosine content of human milk protein, supplied an excessive amount of tyrosine, as judged by markedly elevated plasma concentrations and the presence of catabolites in the urine(11).
The purpose of this study, therefore, was to estimate the requirement for tyrosine, when supplied as GT, during neonatal TPN. The phenylalanine content of the TPN solution was consistent with its relative concentration in milk protein. Estimates of tyrosine requirement were derived from nitrogen retention (percent of intake retained), and tyrosine oxidation (precent of dose and μmol·kg-1·h-1) determined by stochastic modeling techniques(13), after a primed-continuous infusion of L-[1-14C]tyrosine.
METHODS
Animals and study protocol. All procedures used in this study were approved by the Animal Care Committee of the University of Guelph. Male Yorkshire piglets, approximately 3 d of age, were transferred from a specific pathogen-free herd (Arkell Swine Research Unit, University of Guelph, ON, Canada) to the animal holding facilities in the Department of Animal and Poultry Science, University of Guelph, Ontario. After surgical preparation for TPN infusion, piglets were randomly assigned to one of five levels of GT(n = 3/treatment), as described below under “TPN regimen.” Piglets were maintained for 6 d on TPN based on the amino acid profile of human breast milk protein (Vaminolact; Kabi Pharmacia, Stockholm, Sweden), before studies of in vivo tyrosine kinetics on d 6.
Study procedures. The piglets were weighed, anesthetized, and fitted with central venous catheters, using methods previously described(12). Briefly, a central line for TPN administration was inserted into the left femoral vein and advanced to the inferior vena cava. A venous sampling line was inserted into the left external jugular vein and advanced to the superior vena cava. Catheter positions were verified at necropsy. After surgery, the piglets were fitted with an adjustable cotton jacket with an attached anchoring button. The laboratory conditions and piglet housing have been described previously(12).
TPN regimen. The TPN regimen was designed to supply all of the nutrients required by the piglet(14), as described previously(12). This regimen was designed to provide 15 g·kg-1·d-1 of amino acids and 1.1 MJ·kg-1·d-1 of available energy, with glucose and lipid (20% Intralipid; Kabi Pharmacia) each supplying 50% of the nonprotein energy intake. The base amino acid profile of the TPN consisted of (g/100 g of total L-amino acids): Arg 6.1; Asp 6.1; Cys 1.5; Glu 10.5; His 3.1; Ile 4.6; Leu 10.4; Lys 8.2; Met 1.9; Phe 4.1; Pro 8.3; Ser 5.6; Tau 0.5; Thr 5.3; Trp 2.1; and Val 5.3. Phenylalanine was supplied at a level consistent with its concentration in human milk protein. For the preparation of isonitrogenous treatment solutions, the appropriate quantity of GT (ICN Biomedicals, Mississauga, ON, Canada), glycine, and alanine was dissolved in 75 mL of distilled water, and the solutions were filter-sterilized through a 0.22-μm filter (Waters, Millipore, Milford, MA) and quantitatively transferred to the infusion bags containing the TPN, immediately before use. To maintain the glcyine content of the amino acid profile at 3.3 g/100 g of total L-amino acids, crystalline glycine was added to balance for the glycyl moiety of GT. The amino acid alanine was used to make the treatment solutions isonitrogenous(range 9.4-11.3 g/100 g of total L-amino acids), as this amino acid is indispensable for piglets and does not appear to be involved with the main mechanisms of aromatic amino acid oxidation. The tyrosine test intakes were chosen based on previous experiments(11, 15), and represented 0.8, 2.1, 2.7, 3.4, 4.7 g/100 g of total L-amino acids, with the lowest level representing the concentration of crystalline tyrosine in the original amino acid solution (Vaminolact; Kabi Pharmacia), and the highest level representing the concentration of tyrosine in human milk protein. Based on the designed total amino intake goal of 15 g·kg-1·d-1, the projected intakes of tyrosine were 0.11, 0.31, 0.41, 0.51, or 0.71 g·kg-1·d-1.
TPN was administered for a period of 6 d, with full infusion rates established by the 2nd d after surgery. The animals were weighed daily for a period of 6 d, with TPN infusion rates adjusted accordingly. Daily urinary output was collected on ice through a funnel each day. Urinalysis was performed daily (Multistix; Miles, Etobicoke, ON, Canada) to monitor urine pH. Urine pH was consistently below 5.5, therefore the urine samples were not acidified to stabilize them. Each 24-h urine collection was analyzed for total nitrogen, and nitrogen balance and retention were calculated for the final 3 d of the study.
