Abstract
To explore the changes in resting energy expenditure (REE) and whole body protein turnover induced by malaria, 23 children aged 6 to 14 y (23.9 ± 1.0 kg, 1.3 ± 0.02 m) were studied on three separate days after treatment (d 1, d 2, and 15 d later). REE was assessed by indirect calorimetry(hood), whereas whole body protein turnover was estimated using a single dose of [15N]glycine administered p.o. by measuring the isotopic enrichment of [15N]ammonia in urine over 12 h. Within the first 3.5 h after treatment, the body temperature dropped from 39.8 ± 0.1 to 37.8± 0.1°C (p < 0.0001), and REE followed the same pattern, decreasing rapidly from 223 ± 6 to 187 ± 4 kJ/kg/d(p < 0.0001). Whole body protein synthesis and breakdown were significantly higher during the 1st day (5.65 ± 0.38 and 6.21 ± 0.43 g/kg/d, respectively) than at d 15 (2.95 ± 0.17 and 2.77 ± 0.2 g/kg/d). It is concluded that Gambian children suffering from an acute episode of malaria have an increased REE averaging 37% of the control value (d 15) and that this was associated with a substantial increase (by a factor of 2) in whole body protein turnover. A rapid normalization of the hypermetabolism and protein hypercatabolism states after treatment was observed.
Similar content being viewed by others
Main
In developing countries, malaria remains one of the greatest“killers,” especially among children. Studies in various parts of the world(1–3) have shown that growth faltering in children results from both infections and dietary inadequacies. In addition, the marked seasonal differences in growth rate of children observed in many developing countries can be attributed to differences in food availability and to the prevalence of infectious diseases(4).
The nutritional response to infection has been well documented(5–9). Infectious diseases are accompanied by a loss of appetite, often in conjunction with nausea and vomiting, resulting in a decrease in food intake. Simultaneously the rate of energy expenditure (and hence the energy requirement) is increased by the fever which generally accompanies the infectious process(10, 11).
Carbohydrate stores being inadequate to meet the increased energy requirement and lipid stores less effectively used in the infected patient, proteins are therefore mobilized to provide amino acids for gluconeogenesis. IL-1, tumor necrosis factor, and other monokines stimulate muscle breakdown(12), and whole body protein turnover is increased(13). During this period of negative energy balance, the rate of growth is compromised, and this is of clinical importance when affecting children who are already malnourished(14–16).
In a previous study in Gambian children suffering from an acute episode of malaria, we observed an increase in REE averaging 30%. In the present study, our aim was to assess the change in REE during the dynamic phase of treatment by performing REE measurements through the 1st and the 2nd d of treatment and 15 d later (as a control day) and to measure the changes in protein turnover to assess their relation to energy expenditure. The study was also designed to distinguish the effect of fever itself on REE from the disease induced hypermetabolism.
METHODS
This study was performed over a 1-y period in three rural villages of the West Kyiang district in The Gambia (West Africa), i.e. Keneba, Manduar, and Kantonkunda. Detailed descriptions of the environmental conditions prevailing in these villages have been previously published(17–19).
In this part of West Africa, there are two seasons: a rainy season between June and October and a dry season during the rest of the year. Episodes of malaria (Plasmodium falciparum) seldom occur during the dry season, but are frequent in children during the rainy season. Food is also less available during the rainy season because the previous year's food stores become progressively exhausted.
Twenty-three children between the age of 6 and 14 y (mean ± SEM, 10.0 ± 0.5) took part in the study. The diagnostic criteria for malaria were an axillary temperature above 37.5°C and a parasitemia of more than 5000/μL(20). The study did not interfere with the usual treatment. The study was approved by the MRC/Gambian Government Ethical Committee.
