Abstract
In comparison to adults, infants undergoing halothane anesthesia have an increased incidence of clinically significant episodes of bradycardia, hypotension, and cardiac arrest. To examine potential cardiac autonomic regulatory mechanisms that may account for these observations, the relationship between respiratory activity and short-term variations of heart rate was quantified in 10 healthy term nonpremedicated infants (28.4 ± 0.6 wk old) undergoing elective surgery with halothane and low caudal anesthesia. Quantitative respiratory activity, heart rate, and cuff blood pressure data were obtained during the preoperative awake period, and at three depths of halothane-1, 1.3, and 2.0 mean alveolar concentration (MAC). Time and frequency domain analyses were performed on two 2.2-min epochs of data from each condition to yield mean values, spectral measures of low (0.02-0.15 Hz) and high (0.15-0.80 Hz) frequency power (LF and HF), and the LF/HF ratio. The sympathetic (As) and parasympathetic(Ap) components of respiratory sinus arrhythmia were quantified using the transfer relations between respiration and heart rate to derive gain factors As andAp, respectively. Mean heart rate, blood pressure, and respiratory activity all decreased with halothane exposure (p< 0.01), but did not differ by halothane dose. Similarly, LF, HF, LF/HF, and respiratory powers all decreased with halothane, but not between doses. When the effects of respiratory activity on heart rate were accounted for,As decreased at 1.3 and 2.0 MAC only, butAp remained unchanged. Decreased LF and HF power suggests that halothane altered both sympathetic and parasympathetic heart rate control; however, when the ratio between LF and HF and the quantitative effects of respiration are accounted for, halothane appears to cause a reduction in respiratory related sympathetic heart rate control, without a significant change in parasympathetic control.
Similar content being viewed by others
Main
Infants undergoing halothane anesthesia have an increased incidence of clinically significant bradycardia, hypotension, and cardiac arrest, compared with adults(1, 2). These findings may be related to a dose-dependent decrease in heart rate, cardiac output, stroke volume, and ejection fraction(1) and are consistent with the observation that halothane-induced hypotension appears to occur without a compensatory tachycardia, suggesting impairment of the arterial baroreflex. In fact, depression of the arterial baroreceptor reflex with halothane has been studied, and in animals it is due to reductions in the responses of many of the components of the reflex, including the central efferent response to afferent stimuli, the sympathetic ganglionic response to preganglionic stimuli, and the cardiac response to changes of cardiac vagal and postganglionic sympathetic efferent nerve stimulation(2). Other studies in animals have demonstrated a dose-dependent reduction in spontaneous vagal and preganglionic sympathetic efferent activity during halothane administration(4).
The etiology of the depressant effects of halothane on human cardiovascular function has been more difficult to address. A few studies have used heart rate variability measures as a potentially noninvasive means of assessing the cardiac autonomic effects of inhalation anesthesia with either halothane(3) or isoflurane(3, 4). Both of these studies observed reductions of LF and HF heart rate spectral power and concluded that cardiac sympathetic and parasympathetic activity were reduced. However, both studies were in adults, and neither study accounted for the effects of respiratory activity, despite the fact that halothane is known to produce a significant reduction of minute ventilation(5).
Thus, to investigate the effects of halothane on infant cardiac autonomic regulation, the relationship between respiratory activity and short-term variations of heart rate (i.e. respiratory sinus arrhythmia) was quantified in a group of healthy infants undergoing elective genitourinary or orthopedic surgery.
METHODS
Subjects. After approval by the Children's Hospital Committee on Clinical Investigation and with informed parental consent, 10 healthy (ASA I) nonpremedicated (no narcotics or anticholinergics) infants (nine male), were studied while undergoing elective surgery. Mean gestational age at birth was 38.3 ± 2.2 wk, and mean age at the time of the operation was 28.4± 0.6 wk. Mean weight was 7.3 ± 1.7 kg. All were fed orally for a mean of 5.8 ± 2.8 h preoperatively. None of the infants had neurologic, respiratory, or cardiovascular disease. Surgery included urologic(n = 7) and orthopedic procedures (n = 3).