Tracer infusion and14CO2collection. On d 6 of the study, tyrosine flux and oxidation were determined by a primed (5 μCi/kg), constant (3.5μCi·kg-1·h-1) infusion of a tracer solution containing 2.3 μCi/mL L-[1-14C]tyrosine (54 mCi/mmol; Dupont Canada, Mississauga, ON, Canada), as described previously for phenylalanine oxidation(11, 15). Briefly, piglets were placed in a covered plexiglass box and received a continuous infusion of the labeled tyrosine over a 4-h period. Tyrosine oxidation was determined by the complete collection of 14CO2(16), with adjustments for the retention of label in the bicarbonate pool, as previously described(15). The first breath collection period ended at 45 min after the initiation of the tracer infusion and continued half hourly until the end of the study. Blood samples (1.5 mL) were withdrawn into heparinized syringes at 0.5 h before and 0.5 h after the initiation of the primed-constant infusion. Blood samples were transferred to 1.5-mL micro test tubes, centrifuged for 3 min at 7000 × g (Biofuge; Heraeus Instruments, Canlab, Mississauga, ON, Canada) and the plasma collected. Plasma samples were frozen at -20 °C before analysis for tyrosine SRA. Immediately after the oxidation study, piglets were killed by the introduction of 750 mg of sodium pentobarbital into the venous sampling catheter. Tyrosine intake during the oxidation study was determined by weighing the TPN infusion set before and after the study and expressing tyrosine intake as micromoles·kg-1·h-1.
Analytical procedures. Urinary and dietary nitrogen was analyzed by the macro-Kjeldahl procedure(17). Nitrogen balance was calculated as intake minus excretion and expressed as a percentage of body weight, averaged over the final 3 d of the study. Nitrogen retention was calculated as nitrogen balance as a percentage of nitrogen intake.
The rate of expiration of 14CO2 by the piglets was determined for each collection period by liquid scintillation counting of the radioactivity of the CO2-absorbing solution. A 200-μL aliquot of absorbing solution was added to 6 mL of a liquid scintillant (Atomlight; Dupont Canada, Mississauga, ON, Canada) and counted for 10 min using an Auto DPM program for 14C analysis [LS6000SC; Beckman Instruments (Canada), Ltd., Mississauga, ON], with an average counting efficiency of 90% and background of 16 cpm.
The SRA of plasma tyrosine was determined using procedures as previously described(11, 15). Plasma amino acids were determined by reverse-phase HPLC of their phenylisothiocyanate derivatives. The HPLC eluent corresponding to the time window of tyrosine appearance was collected, and the radioactivity associated with the tyrosine was determined by liquid scintillation counting(11, 15).
Calculations. Plasma SRA values corresponding to each time point during the infusion study were plotted. The attainment of a plateau in SRA was verified by visual inspection of the generated plots. Means and standard deviations were generated for the time points within the plateaus.
Tyrosine intake (I) was calculated as:Equation where GT was assumed to be completely hydrolyzed to its constituent amino acids.

Tyrosine flux was calculated, using stochastic modeling principles(13), as:Equation where dose represented the total radioactivity infused.
The rate of 14CO2 expiration(dpm·kg-1·h-1) from oxidation of the[14C]tyrosine tracer was similarly plotted for each collection period. Isotopic steady state was determined, and corrected for retention of the label in the bicarbonate pool as:Equation where 0.93 was the correction factor for bicarbonate recovery(15) and V is volume.


