Abstract
Human intestinal mucosa consists of highly active epithelial cells in continual renewal and differentiation processes located at different portions of the villi. The crypt contains abundant replicating cells which, upon reaching the villus tip, acquire their fully differentiated state. Besides its well recognized role in bone cell homeostasis, calcitriol has been attributed a role in cellular differentiation and proliferation in normal leukocytes and myeloid leukemia cells. We have previously documented the presence and the distribution of specific calcitriol receptors in the cells of the small and large intestine from 13-20-wk-old human fetuses and that calcitriol was able to promote human intestinal epithelium proliferation or differentiation, in organ culture, depending upon fetal age. We now show that, whereas transcripts for calcitriol receptors are abundant from duodenum to colon, those for the 9-kD calcium-binding protein are present mainly in the duodenum and the jejunum and to a lesser extent in the ileum and the colon. Transcripts for 25-hydroxycholecalciferol-24-hydroxylase could not be detected in any of the intestine segments despite a prolonged exposition of the gels. Immunofluorescence staining for the 9-kD calcium-binding protein was exclusively observed in the epithelial cells of the small intestine and colon, the subepithelial layers being always negative. The 9-kD calcium-binding protein distribution along the crypt-villus axis appeared as a gradient, increasing from the developing crypt to the tip of the villus in the duodenum, jejunum, and ileum. Based on the present observations and on the fact that calcitriol promotes human fetal proliferation and differentiation, the presence of transcripts for calcitriol receptors and 9-kD calcium-binding protein in the intestinal cell opens interesting possibilities as of their role in the in utero human gut development and the control of colorectal cancers.
Similar content being viewed by others
Main
The human intestinal mucosa consists of highly active epithelial cells in continual renewal and differentiation processes which are anatomically located at different portions of the villi. The crypt is rich in replicating cells which, upon reaching the villus tip, acquire their fully differentiated state(1). Thus, this tissue is of major interest in the further understanding of the action of hormones and growth factors on developmental processes in physiologic and pathologic states.
Besides its well recognized role in bone cell homeostasis, calcitriol, the hormonal form of vitamin D, has been attributed a role in cellular differentiation and proliferation in normal and cancerous cells(2–5). On our part, we have already reported the presence and the distribution of specific VDR in the cells of the small and large intestine from 13-20-wk-old human fetuses(6). We have also recently shown that, in organ culture, calcitriol could promote either human intestinal epithelium proliferation or differentiation, depending upon fetal age(7).
In vivo and in vitro studies, performed in animals or on isolated cells, have given compelling evidence that calcitriol is also a potent stimulator of intestinal ATP-dependent calcium transport(8–10). On the other hand, vitamin D-dependent CaBP, discovered in fowl intestine by Wasserman and Taylor(11), has been attributed roles in calcium transit from the brush border to the basolateral membrane where calcium is extruded into the circulation(12, 13). Staun et al.(14) have been successful in purifying to homogeneity of a 9-kD CaBP from adult duodenum and in producing MAb against the protein. Howard et al.(15) have on their part, using mixed oligonucleotide primers derived from the conserved region of the rat, cow, and pig sequences, shown the presence of 9-kD CaBP mRNA in adult human proximal small intestinal mucosa and assigned the gene to chromosome X. Also Jeung et al.(16), using reverse transcription and PCR methodology, have independently cloned the full-length cDNA encoding human 9-kD CaBP and shown that mRNA abundance was substantially higher in child than in adult duodenum.
In keeping with our prior reports(6, 7), we have examined the abundance of 9-kD CaBP, VDR, and 25-hydroxy-24-OHase mRNAs along the fetal intestinal tract. We have also studied, by indirect immunofluorescence, the cellular distribution of 9-kD CaBP along the crypt-villus axis of the small intestine and colon.
METHODS
Tissues. Small intestine (duodenum, jejunum, ileum) and colon(proximal, distal) specimens from fetuses of 10-18 wk in age(postfertilization) were obtained from normal elective pregnancy terminations. No tissue was collected from cases associated with known fetal abnormality or fetal death. Studies were approved by the Institutional Human Subject Review Board. The specimens were immersed in Leibovitz L-15 medium (room temperature) containing Garamycin (40 μg/mL) and brought to the culture room within 30 min. The tissues were frozen in liquid nitrogen and kept at -80 °C until further processing for biochemical and PCR analyses. Samples from each fetus were immediately prepared for indirect immunofluorescence. The preparation and embedding of specimens for cryosectioning were performed as previously described(17) using optimum cutting temperature embedding compounds (Tissue Tek, Miles Laboratories, Elkhart, IN).