Procedure and measurements. Each child was used as his or her own control. A baseline recording of heart rate and respiratory activity was made in the preoperative period while supine. The infant spontaneously breathed room air in an awake alert or active sleep(6) state, sucking on a pacifier. After this 10-min baseline recording, infants were taken to the operating room.
Operative procedure. After mask induction with a mixture of halothane (2-3% vol), nitrous oxide, and oxygen, an i.v. line was placed. To replace a portion of the fluid deficit incurred during the 5.8-h period without oral feeding, a 10 mL/kg bolus of Ringer's lactate with 5% dextrose was given. To ensure that afferent nociception from the operative incision itself would not influence heart rate, all infants received a caudal anesthetic with 0.75 mL/kg 0.25% bupivicaine. Monitoring during anesthesia included a precordial stethoscope, pulse oximetry, and automated noninvasive blood pressure (Dinamap, Critikon, Inc). End-tidal expired concentrations of halothane and CO2 were measured with RASCAL II (Ohmeda) and RG 5052(Ohmeda).
After induction, the nitrous oxide was switched off, and while the infants continued to breath spontaneously, the inspired halothane concentration was delivered by mask and adjusted to maintain a MAC of 2 (2.4% vol), 1.3 (1.6% vol), and 1 (1.2% vol), adjusted for age(2). After observation for approximately 10 min at each halothane dose, heart rate and respiratory epochs were selected for analysis. To ensure adequate and uniform sampling of the end-expired halothane concentration, a 14-gauge cannula was inserted into the mask so that the tip rested just above the subject's oral airway.
One lead of the surface ECG, respiratory activity, and blood pressures were recorded before, during, and after the operation. Three standard surface chest electrodes were used to produce continuous ECG, and a two-belt microprocessor-controlled inductance plethysmography system was used to produce a respiratory signal (Respiratrace-Plus, NonInvasive Monitoring Systems, Miami, FL). Both signals were digitally sampled to disk at 360 Hz using a personal computer-based data acquisition system. Calibration of the respiratory signal was performed using a previously described algorithm(7), assuming a tidal volume of 7 mL/kg. Respiratory volumes were normalized to a standard body surface area of 1.73 m2 to enable comparisons between adult and infant respiratory data. Blood pressure was measured at approximately 5-min intervals, before, during, and after the operation.
Data analysis. R waves were detected from the sampled ECG and used to form a smoothed instantaneous 4 Hz heart rate time series(8). The inductance respiratory signal was digitally low pass-filtered and decimated to 4 Hz. Two 2.2-min segments of heart rate and respiratory activity were selected from the preoperative period and at each depth of halothane. The epoch selection criteria were based on 1) qualitative signal stationarity, 2) the absence of infant movement in the presence of a stable behavioral state in the preoperative period(6), and 3) stable inspired and expired concentrations of halothane at the desired concentration for approximately 10 min during the operative periods.
Power spectral estimates of heart rate were quantified using the area(power) of the spectrum in a LF region (0.02-0.15 Hz) and a HF region(0.15-0.80 Hz), as well as by the ratio of LF and HF power (LF/HF), as previously described(9). These parameters were chosen because previous work has shown that, in adult humans and dogs, power at frequencies above 0.15 Hz is due solely to modulation of cardiac vagal activity, primarily by respiratory activity, whereas power at lower frequencies below 0.15 Hz can be due to modulation of both cardiac vagal and sympathetic activity by a variety of stimuli, including LF-respiratory activity descriptive here and the arterial baroreflex(9, 10). Similar measures of respiratory activity were tabulated from the respiratory power spectrum to yield LF-RP quantity here and HF-RP, and total RP (LF-RP + HF-RP).