Tyrosine oxidation, both as a fraction of the dose oxidized and the oxidation rate, were calculated as follows:Equation

Statistical analyses. A completely randomized design was used with level of GT serving as the main treatment effect. The main goal of the experiment was to estimate the tyrosine requirement (when provided as GT) using the outcome variables of plasma tyrosine concentration and tyrosine oxidation. A design, including number of treatments and number of animals, was chosen based on planned regression analysis(18) to estimate a break point and the 95% confidence interval of the estimate. Accordingly, the mean requirements for tyrosine were estimated by break point analysis using a two-phase linear regression crossover model(19), as described previously(20, 21). Briefly, the individual data points were partitioned between two separate linear regression lines, and the intersection of the two lines, or the break point, was determined. The equation for the statistical model was:Equation where Y is the individual observations for tyrosine oxidation or plasma tyrosine concentration; A1 and A2 are the intercepts of the first and second lines, respectively; B1 and B2 are the slopes of the first and second lines, respectively; D equals 0 if the observation is from the first line and 1 if it is from the second line; X is tyrosine intake; and E is the residual error of the model. The 95% confidence interval for the values of the tyrosine intakes at the break point estimate was determined using Fieller's theorem(19).

Differences among treatments were also determined by ANOVA(22) to provide additional information and support the interpretation based on regression analysis. If the overall F value of the ANOVA model was less than 0.05, significant differences between treatment means were assessed using Student-Newman-Keul's multiple comparisons procedure(23).
RESULTS
The piglets were healthy and active over the course of the trial. Full intake levels of the TPN solutions were established by the second morning of the study. The sampling catheter of one animal became occluded on the oxidation study day, precluding measurements of plasma tyrosine kinetics. Measurements made on this animal before the final day were included in the results. An additional animal was therefore studied at 0.31 g·kg-1·d-1 to maintain the designed number of three pigs per treatment for plasma kinetics.
Initial and final body weights (Table 1) were not significantly different between treatments. The intakes of TPN and lipid solutions were not significantly different for the five treatment groups over the 6-d study period, and the intakes were similar to the designed values. The actual intakes of tyrosine achieved during the infusion study were similar to the projected intakes (Table 2). Nitrogen excretion was significantly higher (p < 0.05) in the lowest GT-supplemented group (Table 2). Percentage of nitrogen retained was lowest (p < 0.05) in the group receiving GT at 0.11 g·kg-1·d-1 (Table 2, Fig. 1) and increased dramatically, by 17% units (Table 2) when intake was increased to 0.31 g·kg-1·d-1 with no significant change as intake increased further.
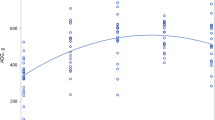
Plasma tyrosine concentrations (Table 3, Fig. 2) were low and similar for the piglets receiving the two lowest tyrosine intakes(0.11 and 0.31 g·kg-1·d-1). Increasing GT intake above 0.31 g·kg-1·d-1 resulted in significantly higher plasma tyrosine concentrations, with the piglets receiving the highest GT intake exhibiting the highest plasma tyrosine concentrations. There was little variability in plasma tyrosine concentrations. There was little variability in plasma tyrosine concentrations among pigs receiving the 0.11 and 0.31 g·kg-1·d-1 treatments (Fig. 2). Variability appeared to become greater as the excess of tyrosine became larger.
Plasma tyrosine concentrations in neonatal piglets receiving graded intakes of tyrosine. Data are presented as individual values, together with the two-phase linear regression line and the associated 95% confidence interval for the break point estimate. Tyrosine intakes were calculated based on the intakes during the infusion period.
Plasma threonine concentrations responded in an unusual fashion, increasing 5-fold (444 versus 2229 μmol/L;p < 0.05) when GT intake was increased from that providing 0.11 g·kg-1·d-1 tyrosine to that providing 0.51 g·kg-1·d-1 tyrosine, respectively. However, those piglets receiving the highest GT intakes had mean plasma threonine concentrations (849 μmol/L) similar to that of the piglets receiving the lowest GT intakes.
Plateaus in 14CO2 production occurred by 2 h postinfusion in all piglets. The coefficients of variation for the data points used in the calculation of the plateau values for each pig were less than 10%. Similar results were observed for the plateau values in plasma tyrosine SRA. Mean values for the plateaus in tyrosine SRA and 14CO2, for the five treatment groups, are reported in Table 4.