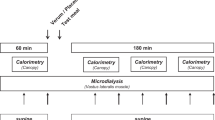
Experimental design (Fig. 1). The study took place on three separate days during the course of the treatment,i.e. on the day the diagnosis was made (d 1), the next day (d 2), and 15 d later (d 15). The study started immediately after the initiation of treatment (chloroquine phosphate, 10 mg/kg p.o. followed by 5 mg/kg 6 h later, and 5 mg/kg daily for 2 d; acetaminophen, 15 mg/kg three times a day for 3 d). On d 1, 2, and 15 the protocol included measurements of resting metabolic rate, HR, and body temperature. On d 1 and 15 the whole body nitrogen flux, whole body protein synthesis, and breakdown were measured. Anthropometric measurements were carried out at the beginning and at the end of the study.
Nutritional intake. Meals were given in the form of a drink, four times a day (immediately after each calorimetric measurement and 3.5 h after the last measurement) and consisted of a protein supplement formula(Casilan 90, GB) and saccharose mixed with water (1.2 kcal/mL). The protein content of the diet corresponded to 0.6 g/protein/kg/12 h, and its energy content was 1.0 times the basal metabolic rate of the child calculated over a 12-h period. The basal metabolic rate was predicted from the equations of FAO/WHO/UNU Expert Committee (1985) using gender, weight, and age as factors. The relative level of protein intake corresponded to 11-12% of the total food energy. This is twice as high as the recommended dietary allowance for this age group (5-6%).
To obtain comparable results, the same diet was provided on the control day. Due to the state of anorexia characterizing the infection process, the energy and protein intakes were fed at the “maintenance” level. The meals were well accepted and tolerated by the children.
Body weight and body composition. Body weight was measured in the fasting condition, using a scale (Kipfer, model DPW 150, Jegendorf, Switzerland) with an accuracy of 0.1 kg. Body composition was assessed using skinfold thicknesses measured at four sites (biceps, triceps, subscapular, and suprailiac) with a caliper (Holtain Ltd., Crymych, UK). Body fat percentage and fat-free mass were determined by Brook's equation(21).
Resting energy expenditure. The REE and the RQ were measured three times a day for 30 min by indirect calorimetry, using a ventilated hood system(22, 23). The subject's head was inserted into a transparent acrylic hood specially built for children. A cloth was secured around the neck to make it airtight. Outside air was drawn continuously through the hood at a constant rate by a ventilator inserted into the outlet tube. The flow rate of air leaving the hood was measured with a digital pneumotachygraph and a differential manometer (AVA200, 0-10 mm H2O full scale, Hartmann & Braun, Frankfurt am Main, Germany). A fraction of the outflowing air was continuously sampled, and its O2 and CO2 concentrations were measured with a thermomagnetic O2 analyzer (Magnos 2T, Hartmann & Braun) and an infrared CO2 analyzer(Uras 2T, Hartmann & Braun). Energy expenditure, calculated from the O2 consumption and CO2 production with classic equations, was averaged for 2-min periods and monitored on-line. For technical reasons, REE could not be measured in two children.
HR and body temperature. During each study day, in free living conditions as well as during the calorimetric measurements, HR was recorded with a portable small recorder (Bauman HR counter, Fleurier, Neuchâtel, Switzerland), the data being averaged over 1-min intervals and stored.
Immediately before and after each indirect calorimetric assessment, the sublingual temperature (T) was measured over a 10-min period with a mercury thermometer: this measurement was used as an estimate of central body temperature. All the measurements were carried out in a room with standardized conditions of temperature (26 ± 1°C) and relative humidity (60± 5%) to avoid any environmental influence on resting energy expenditure.
Measurement of protein metabolism. Four meals were given over a period of 12 h. The first of the four meals of the day was given after the basal metabolic rate measurement. A single dose of [15N]glycine (150 mg, 99.2 atom% excess, Amersham International, Buckinghamshire, UK) was given orally, diluted in water, with the first meal (Fig. 1). Urinary collections were made every 3 h, during the 12-h study period and started before the first meal. The volume of each urine sample was measured, and 60 mL were then immediately acidified to a pH of 1.5 with concentrated HCl and frozen to -20°C. Urine collection could not be completed in two children.
