Abstract
Neurocardiogenic syncope may be caused by enhanced sympathetic activity evoking a vasodepressor-cardioinhibitory reflex. Heart rate variability (HRV) methods can be used to assess the modulation of sympathetic and parasympathetic activity. To determine whether HRV measurements are related to the outcome of head-up tilt testing (HUT), we studied 29 syncopal patients aged 7-19 y. After 30 min supine, patients were tilted to 80 ° for 30 min or until syncope occurred. Sequential beats free from ectopy were analyzed. Time domain indices included SD (SDNN), root mean square successive differences (RMSSD), percent exceeding 50 ms (pNN50). An autoregressive model was used to calculate power spectra. Low frequency power (LFP, 0.05-0.15 Hz), high frequency power (HFP, 0.15-0.40 Hz), and total power (TP, 0.01-0.40 Hz) were compared before and after tilt. Data were obtained supine before tilt(baseline), within 5 min after HUT (early), 5-10 min after HUT (mid), and 15 min after HUT or presyncope (late). Seventeen patients fainted (HUT+), and 12 patients did not (HUT-). Variability indices were different for HUT- and HUT+ at baseline: SDNN was 123 ± 17versus 78 ± 6, RMSSD was 127 ± 23 versus 64± 6 ms, pNN50 was 51 ± 6 versus 31 ± 4, respectively. Spectral data demonstrated decreased HFP and TP in HUT+(834 ± 133 and 2855 ± 420 ms2) versus HUT- (3433 ± 840 and 7062 ± 1500). With tilt, SDNN, RMSSD, and pNN50 decreased proportionately in HUT- and HUT+. However, sympathovagal balance, measured by the ratio LFP/HFP and by normalized LFP, was markedly increased in HUT+ (2.2 ± 0.7 and 0.43 ± 0.03) compared with HUT- (0.8 ± 0.2 and 0.31± 0.02) at baseline and differences increased with tilt. With syncope, sympathetic activity decreased, and parasympathetic activity increased. Decreased RR variability with decreased parasympathetic activity and increased indices of sympathovagal balance before HUT predict a positive tilt test in children referred for evaluation of neurocardiogenic syncope.
Similar content being viewed by others
Main
Although the precise etiology of neurocardiogenic syncope is not completely understood, evidence suggests that it may be precipitated by enhanced sympathetic stimulation in conjunction with underfilling of the left ventricle(1, 2). This stimulates ventricular mechanoreceptors receptors evoking a vasodepressor-cardioinhibitory, Bezold-Jarisch-like reflex mediated through vagal C-fiber afferents. Resultant increased vagal efferent activity and withdrawal of sympathetic stimulation from resistance and capacitance vessels cause hypotension, bradycardia, and finally loss of consciousness(3–5).
HUT testing has been very useful as a provocative test for neurocardiogenic syncope because the orthostatic stress of tilting decreases cardiac filling, increasing sympathetic and reciprocally decreasing parasympathetic activity(6, 7). However, HUT use as a diagnostic tool and as a test of efficacy of medical therapy for neurocardiogenic syncope has been hampered by poor reproducibility(8, 9).
Recently, studies of HRV have provided noninvasive techniques with which to assess sympathetic and parasympathetic activity(10, 11). HRV data can be used to demonstrate the changes in autonomic activity with orthostatic tilt(12, 13).
We hypothesized that the modulation of sympathetic and parasympathetic activity at the time of tilt testing accounted for the variable response of patients with neurocardiogenic syncope to orthostatic stress. The goal of our study was to use HRV techniques to answer the question: does modulation of autonomic activity at the time of HUT determine the outcome of the test?
METHODS
Subjects. To accomplish this goal we studied 29 patients ranging in age from 7 to 19 y (median = 15 y). There were 17 girls and 12 boys. All patients had at least two episodes of syncope. Patients had undergone normal physical examination, electrocardiography, Holter examination, and echocardiography. There were no signs or history of systemic illness. Patients were not taking medications. Informed consent was obtained in all patients.