To determine the contribution of both sympathetic and parasympathetic components to heart rate modulation, the effect of respiratory activity on heart rate was assessed using transfer function analysis, as previously described(10). Autospectra of the heart rate and respiratory signals and the cross-spectrum between them were estimated for each 128 s (512 point) segment(9). The complex transfer function (or frequency response) between RP and heart rate was quantified using the cross-spectral method to yield magnitude (gain) and phase components. A squared coherence spectrum was also computed to define the degree of the linear relation between respiratory activity and heart rate. The coherence varied between 0 and 1.
Specific measures of parasympathetic (Ap) and sympathetic (As) activity were derived from the average coherence weighted transfer gains of the two 2.2-min segments of data chosen for each experimental period in each subject. Previous work using this technique during pharmacologic treatment of adults with either atropine while upright, or propranolol while supine, has demonstrated gain and phase plots characteristic of pure parasympathetic and pure sympathetic modulation of heart rate, respectively(10). A pure sympathetic heart rate response (during standing plus atropine) was characterized by a reduced gain at frequencies >0.10 Hz and a phase delay. In contrast, under pure vagal control (supine plus propranolol), the heart rate response was characterized by higher gain at all frequencies and no phase delay. Values of transfer function gain from these studies were used to derive the specific Ap and As gain factors for infant autonomic modulation of heart rate, as previously described(11), equation 1, equation 2 where L and H are the LF and HF transfer function gains for each data epoch. It should be noted thatAs and Ap can have positive and negative values, given the relative presence or absence of LF transfer gain.


Statistical analysis. The mean and SD of the heart rate and power spectra for each data segment were calculated. A repeated measures analysis of variance was used to compare outcome measures across all periods. Paired t tests were used for post hoc comparisons between periods. A difference was considered significant for p values less than 0.05. For the purposes of display, group average transfer function estimates of both gain and phase from each experimental period were also computed, as previously described(10).
RESULTS
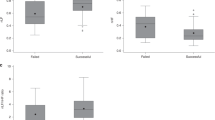
All 10 infants underwent the caudal and inhaled anesthetic without complications. Physiologic data were obtained for 1, 1.3, and 2 MAC for nine infants, and at 1 and 1.3 MAC only for one infant. End-tidal CO2 during all study periods ranged from 35 to 50 mm Hg. Oxygen saturation ranged from 97 to 100%. Mean heart rate (F = 9.07; df(3, 27); p= 0.003) and blood pressure parameters [systolic: (F = 8.41;df(3, 27); p < 0.1); diastolic: (F = 3.34;df(3, 27); p = 0.03)] decreased significantly with halothane, but did not differ significantly between halothane concentrations(Fig. 1).
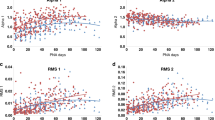
Power spectral estimates. With halothane both LF and HF power decreased significantly from baseline, but remained stable across halothane doses (F = 12.8; df(3, 27), p < 0.01 andF = 16.6; df(3, 27); p < 0.01, respectively)(Fig. 2). Similarly, the LF/HF ratio decreased with halothane (F = 7.0; df(3, 27); p = 0.0002), reflecting a greater decrease in LF than in HF power (Fig. 2). Finally, both LF and HF respiratory spectral power (LF-RP and HF-RP), and their sum (total power) also decreased significantly with anesthetic exposure, but again no differences were observed between halothane doses(Fig. 3).
Transfer function estimates of respiratory sinus arrhythmia. The specific measure of sympathetic modulation (As) derived from the respiratory to heart rate transfer functions decreased significantly with halothane (F = 3.00; df(3, 27);p = 0.05) (Fig. 4). Post hoc tests revealed significant differences between preoperative and 2 MAC dose(p < 0.05) and between the preoperative and 1.3 MAC dose(p < 0.05), but not between the preoperative and 1 MAC dose(p = NS). The specific measure of parasympathetic modulationAp did not change significantly with halothane exposure (F = 0.38; df(3, 27); p = 0.77).