The rate of protein turnover was estimated from the level of isotopic enrichment of urinary ammonia after a single oral dose of [15N]glycine. Details of the method have been described previously by Fern et al.(24, 25). The 15N enrichment of ammonia was corrected for background isotopic enrichment as measured in the first predose sample of urine.
The rate of whole body nitrogen flux (Q) was calculated from the equation given by Waterlow et al.(26) using ammonia as end product: Equation where E is the rate of nitrogen excreted in urine as ammonia (g/12 h), d is the dose of 15N given (g), and e is the total amount of isotope excreted in urine as ammonia during the 12-h collection.
Rates of protein synthesis and breakdown were calculated from the rate of nitrogen flux given by ammonia using the following equations: hence: Equation A factor of 6.25 was used to convert nitrogen into corresponding protein values. Miscellaneous nitrogen losses (e.g. sweat and feces) were considered to be negligible.
Biochemical analysis. Urinary ammonia was measured by an enzymatic method as described by Kun and Kearney(27). Total urinary nitrogen excretion was determined by the Kjeldahl method(Autoanalyser, Technicon, Tarrytown, NY).
Stable isotope analysis (15N). The method was based on trials by Preston and McMillan(28) with some minor modifications; briefly, after neutralizing each urine sample (10-15 mL) to pH= 6.0 ± 0.1 with 5 N NaOH, ammonia nitrogen was isolated using a cationic exchange resin (Bio-Rad Laboratories, Richmond, CA). After discarding the supernatant and after several washings of the resin with purified water, ammonia was extracted with 1.5 mL of 2.5 M potassium hydrogen sulfate and analyzed for 15N enrichment by combustion and continuous flow IRMS on a Roboprep CN-Tracermass (Europa Scientific, Crewe, UK). A 5% urea solution was used as an internal standard.
Statistical analysis. All results are expressed as mean± SEM. Statistical analyses were performed by using an analysis of variance of repeated measurements when comparing more than two tests and a paired t test when comparing two situations. The level of significance was chosen as p < 0.05. Resting energy expenditure, RQ, sublingual temperature, and HR were compared within days and between days.
RESULTS
Anthropometry. As shown in Table 1, body weight and body mass index decreased during the first 2 d (p < 0.0001). The weight loss averaged 0.7 ± 0.1 kg. At the end of the study, body weight was back to its initial level so that there was no significant difference between the first and last day of the study. There was no significant change of the fat-free mass of the children between the first and the last day of the study.
Body temperature, resting energy expenditure, and HR. During the 1st d, the sublingual temperature dropped from 39.8 ± 0.1°C to 37.8 ± 0.1°C (p < 0.0001) within the first 3.5 h and then stabilized to 37.4 ± 0.2°C during the next 3.5 h. The following day and the last day of the study, the child was afebrile, and no significant difference in body temperature was observed between these two measurements. The overall drop in temperature from the study onset up to the time the child was afebrile averaged 2.7 ± 0.1°C.
REE followed almost the same pattern, decreasing rapidly from 223 ± 6 to 187 ± 4 kJ/kg/d (p < 0.0001) within the first 3.5 h and reaching an apparent “steady state” during the next 3.5 h(Fig. 2). HR dropped from 119 ± 3 beats/min to 102± 2 beats/min within the first 3.5 h (p < 0.002). But in contrast to the evolution of internal temperature, the measurements of REE and HR during d 2 were significantly different from the control day. There was a decrease in REE between d 2 and d 15 of 17 ± 3 kJ/kg/d(Table 2, p < 0.0001). Over the same period of time, HR decreased by 7 ± 1 beats/min. (p < 0.005).
It was of interest to study the increase in REE/°C of fever during the acute episode of malaria using two approaches: the first one by taking into account the difference between the acute phase and the recovery phase at the study outset and the second one between the acute phase and the 2nd d of the study where the child was already afebrile. If we compared the results during the acute phase relative with that of the control day at the study outset (1aversus 15a), we observed a significant correlation between theΔREE and the ΔT (r = 0.57, p < 0.008; Fig. 3) with a slope of 8.5% indicating that each degree of change in body temperature was accompanied by a corresponding change in energy expenditure of 8.5%. When the first 2 d were compared with each other (1a versus 2a) the slope of the regression was moderately lower (7.3% change per unit °C; r = 0.66, p < 0.002).