HUT protocol. Subjects were asked to fast overnight. This is our standard procedure for tilt testing and was initiated primarily to avoid potential aspiration. It does tend to reduce the intravascular volume, thus increasing the likelihood of neurocardiogenic syncope. All tests began between 0900 and 1000 h and were conducted in a quiet room with dimmed lighting. Tilt table testing was performed on an electrically driven tilt table(Cardiosystems 600, Dallas, TX) with a foot board for weight bearing. An i.v. line was inserted and patency maintained with a solution of 0.9% saline with 100 U of heparin/mL. The ECG was monitored continuously (Quinton 3000, Seattle, WA), and periodic 12-lead strip chart recordings were made. Two electrocardiographic leads were continuously recorded with a tape recorder(Marquette Systems 8000, Milwaukee, WI) for subsequent calculation of HRV data. Blood pressure was monitored noninvasively with an arm sphygmomanometer.
Baseline recordings in the supine position were obtained near the end of a 30-min resting period. Patients were then tilted to 80 °(14) for a maximum duration of 30 min or until syncope or presyncope occurred. Syncope was defined as hypotension (systolic blood pressure less than 70 mm Hg) with or without a relative bradycardia (heart rate less than 75% of resting heart rate) accompanied by loss of postural tone and loss of consciousness. Presyncope was defined by hypotension and relative bradycardia with loss of postural tone but without loss of consciousness. A positive test was defined as syncope or presyncope before the 30-min cutoff. Patients with a positive test were returned immediately to the supine position. Intravenous 0.9% saline was administered. All had prompt resolution of signs and symptoms of syncope or presyncope.
Data analysis. Continuous electrocardiographic recordings were analyzed, digitally processed, and scanned using the System 8000 Marquette Holter Scanner. Standard Marquette algorithms for QRS labeling were used. Time series of RR intervals were downloaded to a personal computer using the QRSDK list software provided by Marquette. Sequential 256-512-beat epochs, free of ectopy, were used for subsequent analysis, except for late measurements in HUT+ patients. In those patients 100 beats before syncope were used. Epochs were linearly detrended. All calculations were done on a personal computer using custom software.
Time domain indices of HRV were calculated(15). These included the mean RR interval, the SD of the RR interval (SDNN), the root mean square of successive RR differences (RMSSD), and the percent of successive RR intervals differing by >50 ms (pNN50).
An autoregressive model was used to calculate the power spectrum following the methods of Montano et al.(12) and Paganiet al.(16). In brief, RR intervals were acquired as a sequence of discrete point events. The RR intervals were transformed into an equivalent impulse train in which pulses were arranged at equal intervals (equal to the mean RR interval) with impulse heights equal to the RR intervals(17). Autoregression was performed, and digital power spectra were calculated(18, 19). The Levinson-Durbin algorithm on the Yule-Walker equations was followed by Anderson's test, choosing the order of the model which minimized Akaike's final prediction error. This yields the interval spectrum which is converted to the spectrum of counts by dividing by the mean RR interval of the sequence(17, 18). These techniques result in a smoother and more consistent power spectrum estimate and tend to improve statistical consistency compared with the fast Fourier transform without smoothing. The spectral power within a given band was computed by taking the power in the actual frequency band. Spectral power was partitioned into ultra low frequency(<0.01 Hz), very low frequency (VLFP, 0.01-0.05 Hz), low frequency (LFP, 0.05-0.15 Hz), and high frequency (HFP, 0.15-0.40 Hz) power bands. The total power (TP, 0.01-0.40 Hz) was also calculated. Ultra low frequency was not represented accurately for 100-beat sequences and was not used as an index of autonomic activity. The LFP band is thought to reflect baroreceptor sympathetic and parasympathetic influences, whereas the HFP band represents the modulation of parasympathetic activity alone. The LFP/HFP ratio and normalized power (nP) were calculated where nP was defined as Equation and are believed to represent the overall sympathovagal balance(12).

Approximately stationary segments were obtained at baseline and after tilting. For comparison, epochs while supine for at least 20 min (baseline), 3-5 min after tilt (early), 5-10 min after tilt (mid), and 15 min after tilt or just before syncope or presyncope (late) were compared.