Sympathetic (A) and parasympathetic(B) cardiac modulation as a function of halothane dose. The gain factors were derived from the transfer functions as described in the text. Significant changes were observed only for the sympathetic factorAs at halothane concentrations of 1.3 and 2.0 MAC.*p < 0.05 compared with preoperative (Preop) state.
As shown for both an individual subject (no. 19, Fig. 5) and the group (Fig. 6), during the preoperative baseline period, the transfer gain was highest at low frequencies and the phase began at 180 ° at 0 Hz, progressively decreasing to 0 ° at 0.2 Hz, all consistent with a dominance of sympathetic heart rate control. With halothane exposure, the mean respiratory frequency increased(Fig. 5, row 3, 0.52 Hz preoperatively, 0.6 Hz at 1 MAC, 0.7 Hz at 1.3 MAC, and 0.8 Hz at 2 MAC), and the variability of both the respiratory and heart rate signals decreased (Fig. 5, rows 1-3). At 1 MAC, a large part of the reduction in heart rate power could be accounted for by a reduction in respiratory power, particularly at low frequencies. These changes resulted in a reduction of transfer gain at all frequencies, which was most notable at low frequencies, and was accompanied by a shift in the LF phase to near 0 °. The changes in LF and HF gain translated into a large reduction of the sympathetic gain factorAs, and a smaller decrease of the parasympathetic gain factor Ap at 1 MAC (Table 1), shifting the balance of heart rate control toward the parasympathetic system. The phase shift toward 0 ° supports such a conclusion.
Time series, spectra, and transfer functions from subject no. 19 during the preoperative stage and all three concentrations of halothane. Power spectral scales are constant in each condition: heart rate, 0-50 (beats/min)2/Hz, lung volume, 0-0.019 L2/Hz/m2. See text for detailed explanation of results.
At 1.3 and 2 MAC, there were only small changes in LF and HF gain, and the phase remained near 0 °, consistent with the lack of significant changes in As and Ap observed at those doses in subject no. 19 (Table 1). The findings from this subject were somewhat in contrast to those seen for the group, where only As decreased significantly(Fig. 4), due to a selective reduction of LF gain(Fig. 6). However, the phase changes for subject no. 19 were similar to those of the group, with halothane producing a shift toward 0°, consistent with a shift in cardiac autonomic balance toward parasympathetic control.
DISCUSSION
The primary finding of this study is that halothane in infants appears to produce a marked reduction in respiratory related sympathetic heart rate control, without a significant change in respiratory related parasympathetic control. Although this conclusion contrasts somewhat with those of other investigators who have used heart rate spectral techniques to study the effects of inhalation anesthesia on cardiac autonomic regulation(3, 4), this is the first study to specifically include the influence of respiratory rate and volume on the spectral measures. As noted by others(3, 12–14), including the effects of RP may be critical to interpreting heart rate variability measurements.
Power spectra interpretation. The results of this study for LF and HF heart rate spectral power are not precisely comparable to those of previous studies examining the effects of inhalation anesthesia on heart rate variability, in part because the definitions of the various frequency bands are slightly different(3, 4); however, previous studies of halothane(3) and isoflurane(3, 4) in adults, and this study in infants, all found a reduction of lower frequency (<0.15 Hz) and higher frequency(>0.15 Hz) power with anesthesia. The relative reduction of power in each frequency band, as quantified by the LF/HF ratio, did differ, with this study revealing a large (78%) reduction at 1 MAC and smaller reductions at higher doses, whereas Galletly et al.(3) found a much smaller reduction of about 20% at 0.75% delivered halothane, and Katoet al.(4) found no reduction until 2.0 MAC isoflurane, when a 53% reduction from baseline was noted. These differences may be due to infants having a larger predominance of LF over HF power than adults(15, 16), a difference which may have been more marked for the subjects who were Awake Alert during the preoperative period in this study.