The rates of tyrosine intake, flux, and oxidation are reported in Table 4. Tyrosine flux was lowest for the lowest tyrosine intake and highest at the highest tyrosine intake. Tyrosine oxidation, expressed as either a percentage of the dose or as a rate, was not significantly different between the two lowest intakes of GT, despite the 3-fold increase in tyrosine intake, from 0.11 g·kg-1·d-1 to 0.31 g·kg-1·d-1. Increasing tyrosine intake from 0.31 to 0.41 g·kg-1·d-1 resulted in a significant 2-fold increase in tyrosine oxidation (p < 0.05). Further additions of GT to the TPN resulted in further significant increases in oxidation. The distribution of the individual data points for tyrosine oxidation is shown in Figure 3, as percentage of dose oxidized, and Figure 4, as micromoles·kg-1·h-1. There was clearly no effect on tyrosine oxidation when intake was increased from 0.11 to 0.31 g·kg-1·d-1; however, each additional increment significantly increased tyrosine oxidation (Table 4).
Tyrosine oxidation, expressed as a percentage of the dose oxidized, in neonatal piglets receiving graded intakes of tyrosine, as determined during a primed-continuous infusion of L-[14C]tyrosine. Data are presented as individual oxidation values, together with the two-phase linear regression line and the associated 95% confidence interval for the break point estimate. Tyrosine intakes were calculated based on the intakes during the infusion period.
Tyrosine oxidation rates in neonatal piglets receiving graded intakes of tyrosine, as determined during a primed-continuous infusion of L-[14C]tyrosine. Data are presented as individual oxidation values, together with the two-phase linear regression line and the associated 95% confidence interval for the break point estimate. Tyrosine intakes were calculated based on the intakes during the infusion period.
The tyrosine requirement, when supplied as GT, was estimated by partitioning the individual data points between two distinct linear regression equations for the tyrosine oxidation and plasma tyrosine concentration. The response parameters for the projected tyrosine intakes below 0.31 g·kg-1·d-1, representing six observations, were included in the first regression line, and the tyrosine intakes above 0.41, representing nine observations, were included in the second regression line because this minimized the total sums of squares in error. The break points for percentage of the dose oxidized, oxidation rate, and the plasma tyrosine concentrations were 0.24, 0.35, and 0.31 g·kg-1·d-1, respectively, with r2 of 0.96, 0.94, and 0.90, respectively. The upper 95% confidence limits, representing safe intake levels, for the percentage of the dose oxidized, the oxidation rate, and the plasma tyrosine data were 0.32, 0.42, and 0.44 g·kg-1·d-1, and are illustrated in Figures 3 and 4.
DISCUSSION
Previous studies in parenterally fed piglets have confirmed the effectiveness of GT as a source of tyrosine in vivo(11). However, a tyrosine intake of 0.71 g·kg-1·d-1 (supplied as GT), corresponding to a tyrosine level of 4.7 g/100 g of total L-amino acids, was deemed to be excessive. These results were observed when phenylalanine intake was 0.61 g·kg-1·d-1, or 4.1 g/100 g of total L-amino acids. High plasma tyrosine concentrations and the presence of tyrosine catabolites in the urine supported the conclusion that tyrosine intake was excessive. The present study, therefore, was designed to estimate the level of GT intake that would supply sufficient tyrosine to meet the tyrosine requirements for protein synthesis, in an amino acid solution that provided phenylalanine at a level of 4.1 g/100 g of total L-amino acid pattern.
Growth over 6 d was not affected by the level of GT in the TPN solutions. However, growth rates have been shown to be a relatively insensitive measure of the quality of the amino acid source offered to piglets receiving TPN(11, 15, 24). Nitrogen excretion was significantly higher in piglets receiving the lowest tyrosine intake. However, nitrogen retention, as a percent of intake, indicated that a tyrosine intake of 0.11 g·kg-1·d-1 was inadequate. Increasing tyrosine intake to 0.31 g·kg-1·d-1 increased nitrogen retention to a plateau level; further increases in tyrosine intake had no significant effect. The high values for nitrogen retention observed in the present study are consistent with previous studies in growing swine to which a well blanced amino acid source had been offered(25, 26). However, the development of a plateau in nitrogen retention does not necessarily indicate that a maximal response has been obtained. Maximum nitrogen retention will be influenced by the total amino acid profile and the energy level of the diet. These interrelationships have yet to be fully elucidated for either the neonatal piglet or the human infant receiving TPN.