Another alternative to obtain a global estimate of the energy cost of fever was to calculate for each child the increase in REE per degree of fever(ΔREE/ΔT) by simple ratio and not by regression. In that case, we assumed that the relationship had a zero intercept. With this approach the hypermetabolism induced by fever was 13.2%/°C between 1a and 15a and 9.9% per degree of fever for the period 1a-2a.
Parasitemia was positively correlated with the internal temperature(r = 0.46, p < 0.005), indicating that the greater increase in temperature was associated with a greater number of parasites. HR was also correlated with parasitemia (r = 0.49, p < 0.005).
The RQ value was not different within days (Table 3). In addition, the 1st and 2nd d of the study had similar RQ values. At the study outset, the RQ of the control day increased in particular at the beginning of the day (1a 0.79 ± 0.01, 2a 0.80 ± 0.01versus 15a 0.89 ± 0.02, p < 0.0001).
Whole body protein turnover. Whole body protein synthesis and protein breakdown were significantly higher during d 1 (Table 4, p < 0.0001). This difference represented an increase between 92 and 125% of the control values.
The protein balance, equivalent to the difference between protein synthesis and protein breakdown, was negative on d 1 (-0.57 ± 0.11 g/kg/12 h), whereas it was positive on d 15 (0.18 ± 0.05 g/kg/12 h). Day 1 was significantly different from d 15 (p < 0.0001).
In the pooled groups we observed a positive correlation between body temperature and protein synthesis (r = 0.77, p < 0.0001), and protein breakdown (r = 0.81, p < 0.0001). A rise of 1°C increased the protein synthesis by 2 g/kg and the protein breakdown by 2.6 g/kg. There was also a significant correlation between protein synthesis and REE (Fig. 4; r = 0.69,p < 0.0001).
A multiple regression analysis showed the parameters having a major effect on REE. The total increase of REE [ΔREE (kJ/kg/d)] during an acute episode of malaria is expressed by the following equation:
DISCUSSION
The children lost 0.7 kg within 24 h after the onset of malaria treatment. This represents 3% of their initial body weight. This is explained both by a state of negative energy balance and by a shift in water balance.
We observed that within 3.5 h after the treatment onset, dramatic changes occurred in metabolic, cardiovascular, and thermoregulatory parameters. REE dropped by 36 kJ/kg/d, representing a drop of approximately two-thirds of the total difference in REE between the study onset and the end of the study; the sublingual temperature fell by 2°C, i.e. nearly three-fourths of the total decrease observed over the study period. Finally HR decreased by 17 beats/min, representing nearly half of the total decrease. Taken together these results emphasize the need to treat a child as soon as the diagnosis of malaria is established to reduce as much as possible the metabolic stress due to the infection.
The measurements performed 15 d after the malaria treatment were considered as the control (d 15), because the measured clinical signs were back to control level. The difference in REE between the first measurement and the control day averaged 60 ± 4 kJ/kg/d. This represents the global metabolic cost of an acute episode of malaria. This means that malaria infection increased the REE by 37%, confirming the results of a previous investigation in the same community(10) which showed an increase in REE averaging 30%. In contrast, Duggan and Milner(29), who studied energy metabolism in Kenyan children, showed a negative energy balance, but failed to observe an increase in energy expenditure accompanying the episode of fever during measles infection. However, it seems difficult to delineate the respective effect of progressive improvement in nutritional status by treatment and nutrition versus the fading out of the metabolic stress of the disease on the rate of resting energy expenditure.