Using the heart rate spectral data alone, the reductions of LF, HF, and LF/HF observed in this study might be interpreted as a reduction of both parasympathetic (HF) and sympathetic (LF and LF/HF) heart rate modulation, similar to that proposed for the two studies of adult patients referred to above(3, 4); however, we also noted a marked reduction in respiratory signal power, with almost complete elimination of respiratory activity in the LF range (97% reduced). Because LF and HF heart rate fluctuations are strongly influenced by both the frequency(12, 17, 18) and amplitude(19) of RP, such changes in respiratory activity must be accounted for when interpreting the spectral results.
Transfer function interpretation. Although seemingly complex, the transfer function between RP and heart rate is a relatively simple method for quantifying respiratory sinus arrhythmia by effectively normalizing changes of heart rate at a particular frequency by the amplitude of respiratory activity at the same frequency. Thus, the gain, or magnitude, of the transfer function is expressed in beats/min of heart rate per liter of respiratory tidal volume and given as a function of respiratory frequency. In addition, the transfer function automatically provides information on the phase relation, or delay, between the two signals as a function of frequency.
As noted above, respiratory sinus arrhythmia has been quantified using transfer function analysis in normal adults(10, 12, 17) and infants(20) in a variety of autonomic states. In fact, the group average transfer function characteristics from the baseline preoperative period in this study (Fig. 6) are relatively similar to those seen during standing in normal adults(10, 12), suggesting a predominance of sympathetic heart rate control in these infants. In agreement with such an assertion, the values for sympathetic and parasympathetic respiratory sinus arrhythmia gain(As and Ap) during the preoperative period had a value for As about twice that of Ap (Fig. 4).
When the transfer function was used to account for respiratory activity during halothane exposure, sympathetic heart rate control decreased significantly, whereas parasympathetic control remained unchanged. These conclusions are markedly different from those which might be drawn from the heart rate spectral data alone and may demonstrate the importance of accounting for respiratory activity. However, an additional question which is extremely difficult to answer in humans must be asked-are such conclusions reasonable? The dose-dependent reductions of both heart rate and the arterial pressure parameters observed in this and other studies(1, 2) are certainly consistent with diminished cardiac and peripheral sympathetic activity, without a significant offsetting reduction of cardiac parasympathetic activity. Yet it remains possible that halothane has direct nonautonomically mediated effects on the sinus node and smooth muscle vasculature. Thus, a closer examination of the effects of halothane on autonomic control may be necessary.
Cardiac autonomic changes during halothane. Only limited data exist to address directly the changes of cardiac autonomic control that occur with halothane, particularly in human infants. In a study examining the dose-response of heart rate to atropine during halothane anesthesia (titrated to effect) in patients 1 mo to 12 y of age, Palmisano et al.(21) found that complete parasympathetic blockade in patients less than 6 mo of age increased heart rate from a mean of about 128 beats/min to about 175 beats/min, an increase of 37%. Such a large change of heart rate indicates not only that parasympathetic heart rate control(22) was present during halothane, but also suggests that it was near normal levels before atropine administration. Thus, it is unlikely that halothane caused a large reduction of parasympathetic activity in that study.
Yamamura et al.(23) directly measured sympathetic and parasympathetic activity from the cut efferent ends of the right vagosympathetic trunk in adult cats at end-tidal concentrations of halothane varying from 0.5 to 2%. In contrast to the noninvasive findings from this study, they found similar reductions in the activity of both autonomic branches of between 60 and 70%, at the highest halothane dose. However, in part because of the extensive vagal dissection and division in their study, the applicability of those data to infants with intact autonomic regulation is unclear.