The estimates of the mean requirement and safe level of tyrosine intake, when supplied as GT, derived from the oxidation data concur with the nitrogen retention results. The break point analysis for tyrosine oxidation yielded requirement levels of 0.24 and 0.35 g·kg-1·d-1, based on the percentage of the dose oxidized and the oxidation rate, respectively. The break point for the plasma tyrosine data of 0.31 g·kg-1·d-1 was between the two estimates derived from the oxidation data. However, the break point represents the estimate of the average requirement for the amino acid. To meet the needs of 95% of the population, confidence intervals were calculated. The upper 95% confidence limits for the percentage of the dose oxidized, the oxidation rate, and plasma tyrosine were 0.32, 0.42, and 0.44 g·kg-1·d-1, respectively. Tyrosine oxidation, expressed as a percentage of the dose oxidized, produced the least variable estimate of the break point. Although the estimates of the mean tyrosine requirements and the safe levels of intake derived from oxidation and plasma data differ, they nonetheless support the nitrogen retention results. Therefore, the provision of GT at rates sufficient to provide a total tyrosine intake of 0.31 g·kg-1·d-1 represents the approximate mean intake required to achieve adequate nitrogen retention and plasma tyrosine and minimize tyrosine oxidation in piglets receiving total parenteral nutrition. A safe level of intake, as supported by the upper 95% confidence interval, would be met by providing 0.41 g·kg-1·d-1 of tyrosine, equivalent to 2.7 g/100 g of the total L-amino acid supply. These values were determined with TPN providing 0.61 g·kg-1·d-1 of phenylalanine or 4.1 g/100 g of the total L-amino acid supply, resulting in a total phenylalanine plus tyrosine intake of 1.02 g·kg-1·d-1, or 6.8 g/100 g of the total L-amino acid supply. However, the specific requirements for phenylalanine must still be determined.
If was assumed for calculation of tyrosine requirements that the hydrolysis of GT to its constituent amino acids was complete. Although studies in humans have shown that GT disappears rapidly from plasma after a bolus injection(35), studies providing evidence of complete hydrolysis in pigs have not been performed. The current results, as well as previous work in parenterally fed piglets(11), clearly indicate that the administration of GT is followed by increases in plasma tyrosine concentrations, nitrogen retention, and tyrosine oxidation. However, these studies cannot confirm the complete hydrolysis of this dipeptide. Because the present work was concerned with identifying a level of GT administration that would supply sufficient tyrosine in vivo, the question of whether the dipeptide has been completely hydrolyzed has limited bearing on the conclusions. When complete information on tyrosine availability from GT becomes available, these data can be recalculated if necessary.
We believe the changes in plasma glutamine, asparagine, and histidine concentration observed with the changes in tyrosine intake are consistent with the alterations in whole body introgen economy. High plasma concentrations of glutamine and asparagine during low tyrosine intake may reflect an increased availability of amino groups for the transamination of both glutamate and aspartate, respectively, due to a greater rate of amino acid catabolism when protein synthesis is limited by low tyrosine intake. The provision of additional tyrosine to a TPN solution limiting in total aromatic amino acids should increase the rates of protein synthesis, resulting in a reduction in the concentration of plasma amino acids. Histidine levels would therefore fall as the incorporation of this amino acid into protein increased due to an improvement in the amino acid pattern. The failure to find significant differences in the concentrations of most of the other amino acids may indicate that the regulation of glutamine, asparagine, and histidine concentrations was more sensitive to imbalances in the total amino acid supply. Because histidine was the only indispensable amino acid affected, a decrease in its plasma concentration might indicate that the ability to remove the excess histidine was compromised when tyrosine was limiting for protein synthesis.
Increasing the GT intake to deliver 0.51 g·kg-1·d-1 of tyrosine resulted in a 5-fold increase in plasma theronine concentrations. However, speculation on a potential mechanism for an alteration in threonine metabolism or disposal should not occur without considering the significant reduction in theronine concentration as GT supply was further increased to 0.71 g·kg-1·d-1. The return of plasma threonine concentration values to near control values with the highest level of GT supplementation suggests an interaction of the treatment regime with the enzymes of threonine catabolism. Increased plasma threonine concentrations could result from either a reduced transport rate across membranes or a reduction in threonine catabolism. If transport was significantly affected, threonine would have become limiting at the site of protein synthesis. Because nitrogen retention values improved with GT supplementation, this suggests that threonine transport was either not affected or that any effect on transport was inadequate to reduce protein synthesis.