Stationarity. Interpretation of heart rate spectra depends on stationary. Stationarity issues are particularly relevant to studies of HRV in which measurements and statistics are to some degree time dependent. Our methods, based on autoregression calculations, implicitly assume that the sample is drawn from a stationary, or wide sense stationary time series which is ergodic in its first two moments. This is distinguished from many methods using the FFT in which discrete time and frequency domain data are modeled as periodic functions. Calculations of baseline data may most closely approximate calculations made on a stationary time series because conditions are not changing rapidly. To address these issues we have empirically tested stationarity by calculating mean values and standard deviations on 128- and 256-beat records and compared them with 512-beat records. No statistical difference in mean values were observed. The 512-beat sequences were not available for some segments after tilt. However, when means and standard deviations were compared from 128- and 256-beat sequences obtainable after tilt, no statistical differences were observed.
Statistics. Data were compared by two-way analysis of variance for repeated measures. When significant interactions were demonstrated the ratio of F values was converted to a t distribution using Scheffe's test, and probabilities were thereafter determined. All results are reported as mean ± SEM. Statistically significant differences are reported for p < 0.05.
RESULTS
Of the 29 patients, 17 fainted, and are designated HUT positive(HUT+), and 12 did not and are designated HUT negative (HUT-). The median time to syncope was 13 min (range, 6-20 min). Fainting episodes were all mixed depressor-cardioinhibitory responses with a decrease in blood pressure preceding a fall in heart rate.
A representative tachogram of RR intervals and power spectral analysis of the tachogram are shown in Fig. 1. Discrete low frequency and high frequency peaks were almost always present both before and after HUT.Figure 2 shows complete tachograms for a HUT+ patient with presyncope and an HUT- patient. The characteristic decrease in RR interval after tilt is indicated. With presyncope, the RR interval increased. On resumption of the supine position, RR intervals returned to baseline values.
Tachograms during the tilt test. Continuous records from a HUT+ patient with presyncope are shown in the top panel and a HUT- patient in the bottom panel. The characteristic decrease in RR interval with tilt is indicated. With presyncope, the RR interval increased. On resumption of the supine position, RR intervals returned to baseline values.
Data obtained at baseline, early, mid, and late phases of tilt are shown inTables 1 and 2. There were no significant differences among data for HUT- patients during early, mid, or late phases. There were no differences between data for HUT+ patients during early and mid phases.
Time domain results. Time domain HRV indices for HUT+ and HUT- are shown in Table 1. Similar decreases in RR interval and thus increases in heart rate occurred with tilt for HUT+ and HUT- groups. Baseline indices of HRV were uniformly lower in the HUT+ compared with HUT- patients. With tilt, the RR interval decreased similarly for both groups, whereas all time domain indices of HRV decreased proportionately in HUT+ and HUT-. Therefore, all time domain indices remained significantly lower in HUT+ compared with HUT- patients.
Frequency domain results. Spectral analysis data are shown inTable 1. Both TP and HFP were markedly lower in HUT+ compared with HUT- patients, whereas LFP was not different. With tilt, all power decreased proportionately in HUT+ and HUT- groups. HFP decreased proportionately more than LFP.
Indices of sympathovagal balance. Indices of sympathovagal balance are shown in Table 2. During resting conditions at baseline, HUT+ patients have a markedly larger LFP/HFP ratio, larger nLFP, and smaller nHFP compared with HUT- patients. With tilt, the LFP/HFP increases for both HUT+ and HUT- groups because HUT causes a relative increase in the modulation of sympathetic activity in all patients, whereas vagal activity is withdrawn. However, the LFP/HFP ratio remains much larger in HUT+ patients, reflecting relatively enhanced sympathetic over parasympathetic activity compared with HUT- patients.