Finally, examination of the effects of halothane on arterial baroreflex control of heart rate has revealed a dose-dependent reduction of baroreflex gain in adult dogs(24), piglets ranging in age from 1 to 60 d(21), and humans(25). Seagard et al.(24) identified a number of abnormalities which might account for the reduction of baroreflex gain, including the central efferent response to afferent stimuli, the sympathetic ganglionic response to efferent preganglionic stimuli, and the cardiac response to changes of vagal and postganglionic sympathetic efferent nerve stimulation. Of note, those investigators found that the response of heart rate to sympathetic efferent activation was blunted much more than the response to vagal activation. When combined with the observed reduction of sympathetic ganglionic transmission noted in the same study, the animal reports strongly support the notion that reflex sympathetic heart rate control is significantly perturbed by halothane, consistent with the findings from this study. However, the results presented here also suggest that respiratory related parasympathetic modulation of heart rate is relatively preserved during halothane, somewhat in contrast with both the results of direct nerve recordings in cats noted above(23) and the notion that baroreflex control of heart rate is mediated primarily by the cardiac parasympathetic response(26). This apparent paradox may have its resolution in a few additional observations.
Baroreflex control of heart rate does have a significant sympathetic component; however, it operates with a smaller gain and at lower frequencies than the parasympathetic response(10, 27). In addition, baroreflex effects are dependent entirely on central processing of afferent inputs, whereas respiratory sinus arrhythmia has multiple components, including arterial baroreflex responses to mechanically induced changes of arterial pressure, cardiopulmonary reflex responses, and importantly, direct central modulation of cardiac parasympathetic and sympathetic outflow(28). Thus, it is possible that halothane has greater effects on arterial baroreflex control of heart rate than on respiratory sinus arrhythmia. The decrease we observed in the portion of respiratory sinus arrhythmia mediated by sympathetic control may then be due to the halothane-mediated reductions of sympathetic ganglionic transmission and sympathetic efferent modulation of heart rate previously described by Seagardet al.(24).
LIMITATIONS
There are a few potentially important limitations to the study. The first is that, to eliminate the effects of nociceptive stimuli, the infants were all studied after the placement of a caudal anesthetic. Further, in light of the 5.8-h average period without fluids, and to offset any significant effects of the caudal anesthesia on venous capacitance, the infants were administered 10 mL/kg Ringer's lactate. Using similar analysis techniques, our laboratory has previously demonstrated in a group of slightly younger infants that even high thoracic spinal anesthesia does not significantly reduce sympathetic modulation of respiratory sinus arrhythmia (As)(11). Thus, given the caudal location and the small dose and low concentration of Marcaine (Winthrop, New York) used in this study, it seems unlikely that the caudal anesthetic significantly influenced sympathetic heart rate control. In addition, a reflex enhancement of sympathetic activation due to peripheral vasodilation was unlikely, because infants and young children undergoing similar epidural blocks have not been found to have significant changes in either mean arterial pressure or heart rate(29).
Second, the effects of halothane were compared with a preoperative state which varied between awake alert and active sleep(6), and had an unquantifiable range of infant awareness. This variation may have been associated with a wide range of autonomic control states preoperatively, potentially biasing the outcome, particularly because there is no control group that did not receive halothane. A related issue concerns our observation that the autonomic control responses in this study were variable, as highlighted by the reductions of both sympathetic and parasympathetic heart rate control found for subject no. 19 (Table 1, Fig. 5), in contrast to the findings for the group. These last two issues may have led to a combination of physiologic and technical issues which could markedly enhance the variance and reduce the significance of the results. Thus, although it may be unlikely that our positive finding of halothane reducing sympathetic heart rate control is incorrect, an additional undetected smaller effect on parasympathetic control cannot be ruled out. A lack of halothane dose dependency may have also been the result of high variance due to small sample size.
Finally, the interpretation of transfer functions, which effectively normalize heart rate variability by the amplitude of respiratory activity, is dependent in part on the amplitude of respiratory sinus arrhythmia being a function of tidal volume. Although such an effect has been confirmed in adults undergoing voluntary controlled breathing(17, 19), the effect is not entirely linear(19) and has never been confirmed with negative pressure breathing in infants or during inhalation anesthesia. Although we have no specific reason to doubt that tidal volume had an effect on respiratory sinus arrhythmia in this study, our interpretations of autonomic heart rate control are highly dependent on that effect being present.