GT addition to TPN may have affected the enzymes of threonine degradation(27, 28). In growing pigs, ≅80% of threonine catabolism occurs through the threonine dehydrogenase pathway(29), yielding aminoacetone or acetyl-CoA and glycine. The dipeptide GT may result in the inhibition of this enzyme, via the glycyl moiety. Even though total glycine intakes were kept constant across treatments, the presentation of an increasing amount of glycine in a peptide form may have had localized effects at the level of threonine catabolism. However, the possibility that the dipeptide itself or its tyrosyl moiety may have influenced threonine catabolism cannot be dismissed.
The precise mechanisms involved with the apparent perturbations in threonine metabolism, as a result of GT supplementation, but require further study, especially if GT is to be recommended for clinical application in neonates. Alternatively, the use of other tyrosine peptides should be considered.
Tyrosine flux increased significantly as the level of GT in the TPN increased. This observation is consistent with studies in humans, which demonstrated increases in the fluxes of leucine(30), valine(31), lysine(32), threonine(33), and phenylalanine(21), with increases in the intake of the respective amino acids. Tyrosine intake accounted for 20% of the flux at the lowest GT intake, and 43% of the flux at the highest GT intake. In contrast, sick preterm neonates, receiving an amino acid solution with a low tyrosine-high phenylalanine content, exhibited tyrosine flux rates of 66μmol·kg-1·h-1, with tyrosine intake accounting for less than 1% of the flux(34). This low contribution of tyrosine intake to flux in the sick preterm infant was attributed to an overall elevation of the flux rate, due to an increased rate of protein breakdown. However, the intake to flux ratio would have been underestimated by an amount equivalent to that quantity of tyrosine arising from the hydroxylation of phenylalanine.
The unknown amount of tyrosine arising from the hydroxylation of phenylalanine needs to be considered. The total aromatic amino acid supply to the piglets receiving a tyrosine intake of 0.11 g·kg-1·d-1 was below the requirement level, based on all the metabolic measurements. In this treatment group, tyrosine supplied 10% of the total aromatic amino acids. However, because tyrosine accounts for≅43% of the aromatic amino acids present in piglet tissue(36), a significant amount of the tyrosine deposited over the 6-d period must have arisen from the hydroxylation of phenylalanine(11). Tyrosine arising from phenylalanine hydroxylation must be accounted for to prevent an underestimation of the total amount of tyrosine entering the body's free pool of amino acids.
Table 5 provides a comparison of the estimates of net tyrosine gain derived from both the nitrogen retention and kinetic data. There is excellent agreement between the mean estimates of tyrosine retention derived from either the nitrogen retention or the kinetic data for those piglets receiving tyrosine intakes equivalent to 0.41 g·kg-1·d-1 or higher. At the tyrosine intake of 0.11 g·kg-1·d-1, this agreement is no longer apparent. The difference between the two estimates likely represents that fraction of deposited tyrosine which has arisen from the hydroxylation of phenylalanine. These findings could provide some insight into the slight differences observed in the break point estimates of tyrosine requirement. The contribution of tyrosine from phenylalanine hydroxylation could not be included as part of the tyrosine intake, therefore the slope of the first phase of the model may be overestimated. Despite this limitation in the model, it must be appreciated that the estimates of tyrosine requirement derived by regression analysis from the oxidation and plasma amino acid data agree well with the nitrogen retention results. The evidence of significant phenylalanine hydroxylation in the piglets receiving low levels of tyrosine in the present study supports previous work by us in piglets(15) and work by others in LBW infants(34, 37, 38) that phenylalanine hydroxylation does occur at significant levels in neonates receiving TPN. The question remains, however, as to the cause of the observed incidences of hyperphenylalanemia in LBW infants receiving TPN containing greater than 8 g/100 g of the total L-amino acids as phenylalanine. The ability to hydroxylate phenylalanine may be challenged at these high phenylalanine intakes.
The evidence from the present study tentatively supports a total aromatic amino acid intake of 6.8 g/100 g of L-amino acids as meeting the requirements of these amino acids. This is markedly below the total aromatic amino acid concentration of some amino acid solutions (9 g/100 g of L-amino acids). Therefore, the possibility of an excessive phenylalanine supply cannot be ruled out and awaits further studies involving the elucidation of the requirement for phenylalanine in the presence of adequate tyrosine.