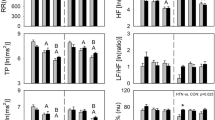
HRV with syncope. The response of HRV indices in HUT+ patients during the late phase after tilt is shown in Figure 3. There is a large increase in time domain and frequency domain indices compared with baseline. The largest is in HFP, suggesting a massive surge in parasympathetic modulation. The ratio of LFP/HFP fell from approximately 5± 1 to 0.8 ± 0.2, whereas nLFP fell to 0.35 ± 0.04 and nHFP increased to 0.52 ± 0.06. These changes reflect a relative decrease in sympathetic heart rate modulation and increase in parasympathetic heart rate modulation, but may also in part reflect a decrease in heart rate preceding syncope.
HRV preceding syncope. Data were obtained near the time of syncope or presyncope in HUT+ patients. There is an increase in those time and frequency domain indices, reflecting enhanced parasympathetic and diminished sympathetic activity. This is best shown by changes in the ratio of LFP to HFP and in nLFP, whereas nHFP increases. *p< 0.05 compared with baseline data.
Results are exemplified by data shown in Figure 4 which presents RR intervals and corresponding spectra from representative HUT- and HUT+ patients. The overall variability is quite different for these patients with much greater HFP at baseline in the HUT- patient. RR interval decreases and overall power decreases with tilt for both patients with proportionately greater LFP. HFP is nearly absent in the HUT+ patient after tilt until the late phase, when a large upsurge of HFP and total power is observed.
DISCUSSION
Summary and main results. Our data indicate that relatively increased sympathetic heart rate modulation compared with parasympathetic heart rate modulation is associated with a positive HUT test in children referred for evaluation of neurocardiogenic syncope. The data further suggest that the results of tilt testing may be predicted by measuring indices of HRV in the supine, resting, baseline condition. We have posed and affirmatively answered a specific question: whether HRV can predict HUT outcome in children referred for evaluation of neurocardiogenic syncope. All of our children hadno other discernible etiology for syncope as determined by history, physical examination, electrocardiography, Holter monitoring, and echocardiography.
The HRV indices which we measured do not yield straight-forward yes/no results. Nevertheless, with certain assumptions, we have calculated sensitivity, specificity, and positive and negative predictive values of a subset of the HRV indices at baseline before the induction of syncope. We chose two time domain indices-the RMSSD, which measures succeeding beat variability, and the SDNN, which measures overall variability-and two frequency domain indices-HFP and LFP/HFP ratio. To create yes/no tests from these HRV measurements, data from each index were collected and a“cutoff” value estimated which maximized the sum of sensitivity and specificity for each.
Sensitivity was calculated as TrN/(FP + TrN), specificity was calculated as TrN/(FN + TrP), PPV was calculated as TrP/(FP + TrP), and NPV was calculated as TRN/(FN + TrN). TrP denotes true positive (HUT+ and HRV+), FP denotes false positive (HUT+ and HRV-), TrN denotes true negatives (HUT- and HRV-), and FN denotes false negatives(HUT- and HRV+), with PPV and NPV as abbreviations for positive predictive value and negative predictive value, respectively.
The results of these computations appear in Table 3. The statistics suggest that representative HRV indices can predict the results of the HUT test with fair accuracy. Discrepancies in such predictions may result from inadequacies of the method but may also reflect inclusion of some patients who do not have true neurocardiogenic syncope but rather some alternative cause for syncope.
HRV reflects sympathovagal balance that relates to syncope in HUT. That HRV indices relate to HUT outcome should not be surprising. HRV data have been shown to reliably reflect modulation of sympathetic and parasympathetic nervous system activity during HUT. HFP reflects modulation of parasympathetic activity only, whereas LFP variability reflects both sympathetic and parasympathetic activity(20). The ratios of LFP to HFP and nLFP and nHFP are believed to represent changes in relative sympathovagal balance with HUT. Our data indicate that children referred for tilt testing who faint during testing have indices of sympathovagal balance skewed markedly toward enhanced sympathetic and diminished parasympathetic activity when studies are performed immediately before HUT. Enhanced sympathetic activity upon HUT compared with the supine position was seen in both HUT- and HUT+ patients and has been observed in normal patients with HUT(21). Sympathovagal balance, as measured by the LFP/HFP ratio and by nLFP and nHFP, however, was markedly shifted toward increased sympathetic modulation in patients who subsequently fainted compared with patients who did not. This is consistent with prevailing theories of neurocardiogenic syncope, in which enhanced sympathetic tone is an important precedent(1, 2), resulting in hypercontraction of an underfilled left ventricle which evokes the vagal C-fiber-mediated reflex(3, 5). With syncope or presyncope, vagal activity increases abruptly in association with reversal of LFP/HFP ratio, and of normalized power spectra.