CONCLUSIONS
Analysis of heart rate variability and its relation to respiratory activity in a group of infants undergoing halothane anesthesia revealed reductions of both the higher frequency variations of heart rate (HF) mediated by respiratory modulation of parasympathetic activity, and the lower frequency variations of heart rate (LF) mediated by sympathetic and parasympathetic activity. However, when both the balance between the LF and HF variations(LF/HF) and the quantitative effects of respiratory frequency and amplitude were accounted for (As andAp), halothane appeared to cause a reduction in only sympathetically mediated respiratory sinus arrhythmia, with preservation of parasympathetic effects. These results may have implications for the management of infants during halothane anesthesia.
Abbreviations
- MAC:
-
mean alveolar concentration
- LF:
-
low frequency
- HF:
-
high frequency
- RP:
-
respiration
- A s :
-
sympathetic gain factor
- A p :
-
parasympathetic gain factor
References
Murray DJ, Mahoney LT 1992 Comparative hemodynamic depression of halothane versus isoflurane in neonates and infants: an echocardiographic study. Anesth Analg 74: 329–337.
Lerman J, Willis MM, Gregory GA 1983 Anesthetic requirements to halothane in young children 0-1 months and 1-6 months of age. Anesthesiology 59: 421–424.
Galletly DC, Westenberg BJ, Robinson BJ, Corfiatis T 1994 Effect of halothane, isoflurane and fentanyl on spectral components of heart rate variability. Br J Anaesth 72: 177–180.
Kato M, Komatsu T, Kimura T, Sugiyama F, Nakashima K, Shimada Y 1992 Spectral analysis of heart rate variability during isoflurane anesthesia. Anesthesiology 77: 669–674.
Brown KA, Bissonnette B, Holtby H, Ein S, Shandling B 1993 Minute ventilation during mask halothane anaesthesia in infants and children. Can J Anaesth 40: 112–118.
Prechtl HF 1974 The behavioral states of the newborn infant (a review). Brain Res 76: 185–212.
Brouillette RT, Morrow AS, Weese-Mayer DE, Hunt CE 1987 Comparison of respiratory inductive plethysmography and thoracic impedance for apnea monitoring. J Pediatr 111: 377–383.
Berger RD, Akselrod S, Gordon D, Cohen RJ 1986 An efficient algorithm for spectral analysis of heart rate variability. IEEE Trans Biomed Eng BME- 33: 900–904.
Berger RD, Saul JP, Cohen RJ 1989 Transfer function analysis of autonomic regulation. I. The canine atrial rate response. Am J Physiol 256:H142–H152.
Saul JP, Berger RD, Albrecht P, Stein SP, Chen MH, Cohen RJ 1991 Transfer function analysis of the circulation: unique insights into cardiovascular regulation. Am J Physiol 261:H1231–H1245.
Oberlander TF, Berde CB, Lam KH, Rappaport LA, Saul JP 1995 Infants tolerate spinal anesthesia with minimal overall autonomic changes: analysis of heart rate variability in former premature infants undergoing hernia repair. Anesth Analg 80: 20–27.
Saul JP, Berger RD, Chen MH, Cohen RJ 1989 Transfer function analysis of autonomic regulation. II. Respiratory sinus arrhythmia. Am J Physiol 256:H153–H161.
Saul JP, Arai Y, Berger RD, Lilly LS, Colucci WS, Cohen RJ 1988 Assessment of autonomic regulation in chronic congestive heart failure by heart rate spectral analysis. Am J Cardiol 61: 1292–1299.
Saul JP 1990 Beat-to-beat variations of heart rate reflect modulation of cardiac autonomic outflow. News Physiol Sci 5: 32–37.
Korkushko OV, Shatilo VB, Plachinda YI, Shatilo TV 1991 Autonomic control of cardiac chronotropic function in man as a function of age: assessment by power spectral analysis of heart rate variability. J Auton Nerv Syst 32: 191–198.