The key symptom of malaria being fever, the present study offered the opportunity to determine the metabolic cost of fever. At the beginning of the century Dubois(30) and Barr and Du Bois(31) observed that in adult patients the increase in metabolic rate for each degree centigrade of fever averaged 13%. Another study, performed in The Gambia, reported similar results(10) with an average increase of 11.7% per unit rise(°C) in axillary temperature. Our results were similar with an increase of REE of 13.2%/°C by simple arithmetic ratio, and 8.5%/°C by linear regression of REE versus internal temperature. The design of the study allowed us to distinguish the febrile infected state (d 1) from the afebrile but still infected state (d 2). We can thus determine the part of the total increase in REE which is not attributable to fever(Fig. 5). This represented 28% of the total increase in REE, resulting from the infection process. To assess the energy cost of fever it is appropriate to use the febrile period (1a-2a): each degree of fever increased the REE by 9.9%. By contrast, the linear regression approach which includes the nonfebrile days, gives a slightly lower value (i.e. 7.3%/°C) for the energy cost of fever. The former approach, i.e. an increase in REE of 9.9%/°C, is to be preferred.
Fever is known to have a strong influence on HR. The latter dropped from 119 to 88 beats/min from the acute phase (1a) to the afebrile infected state(2a), a decrease which represented 80% of the total decrease. Therefore if we consider the interval where the influence of fever and the drop of HR were maximal (1a-2a), we can predict the drop of REE during that period by using a multiple regression integrating the variations in temperature and in HR: Equation
RQ. It is of interest to evaluate the changes in RQ during the dynamic phase of the treatment, because it represents an important indicator of both nutritional status (anabolic versus catabolic state) and relative pattern of substrate utilization.
Infection is typically accompanied by a change in the pattern of substrate oxidation, with a rise in fat oxidation and a decrease in glucose oxidation(as evidenced by a low RQ), the latter being partly explained by peripheral insulin resistance(32).
The fairly low RQ observed at the onset of treatment can be explained by the typical catabolic state which accompanies the infectious disease. The RQ at the study outset was significantly higher than initially and nearly identical to that obtained in a larger sample of healthy children who were investigated in a parallel study (P.-Y. Berclaz, C. Benedek, E. Jéquier, and Y. Schutz, unpublished results). The improvement in the nutritional status undoubtedly constitutes one of the major parameters explaining the net increase in RQ to values similar to those observed in healthy children. The fact that the body weight at the end of the study was slightly higher than on the 2nd d of the study suggests that the children had initiated their “nutritional recovery phase.” In this situation, RQ increases because the child is in positive energy balance.
Whole body protein turnover. In the present study we used the15 N “end product” method for assessing protein turnover rather than the so called “precursor” method. The latter is based on plasma amino acid enrichment and breath 13CO2 measurements after the infusion of an amino acid labeled with 13C (typically 1-[13C]leucine). In field studies, the end product method has the definitive advantage of being totally noninvasive because the 15N tracer can be given p.o., and no blood sample is required when[15N]ammonia is used as end product.
The major assumption of the end product method is that the fraction of the dose of isotope excreted in ammonia (and urea) is the same as the excretion of unlabeled nitrogen in these end products expressed as a fraction of the flux. If this is true, the metabolic pool should not be compartmented. Evidence of metabolic compartmentation of nitrogen metabolism can be shown by administration of different labeled amino acids(33). Anatomical compartmentation has been implied due to the different rates of protein turnover calculated from urea and ammonia after a single dose of[15N]glycine(25): the two end products do not yield the same turnover rates because the rate derived from urea is generally higher than that from ammonia. Fern et al.(25) have attributed these differences to the site of urea synthesis in the liver compared with the localization of ammonia synthesis in the kidney. The major issue in the present study is to know the extent to which the different approaches used (end product method versus “precursor” method) are able to accurately detect changes in turnover rates over time when these occur. Previous studies in which the reproducibility of the methods was tested on the same subject have shown similar precision values between the two methods: for example, Bier et al.(34) have reported a coefficient of variation for the primed 4-h infusion of 1-[13C]leucine technique of 6.8% which is almost identical to the value reported by Fern et al.(24) with the end product method using a single dose of [15N]glycine (6.7%).