Comparison with previous studies of HRV in syncope. Our data and conclusions are not strictly comparable to data in the literature because our research question was different from questions addressed in those investigations. Some questions which may be examined in the context of the literature are: Is autonomic function normal in neurocardiogenic syncope? How does modulation of autonomic activity relate to the results of HUT? Have HRV indices been measured in normal and syncopal children? Are there data concerning HRV at the time of HUT in patients with presumed neurocardiogenic syncope? If such data exist for adults, are results from children likely to be comparable?
When autonomic function testing is performed in patients with presumed neurocardiogenic syncope, results are usually normal. When HRV data have been obtained from such patients, no obvious differences have been found. However, until recently, HRV measurements have not been made near the time of syncope as in our study. Rather, often an entire day's data may be compared with the outcome of tilt testing performed at some other time(22, 23).
Recently, however, several researchers have performed tilt testing in conjunction with studies of HRV. Baharav et al.(24), for example, studied HRV just before and during HUT in nine children with a history of syncope and compared them with nine children who were healthy with no history of syncope. Using a table inclination to 60 °, they found higher baseline LFP in control than in syncopal children. However, 6/9 syncopal patients fainted during tilt, whereas 2/9 control patients also fainted. Their data are not really comparable to ours, because they used different initial conditions and angle of tilt. They also compared syncopal with nonsyncopal patients based on previous history of fainting, whereas we looked at HRV in patients with a history of syncope, comparing those with positive and negative HUT results. Morillo et al.(25), however, compared adult patients with a history of syncope and positive HUT response with patients with syncope and negative HUT, and to control patients. They found that there were no differences among their groups during baseline supine measurements. During tilt (to 60 °) they found results in adults, which were almost exactly opposite of our results in children: their data indicated that low LFP/HFP ratios in the first 5 min of tilt were associated with a positive outcome, and that early HFP was significantly greater in patients who later fainted. These results are puzzling, because enhanced sympathetic activity is an important part of the neurocardiogenic response and should have been manifested by an increased LFP/HFP ratio and increased nLFP. Partial explanation might include differences in autonomic activity between adults and children. However, our results are quite consistent with prior data of Lipsitz et al.(26) who studied adults and found that LFP did increase with tilt significantly more in syncopal than in nonsyncopal patients. In addition, that investigation suggested a trend toward lower baseline power in syncopal patients.
Some differences in HRV data from adults and children may relate to age. The literature and experience indicate that the modulation of autonomic control depends on age(27–29). Age-related differences may in part explain the increase of LFP with upright position in children(27), although mechanisms for these differences are not completely known. Korkushko et al.(29), in a large study of subjects ranging from 3 mo to 89 y, demonstrated a maximum level for HFP between 15 and 19 y, whereas LFP(which he called mid frequency power) peaked about a decade later.
Limitations. The primary methodologic limitation concerns the requirement of stationarity to apply spectral methods to relatively short sequences of heart beats. This is particularly true at times of rapid change, such as that seen to precede and accompany syncope during the late phase of measurement in HUT+ patients. These data are presented mostly for heuristic purposes, and their inclusion or exclusion does not affect our main conclusions.
Also, the computations of sensitivity, specificity, and positive and negative predictive values were based on a posteriori criteria. The calculations were developed for illustrative purposes from continuous measurements of HRV indices and need to be refined and retested to determine their practical importance. Testing of further patients may help to improve such methods.