Abbreviations
- TPN:
-
total parenteral nutrition
- LBW:
-
low birth weight
- GT:
-
glycyl-L-tyrosine
- SRA:
-
specific radioactivity
- ANOVA:
-
analysis of variance
REFERENCES
Moss AR, Schoenheimer R 1941 The conversion of phenylalanine to tyrosine in normal rats. J Biol Chem 135: 415–429
Snyderman SE 1971 The protein and amino acid requirements of the premature infant. In: Jonxis JHP, Visser HKA, Troelstra JA (eds) Metabolic Processes in the Foetus and Newborn. Stenfert Kroese, Leiden, The Netherlands, pp 128–141
Puntis JWL, Edwards MA, Green A, Morgan I, Booth IW, Ball PA 1986 Hyperphenylalanaemia in parenterally fed newborn babies. Lancet 2: 1105–1106
Walker VA, Hall MA, Bulusu S, Allan A 1986 Hyperphenylalanaemia in parenterally fed newborn babies. Lancet 2: 1284
Thornton L, Griffin E 1991 Evaluation of a taurine containing amino acid solution in parenteral nutrition. Arch Dis Child 66: 21–25
Im HA, Meyer PD, Stegink LD 1985 N- Acetyltyrosine as a tyrosine source during total parenteral nutrition in adult rats. Pediatr Res 19: 514–518
Neuhäuser M, Wandira JA, Göttmann U, Bassler KH, Langer K 1985 Utilization of N-acetyltyrosine and glycyltyrosine during long term parenteral nutrition in the growing rat. Am J Clin Nutr 42: 585–596
Magnusson I, Ekman L, Wångdahl M, Wahren J 1989 N- Acetyl-L-tyrosine and N-acetyl-L-cysteine as tyrosine and cysteine precursors during intravenous infusion in humans. Metabolism 38: 957–961
Druml W, Lochs H, Roth E, Hübl W, Balcke P, Lenz K 1991 Utilization of tyrosine dipeptides and acetyltyrosine in normal and uremic humans. Am J Physiol 260:E280–E285
Van Goudoever JB, Sulkers EJ, Timmerman M, Humann JGM, Langer K, Carnielli VP, Sauer PJJ 1994 Amino acid solutions for premature neonates during the first week of life: the role of N- acetyl-L-cysteine and N-acetyl-L-tyrosine. J Parenter Enteral Nutr 18: 404–408
Wykes LJ, House JD, Ball RO, Pencharz PB 1994 Aromatic amino acid metabolism of neonatal piglets receiving TPN: effect of tyrosine precursors. Am J Physiol 267:E672–E679
Wykes LJ, Ball RO, Pencharz PB 1993 The development and validation of a total parenteral nutrition model in the neonatal piglet. J Nutr 123: 1248–1259
Waterlow JC, Garlick PJ, Millward DJ 1978 Protein Turnover in Mammalian Tissues and in the Whole Body. Elsevier/North-Holland, Amsterdam, The Netherlands, pp 225–249
National Research Council 1988 Nutrient Requirements of Swine, 9th ed. National Academy Press, Washington, DC
Wykes LJ, House JD, Ball RO, Pencharz PB 1994 Amino acid profile and aromatic amino acid concentration in total parenteral nutrition: effect on growth, protein metabolism and aromatic amino acid metabolism in the neonatal piglet. Clin Sci 87: 75–84
Ball RO, Bayley HS 1985 Time course of evolution of total and 14C-labelled carbon dioxide by young pigs receiving diets containing 14C-phenylalanine. Can J Physiol Pharmacol 63: 1170–1174
AOAC 1990 Official Methods of Analysis, 15th Ed. Association of Official Analytical Chemists, Washington, DC, 69–79
Draper NR, Smith H 1981 Applied Regression Analysis, Ed 2. John Wiley & Sons, New York, 51–55
Seber GAF 1977 Linear Regression Analysis. John Wiley, New York
Kim KI, McMillan I, Bayley HS 1983 Determination of amino acid requirements of young pigs using an indicator amino acid. Br J Nutr 50: 369–382
Zello GA, Pencharz PB, Ball RO 1990 Phenylalanine flux, oxidation, and conversion to tyrosine in humans studied with L-[1-13C]phenylalanine. Am J Physiol 259:E835–E843
SAS V. 6.06 SAS Institute, Cary, NC
Steel RGD, Torrie JH 1960 Principles and Procedures of Statistics. McGraw-Hill, Toronto, Canada