In the present study the nitrogen flux, the whole body protein synthesis, and protein breakdown were significantly higher during the acute phase of the disease, a difference representing up to 125% of the control values(Table 4). Previous studies in humans have assessed the changes in protein turnover occurring after infection and trauma by both the“end product” and the “precursor” methods(35–39). It should be stressed that there was a remarkable agreement among the different methods used: when the protein synthesis was expressed as the percentage of the control group, the [15N]glycine methods using urinary NH3 as end product yielded values between 117 and 137% of the control value(37, 39) versus 121 and 137% using[15N]alanine (end product methods)(35, 38) and 126 and 147% with [14C]leucine administration (precursor method)(35, 36).
Edirisinghe et al.(40, 41) suggested that malarial infection could be attenuated by reducing the content of protein in the diet, whereas a protein-free diet could suppress the infection to very low levels through depriving the parasite of essential nutrients. However, the nutritional implication of malaria for the child and its effect on growth(1, 9, 5, 3, 42) emphasize the need of a nutritional strategy quite different from Edirisinghe's concepts.
Tomkins et al.(43) studied protein metabolism in a group of five Nigerian children, receiving a diet providing 70 mg of nitrogen/kg/d, during the acute stage and convalescent phase of measles. They showed that the rate of whole body protein breakdown was raised during acute infection, whereas the protein synthesis was not significantly different. In another study performed by the same authors, five Gambian children, with a diet providing 200 mg of nitrogen/kg/d, during an acute episode of malaria, had an increase in both protein synthesis and breakdown, suggesting that the level of protein intake may play a role in the stimulation of protein synthesis(39).
The diet formula we used provided maintenance level of nitrogen (100 mg/kg/d). In our study the rates of whole body protein synthesis and breakdown were increased by 92 and 125%, respectively, during the acute episode of malaria. Without stimulation by large amounts of dietary protein, the whole body protein synthesis was nearly doubled (Table 4). This might be explained by the acute phase response during inflammation(stimulation of the immune system, repair processes, and production of acute phase proteins)(44).
The increase in protein breakdown could contribute to the increased gluconeogenesis to supply glucose for cells involved in the inflammation process and to provide branched-chain amino acids that will be oxidized in the muscle. Although the protein synthesis was also increased, the nitrogen balance was negative during the infection. Based on the correlation between internal body temperature, protein synthesis, and protein breakdown, a child can easily triple his nitrogen metabolism during fever. This can explain the results on growth faltering resulting from repeated infections. Unfortunately our study does not provide arguments for a nutritionnal strategy preventing this deleterious loss of body nitrogen by an increased intake of protein. The biochemical cost of protein synthesis approximates 4.2 kJ/g protein(45). As shown in Figure 4, during malaria infection, the net experimental cost of protein synthesis was 9.2 kJ/g protein.
In conclusion, the present study showed a 37% increase in REE during an episode of malaria, which, combined with the restricted food intake (anorexia, vomiting), could easily explain the growth failure resulting from repeated infections. The rapid normalization of this hypermetabolism after treatment emphasizes the need to intervene promptly to control the infection and interrupt the effect of fever. The metabolic cost of fever was an increase of 9.9% in REE per C° in fever.
During an acute episode of malaria the rates of whole body nitrogen flux, protein synthesis and protein breakdown were twice as high as during the control day. With maintenance level of dietary nitrogen, the nitrogen balance was negative during the infection, having potentially negative effects on growth.





Abbreviations
- REE:
-
resting energy expenditure
- HR:
-
heart rate
References
Mata LJ, Urrutia JJ, Albertazzi BS, Pellecer O, Arellano E 1972 Influence of recurrent infections on nutrition and growth of children in Guatemala. Am J Clin Nutr 25: 1267–1275.
Martorell R, Yarbrough C, Yarbrough S, Klein RE 1980 The impact of ordinary illnesses on dietary intakes of malnourished children. Am J Clin Nutr 33: 345–350.
Cole TJ, Parkin JM 1977 Infection and its effect on the growth of young children: a comparison of the Gambia and Uganda. Trans R Soc Trop Med Hyg 71: 196–199.