Finally, our study could be viewed as limited by the exclusion of normal subjects. However, we addressed a specific research question-whether HRV at the time of tilt testing is related to test outcome in syncopal patients. Therefore, the results pertain only to patients with presumed neurocardiogenic syncope which formed the patient population for this investigation.
Abbreviations
- HRV:
-
heart rate variability
- HUT:
-
head-up tilt
- HUT+:
-
positive head-up tilt test
- HUT-:
-
negative head-up tilt test
- SDNN:
-
SD of normal to normal RR intervals
- RMSSD:
-
root mean square successive RR difference
- pNN50:
-
percent of successive RR intervals differing by at least 50 ms
- LFP:
-
low frequency spectral power
- HFP:
-
high frequency spectral power
- TP:
-
total spectral power
- nLFP:
-
normalized low frequency spectral power
- nHFP:
-
normalized high frequency spectral power
References
Abboud FM 1982 Ventricular syncope. Is the heart a sensory organ?. N Engl J Med 320: 390–392.
van Lieshout JJ, Wieling W, Karemaker JM, Eckberg DL 1991 The vasovagal response. Clin Sci 81: 575–586.
Mark AL 1983 The Bezold-Jarisch reflex revisited: clinical implications of inhibitory reflexes originating from the heart. J Am Coll Cardiol 1: 90–102.
Epstein SE, Stampfer M, Beiser GD 1968 Role of the capacitance and resistance vessels in vasovagal syncope. Circulation 37: 524–533.
Hainsworth R 1991 Reflexes from the heart. Physiol Rev 71: 617–658.
Blomqvist CG, Stone HL 1983 Cardiovascular adjustments to gravitational stress. In: Shepherd JT, Abboud FM (eds) The Handbook of Physiology, Section 2: The Cardiovascular System. Volume III: Peripheral Circulation. American Physiology Society, Bethesda, MD, pp 1025–1063.
Abi-Samra A, Maloney JD, Fouad-Tarazi FM, Castle LW 1988 The usefulness of head-up tilt testing and hemodynamic investigations in the workup of syncope of unknown origin. PACE 11: 1202–1214.
Maya A, Permanyer-Miralda G, Sagrista-Sauleda J, Carne X, Rius T, Mont L, Soler-Soler J 1995 Limitations of head-up tilt for evaluating the efficacy of therapeutic interventions in patients with vasovagal syncope: results of a controlled study of etilephrine versus placebo. J Am Coll Cardiol 25: 65–69.
Brooks R, Ruskin JN, Powell AC, Newell J, Garan H, McGovern BA 1993 Prospective evaluation of day-to-day reproducibility of upright tilt-table testing in unexplained syncope. Am J Cardiol 71: 1289–1292.
Akselrod S, Gordon D, Hubel FA, Shannon DC, Barger AC, Cohen RJ 1981 Power spectrum analysis of heart rate variability: a quantitative probe of beat-to-beat cardiovascular control. Science 213: 220–222.
Malliani A, Pagani M, Lombardi F, Cerutti S 1991 Cardiovascular neural regulation explored in the frequency domain. Circulation. 84: 482–492.
Montano N, Ruscone TG, Porta A, Lombardi F, Pagani M, Malliani A 1994 Power spectrum analysis of heart rate variability to assess the changes in sympathovagal balance during graded orthostatic tilt. Circulation 90: 1826–1831.
Lipsitz LA, Mietus J, Moody GB, Goldberger AL 1990 Spectral characteristics of heart rate variability before and during postural tilt. Circulation 81: 1803–1810.
Strieper MJ, Auld DO, Hulse JE, Campbell RM 1994 Evaluation of recurrent pediatric syncope: role of tilt table testing. Pediatrics 93: 660–668.
Bigger JT, Fleiss JL, Rolnitsky LM, Steinman RC, Schneider WJ 1991 Time course of recovery of heart period variability after myocardial infarction. J Am Coll Cardiol 18: 1643–1649.