Finley JP, Nugent ST 1995 Heart rate variability in infants, children and young adults. J Auton Nerv Syst 51: 103–108.
Hirsch JA, Bishop B 1981 Respiratory sinus arrhythmia in humans: how breathing pattern modulates heart rate. Am J Physiol 241:H620–H629.
Angelone A, Coulter NA 1964 Respiratory sinus arrhythmia: a frequency dependent phenomenon. J Appl Physiol 19: 479–482.
Eckberg DL 1983 Human sinus arrhythmia as an index of vagal cardiac outflow. J Appl Physiol 54: 961–966.
Hanna BD, Saul JP, Cohen RJ, Stark AR 1990 Transfer function analysis of respiratory sinus arrhythmia: developmental changes in sleeping premature infants. Circulation 82( suppl III): 334.
Palmisano BW, Setlock MA, Brown MP, Siker D, Tripuraneni R 1991 Dose-response for atropine and heart rate in infants and children anesthetized with halothane and nitrous oxide. Anesthesiology 75: 238–242.
Fouad FM, Tarazi RC, Ferrario CM, Fighaly S, Alicandri C 1984 Assessment of parasympathetic control of heart rate by a noninvasive method. Am J Physiol 246:H838–H842.
Yamamura T, Kimura T, Furukawa K 1983 Effects of halothane, thiamylal, and ketamine on central sympathetic and vagal tone. Anesth Analg 62: 129–134.
Seagard JL, Hopp FA, Donegan JH, Kalbfleisch JH, Kampine JP 1982 Halothane and the carotid sinus reflex: evidence for multiple sites of action. Anesthesiology 57: 191–202.
Muzi M, Ebert TJ 1994 Randomized prospective comparison of halothane, isoflurane and enflurane on baroreflex control of heart rate in humans. Adv Pharmacol 31: 379–387.
Pickering TG, Gribbin B, Petersen S, Cunningham DJC, Sleight P 1972 Effects of autonomic blockade on the baroreflex in man at rest and during exercise. Circ Res 30: 177–185.
Triedman JK, Saul JP 1994 Comparison of intraarterial with continuous noninvasive blood pressure measurement in postoperative pediatric patients. J Clin Monit 10: 11–20.
Saul JP, Cohen RJ 1994 Respiratory sinus arrhythmias. In: Schwartz, P., Levy, M. (eds) Vagal Control of the Heart. Futura Publishing, Armonk, NY, pp 511–536.
Murat I, Delleur MM, Esteve C, Egu JF, Raynaud P, Saint-Maurice C 1987 Continuous extradural anaesthesia in children. Clinical and haemodynamic implications. Br J Anaesth 59: 1441–1450.
Acknowledgements
The authors gratefully acknowledge the excellent assistance of Robyn Doody in preparing the manuscript, and of Jamil Sobh and Emily Flynn McIntosh in preparing the figures.
Author information
Authors and Affiliations
Additional information
Supported by grants from the Whitaker Foundation, Mechanicsburg, Pennsylvania, a Milton Fund Research Grant from Harvard Medical School, and by National Institutes of Health Grant R01-HL48012-01. T.F.O. was a Clinical Research Fellow of the National Cancer Institute of Canada, and received additional support from a training grant from the Bureau of Maternal and Child Health, National Institutes of Health. J.P.S. is supported by a National Institutes of Health Clinical Investigators Award K08-HL02380-03. C.B.B. is supported by the Anesthesia Patient Safety Foundation and the Christopher Coakley Fund.
Rights and permissions
About this article
Cite this article
Oberlander, T., Berde, C. & Saul, J. Halothane and Cardiac Autonomic Control in Infants: Assessment with Quantitative Respiratory Sinus Arrhythmia. Pediatr Res 40, 710–717 (1996). https://doi.org/10.1203/00006450-199611000-00010
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1203/00006450-199611000-00010