Brewster DR, Greenwood BM 1993 Seasonal variation of paediatric diseases in The Gambia, West Africa. Ann Trop Paediatr 13: 133–146.
Scrimshaw NS 1991 Effect of infection on nutrient requirements. J Paren Enteral Nutr 15: 589–600.
Beisel WR 1977 Magnitude of the host nutritional responses to infection. Am J Clin Nutr 30: 1236–1247.
Kluger MJ 1980 Fever. Pediatrics 66: 720–724.
Wan JM-F, Haw MP, Blackburn GL 1989 Nutrition, immune function, and inflammation: an overview. Proc Nutr Soc 48: 315–335.
McGregor IA 1982 Malaria: nutritional implications. Rev Infect Dis 4: 798–804.
Stettler N, Schutz Y, Whitehead RG, Jequier E 1992 Effect of malaria and fever on energy metabolism in Gambian children. Pediatr Res 31: 102–106.
Baracos VE, Whitmore WT, Gale R 1986 The metabolic cost of fever. Can J Physiol 65: 1248–1254.
Goldberg AL, Kettelhut IC, Furuno K, Fagan JM, Baracos V 1988 Activation of protein breakdown and prostaglandin E2 production in rat skeletal muscle in fever is signaled by a macrophage product distinct from interleukin 1 or other known monokines. J Clin Invest 81: 1378–1383.
Sjolin J, Stjernstrom H, Friman G, Larsson J, Wharen J 1990 Total and net muscle protein breakdown in infection determined by amino acid effluxes. Am J Physiol 258:E856–E863.
Duggan MB, Milner RDG 1986 The maintenance energy requirement for children: an estimate based on study of children with infection associated underfeeding. Am J Clin Nutr 43: 870–878.
Fischer PR 1994 Tropical pediatrics. Pediatr Rev 14: 95–99.
Frood JD, Whitehead RG, Coward WA 1971 Relationship between pattern of infection and development of hypoalbuminaemia and hypo--lipoproteinaemia in rural Uganda children. Lancet 2: 1047–1049.
Prentice AM, Prentice A 1988 Reproduction against the odds. New Sci 14: 42–46.
Mc Gregor IA, Rahman AK, Thomson AM, Billewicz WZ, Thomson B 1970 The health of young children in a West African (Gambian) village. Trans R Soc Trop Med Hyg 64: 48–77.
Eccles MP, Cole TJ, Whitehead RG 1989 Factors influencing sleeping metabolic rate in infants. Eur J Clin Nutr 43: 485–492.
WHO Secretariat for the Coordination of Malaria Training in Asia and the Pacific 1983 Aids to human malaria diagnosis. World Health Organisation, Geneva
Brook CGD 1971 Determination of body composition of children from skinfold measurements. Arch Dis Child 46: 182–184.
Charbonnier A, Jones CDR, Schutz Y 1990 A whole body transportable indirect calorimeter for human use in the tropics. Eur J Clin Nutr 44: 725–731.
Jequier E, Schutz Y 1983 Long-term measurements of energy expenditure in humans using a respiration chamber. Am J Clin Nutr 38: 989–998.
Fern EB, Garlick PJ, Sheppard HG, Fern M 1984 The precision of measuring the rate of whole-body nitrogen flux and protein synthesis in man with a single dose of [15N]glycine. Hum Nutr Clin Nutr 38C: 63–73.
Fern EB, Garlick PJ, Mc Nurlan MA, Waterlow JC 1981 The excretion of isotope in urea and ammonia for estimating protein turnover in man with [15N]glycine. Clin Sci 61: 217–228.
Waterlow JC, Golden MH, Garlick PJ 1978 Protein turnover in man measured with 15N: comparison of end products and dose regimes. Am J Physiol 235: E165–E174.
Kun E, Kearney EB 1974 Methods of Enzymatic Analysis. Academic Press, New York, pp 1802–1806.