Pagani M, Lombardi F, Guzzeti S, Rimoldi O, Furlan R, Pizzinelli P, Sandrone G, Malfatto G, Dell'Orto S, Piccaluga E, Turiel M, Baselli G, Cerutti S, Malliani A 1986 Power spectral analysis of heart rate and arterial pressure variabilities as a marker of sympatho-vagal interaction in man and conscious dog. Circ Res 59: 178–193.
DeBoer RW, Karemaker JM, Strackee J 1984 Comparing spectra of a series of point events particularly for heart rate variability data. IEEE Trans Biomed Eng 4: 384–387.
Kay SM, Marple SL 1981 Spectrum analysis-a modern perspective. Proc IEEE 69: 1380–1419.
Roberts RA, Mullis CT 1987 Spectral estimation. In: Digital Signal Processing. Addison-Wesley, Reading, MA, pp 483–565.
Pomeranz B, Macaulay RJB, Caudill MA, Kutz I, Adam D, Gordon D, Kilborn KM, Barger AC, Shannon DC, Cohen RJ, Benson H 1985 Assessment of autonomic tone in humans by heart rate spectral analysis. Am J Physiol 248:H151–H153.
Vybiral T, Bryg RJ, Maddens ME, Boden WE 1989 Effect of passive tilt on sympathetic and parasympathetic components of heart rate variability in normal subjects. Am J Cardiol 63: 1117–1120.
Simone F, Buonomo C, Nozzoli C, Grossi D, Roca ME, Santostasi R 1990 Vasovagal reactions induced by head-up tilt and test of vagal cardiac function. J Auton Nerv Syst 30:S145–S148.
Sneddon JF, Bashir Y, Muragatroyd FD, Ward DE, Camm AJ, Malik M 1993 Do patients with neurally mediated syncope have augmented vagal tone?. Am J Cardiol 72: 1314–1315.
Baharav A, Mimouni M, Lehrman-Sagie T, Izraeli S, Akselrod S 1993 Spectral analysis of heart rate in vasovagal syncope: the autonomic nervous system in vasovagal syncope. Clin Auton Res 3: 261–269.
Morillo CA, Klein GJ, Jones DL, Yee R 1994 Time and frequency domain analyses of heart rate variability during orthostatic stress in patients with neurally mediated syncope. Am J Cardiol 74: 1258–1262.
Lipsitz LA, Mietus J, Moody GB, Goldberger AL 1990 Spectral characteristics of heart rate variability before and during postural tilt. Circulation 81: 1803–1810.
Yeragani VK, Pohl R, Berger R, Balon R Srinivasan K 1994 Relationship between age and heart rate variability in supine and standing postures: a study of spectral analysis of heart rate. Pediatr Cardiol 15: 14–20.
Finley JP, Nugent ST, Hellenbrand W 1986 Heart-rate variability in children. Spectral analysis of developmental changes between 5 and 24 years. Can J Physiol 65: 2048–2052.
Korkushko OV, Shatilo VB, Plachinda YI, Shatilo TV 1991 Autonomic control of cardiac chronotropic function in man as a function of age: assessment by power spectral analysis of heart rate variability. J Auton Nerv Syst 32: 191–198.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Stewart, J., Erb, M. & Sorbera, C. Heart Rate Variability and the Outcome of Head-Up Tilt in Syncopal Children. Pediatr Res 40, 702–709 (1996). https://doi.org/10.1203/00006450-199611000-00009
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1203/00006450-199611000-00009
This article is cited by
-
On the feasibility of tilt test outcome early prediction using ECG and pressure parameters
EURASIP Journal on Advances in Signal Processing (2011)
-
Orthostatic tolerance is difficult to predict in recurrent syncope patients
Clinical Autonomic Research (2011)
-
Autonomic Nervous System Function in Infants and Adolescents: Impact of Autonomic Tests on Heart Rate Variability
Pediatric Cardiology (2009)
-
Autonomic Function in Children and Adolescents with Neurocardiogenic Syncope
Pediatric Cardiology (2008)
-
Spectral and Time-Domain Analyses of Heart-Rate Variability During Head-Upright Tilt-Table Testing in Children with Neurally Mediated Syncope
Pediatric Cardiology (2006)