House JD, Pencharz PB, Ball RO 1994 Glutamine supplementation to total parenteral nutrition promotes extracellular fluid expansion in piglets. J Nutr 124: 396–405
Zhang Y, Partridge IG, Keal HD, Mitchell KG 1984 Dietary amino acid balance and requirements for pigs weaned at 3 wk of age. Anim Prod 39: 441–448
Wang TC, Fuller MF 1989 The optimum dietary amino acid pattern for growing pigs. I. Experiments by amino acid deletion. Br J Nutr 62: 77–89
Bird MI, Nunn PB 1983 Metabolic homeostasis of L-threonine in the normally-fed rat. Biochem J 214: 687–694
Kang-Lee Y, Harper AE 1978 Effect of threonine intake and prior induction of threonine dehydratase in rats. J Nutr 108: 163–175
Ballevre O, Cadenhead A, Calder AG, Rees WD, Lobley GE, Fuller MF, Garlick PJ 1990 Quantitative partition of threonine oxidation in pigs: effect of dietary threonine. Am J Physiol 259:E483–E491
Meguid MM, Matthews DE, Bier DM, Meredith CN, Soeldner JS, Young VR 1986 Leucine kinetics at graded leucine intakes in young men. Am J Clin Nutr 43: 770–780
Meguid MM, Matthews DE, Bier DM, Meredith CN, Young VR 1986 Valine kinetics at graded valine intakes in young men. Am J Clin Nutr 43: 781–786
Meredith CN, Wen Z, Bier DM, Matthews DE, Young VR 1986 Lysine kinetics at graded lysine intakes in young men. Am J Clin Nutr 43: 787–794
Zhao X, Wen Z, Meredith CN, Matthews DE, Bier DM, Young VR 1986 Threonine kinetics at graded threonine intakes in young men. Am J Clin Nutr 43: 795–802
Castillo L, Ming Y, Marchini S, Chapman TE, Sanchez M, Young VR, Burke JF 1994 Phenylalanine kinetics in critically ill children with sepsis. Pediatr Res 35: 580–588
Albers S, Wernerman J, Stehle P, Vinnars E, Fhrst P 1988 Availability of amino acids supplied intravenously in healthy man as synthetic dipeptides: kinetic evaluation of L-alanyl-L-glutamine and glycyl-L-tyrosine. Clin Sci 75: 463–468
Aumaitre A, Dueé PH 1974 Composition en acides amines des proteines corporelles du porcelet entre la naissance et l'age de huit semaines. Ann Zootech 23: 231–236
Shortland GJ, Walter JH, Fleming PJ, Halliday D 1994 Phenylalanine kinetics in sick preterm neonates with respiratory distress syndrome. Pediatr Res 36: 713–718
Kilani RA, Cole FS, Bier DM 1995 Phenylalanine hydroxylase activity in preterm infants: is tyrosine a conditionally essential amino acid?. Am J Clin Nutr 61: 1218–1223
Acknowledgements
The authors are indebted to Janice Murphy, Cathy Chen, and Susan Murch for their technical assistance, and Dr. André Bahoric for expertise with the catheters. Total parenteral nutrition solutions were manufactured by the staff of the Parenteral Service Pharmacy, the Hospital for Sick Children.
Author information
Authors and Affiliations
Additional information
Supported by Medical Research Council of Canada Grant MT12928 and the Hospital for Sick Children Foundation. Crystalline amino acids were kindly donated by Kabi Vitrum-Pharmacia-Up-John, Stockholm, Sweden.
Portions of this work were presented in abstract form at the 1995 Experimental Biology Meetings in Atlanta, Georgia, and the 1995 Canadian Federation of Biological Sciences, Saskatoon, Saskatchewan.
Rights and permissions
About this article
Cite this article
House, J., Pencharz, P. & Ball, R. Tyrosine Kinetics and Requirements during Total Parenteral Nutrition in the Neonatal Piglet: The Effect of Glycyl-L-Tyrosine Supplementation. Pediatr Res 41, 575–583 (1997). https://doi.org/10.1203/00006450-199704000-00020
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1203/00006450-199704000-00020