Preston T, McMillan D 1988 Rapid sample throughput for biomedical stable isotope tracer studies. Acta Paediatr Scand 16: 229–235.
Duggan MB, Milner RDG 1986 Energy cost of measles infection. Arch Dis Child 61: 436–439.
Du Bois EF 1921 The basal metabolism in fever. JAMA 775: 352–355.
Barr DP, Du Bois EF 1918 The metabolism in malarial fever. Arch Intern Med 21: 627–658.
Goldstein SA, Elwyn DH 1989 Effects of injury and sepsis on fuel utilization. Annu Rev Nutr 9: 445–473.
Fern EB, Garlick PJ, Waterlow JC 1985 Apparent compartmentation of body nitrogen in one human subject: its consequences in measuring the rate of whole-body protein synthesis with 13N. Clin Sci 68: 271–282.
Bier DM, Motil KJ, Matthews DE, Burke JF, Young VR 1981 Energy intake and whole body protein dynamics in man. In: Tsang RC, Nichols BL(eds) Nutrition and Child Health: Perspectives for the 1980s. Alan R. Liss, New York, pp 161–180.
Birkhahn RH, Long CL, Fitkin D, Jeevanandam M, Blakemore WS 1981 Whole-body protein metabolism due to trauma in man as estimated by L-[15N]alanine. Am J Physiol 241: E64–E71.
Clague MB, Keir MJ, Wright PD, Johnston IDA 1983 The effects of nutrition and trauma on whole-body protein metabolism in man. Clin Sci 65: 165–175.
Garlick PJ, McNurlan MA, Fern EB, Tomkins AM, Waterloo JC 1980 Stimulation of protein synthesis and breakdown by vaccination. BMJ 281: 263–264.
Long CL, Jeevanandam M, Kim BM, Kinney JM 1977 Whole body protein synthesis and catabolism in septic man. Am J Clin Nutr 30: 1349–1352.
Tomkins A, Garlick PJ, Fern EB, Waterlow JC 1984 The effect of acute malaria infection on nitrogen metabolism in young children. Proc Nutr Soc 43: 138A
Edirisinghe JS, Fern EB, Targett GAT 1981 The influence of dietary protein on the development of malaria. Ann Trop Paediatr 1: 87–91.
Edirisinghe JS 1986 Infections in the malnourished: with special reference to malaria and malnutrition in the tropics. Ann Trop Paediatr 6: 233–237.
Rowland MGM, Cole TJ, Whitehead RG 1977 A quantitative study into the role of infection in determining nutritional status in Gambian village children. Br J Nutr 37: 441–450.
Tomkins A, Garlick PJ, Schofield WN, Waterlow JC 1983 The combined effects of infection and malnutrition on protein metabolism in children. Clin Sci 65: 313–324.
Young VR, Yu YM, Fukagawa K 1992 Energy and protein turnover. In: Kinney J M, Tucker H N (eds) Energy Metabolism. Raven Press, New York, pp 439–467.
Flatt JP 1978 The biochemistry of energy expenditure. In: Bray E (ed) Recent Advances in Obesity Research. Newman Publishing, London, pp 211–228.
Acknowledgements
We thank the numerous field workers in The Gambia who have contributed to the study.
Author information
Authors and Affiliations
Additional information
Supported by the Nestlé Foundation, Lausanne, Switzerland.
Rights and permissions
About this article
Cite this article
Berclaz, PY., Benedek, C., Jéquier, E. et al. Changes in Protein Turnover and Resting Energy Expenditure after Treatment of Malaria in Gambian Children. Pediatr Res 39, 401–409 (1996). https://doi.org/10.1203/00006450-199603000-00005
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1203/00006450-199603000-00005
This article is cited by
-
Factors related to hypermetabolism in individuals with type 2 diabetes mellitus and non-alcoholic fatty liver disease
Scientific Reports (2023)
-
Laboratory prediction of the requirement for renal replacement in acute falciparum malaria
Malaria Journal (2011)
-
Energetic cost of protein turnover in healthy elderly humans
International Journal of Obesity (2001)