Sample extraction and reverse transcription-PCR analysis. Total RNA was extracted by the method of Chomczynski and Sacchi(18). Briefly, frozen tissues were homogenized in 4.0 M guanidinium thiocyanate. RNA was extracted twice with phenol-chloroform, precipitated twice with propan-2-ol, dissolved in RNase-free distilled deionized water, and digested with RQ1 RNase-free DNase (Promega, Montréal, Canada). Under our extraction conditions, 28 and 18 S RNA populations were intact as judged by agarose gel electrophoresis, thereby excluding the possibility of having RNA degradation by the action of pancreatic RNase. DNase was removed by extraction with phenol/chloroform/isoamyl alcohol (25/24/1). Single-stranded cDNA was synthetized from 10 μg of the purified total RNA by the reverse transcriptase reaction using the following conditions: 50 mM Tris-HCl (pH 8.3), 75 mM KCl, 3 mM MgCl2, 10 mM DTT, 2 mM of each of the deoxynucleotide triphosphates, 30 U of RNAguard (Pharmacia Canada, Montreal, Canada), and 100 pmol of oligo(dT)12-18 primers (Pharmacia), all in 19μL of RNase-free distilled deionized water. The mixture was heated for 15 min at 65 °C and chilled on ice. Superscript Moloney murine leukemia virus RNase H-reverse transcriptase (Life Technologies, Inc., Burlington, Canada) was then added at a concentration of 20 μg of al RNA. After a 2-h incubation at 37 °C, the reaction was stopped by heating at 95 °C for 5 min and quickly chilling on melting ice. Two units of RNase H (Boehringer Mannheim, Montreal, Canada) were then added to the reaction mixture which was incubated for 20 min at 37 °C. The reaction was stopped by heating at 95°C for 5 min followed by immediately chilling on ice. The contents were extracted once with phenol/chloroform/isoamyl alcohol (25/24/1). Oligo(dT) primers were removed by precipitating the reaction products with 2.5 M ammonium acetate and 1 volume of 100% ethanol at room temperature. After washing twice with 70% ethanol, the pellet was redissolved in 20 μL of distilled deionized water. Aliquots of the first strand cDNA were added to the labeled PCR amplification buffer consisting of 10 mM Tris-HCl (pH 8.3), 50 mM KCl, 1.5 mM MgCl2, 0.2 mM of each of the nucleotides, 2 μM up- and down-stream primers, 2 μCi of [32P]dCTP (3 000 Ci/mmol, Dupont Chemicals, Montreal, Canada), and 2.5 U of Taq DNA polymerase(Boehringer Mannheim) in a final volume of 50 μL. The reaction was carried out on a Perkin-Elmer thermocycler model 9600 (Perkin-Elmer, Norwalk, CT). For identification of RNA species, 30 cycles of amplification were used under the following conditions: denaturation at 94 °C for 30 s, annealing at the computer-derived annealing temperatures of the respective primers for 1 min, and extension at 72 °C for 2 min. The last cycle was followed by a 10-min incubation at 72 °C. Primer sets, derived from published sequences(16, 20–23) and located on different exons (whenever the genomic sequence was known), are as listed inTable 1 with their respective base numbers, exon location, and annealing temperature.
Samples (20 μL) were electrophoresed on 5% polyacrylamide gel according to established methods(24), exposed to sensitized Kodak XAR-5 films (Kodak Canada, Toronto, Canada), and quantified by laser densitometry (ScanJet Plus, Hewlett-Packard Canada Ltd., Mississauga, Canada). Blank reactions consisting of the PCR reaction mixture and water instead of the cDNA were included during the amplification procedures. Under no circumstance did any product band appear, thereby excluding contamination of samples. Furthermore, reactions performed as described above but without the addition of the reverse transcriptase excluded the presence of genomic DNA. The relative abundance of the different PCR products was expressed as a ratio to GAPDH taken as the housekeeping gene.
Indirect immunofluorescence. Cryosections 3-4 μm thick, cut on a Jung Frigocut 2800 N cryostat (Leica Canada Inc., Saint-Laurent, Canada), were spread on silane-coated glass slides and air-dried for 45 min at 32°C before storage at -80 °C. Tissue sections were fixed in acetone-chloroform (1:1) for 5 min at 4 °C before immunostaining. Rabbit antibody against human intestinal 9-kD calbindin(14, 25) was kindly provided by Dr. M. Staun (Rigshospitalet, Copenhagen, Denmark). Antibody was diluted 1:100 in PBS (pH 7.4) containing 2% BSA. 5(6)-Carboxyfluorescein-N-hydroxysuccinimide-conjugated anti-rabbit IgG (Boehringer Mannheim) was used as a second antibody at a working dilution of 1:50. Sections were stained with 0.01% Evans blue in PBS. Preparations were mounted in glycerol-PBS (9:1) containing 0.1% p-phenylenediamine and viewed with a Reichert Polyvar 2 microscope (Leica, Montreal Canada) equipped for epifluorescence with 25× and 100× objectives. For immunolocalization observations, FITC-fluorescence was exited with the output of an Osram HBO 100W/2 lamp filtered with a Leica B1 module (exitation filter 450-490 nm; dichroic mirror DS 510; barrier filter LP520). Black and white photographs were taken with Kodak TX-400 films. In all cases, no immunofluorescent staining was observed when primary antibodies were omitted or replaced by appropriate nonimmune sera.
Double immunofluorescence was also performed to verify, at the cellular level, a possible relationship between the localization of 9-kD CaBP and that of sucrase, a specific marker of the intestinal brush-border(26). Briefly, sections were fixed, blocked in 5% blotto-PBS (30 min at 23 °C), and incubated with primary antibodies diluted 1:200 (rabbit polyclonal anti-human intestinal 9-kD CaBP) or 1:100(HSI-14 mouse monoclonal anti-human sucrase(27) in 5% blotto-PBS (pH 7.4). Secondary antibodies were rhodamine-conjugated goat anti-rabbit IgG and fluorescein-conjugated goat anti-mouse IgG, both from Boehringer Mannheim Canada (Laval, Quebec) used at a final dilution of 1:30 in 5% blotto-PBS. In all cases, no fluorescence was observed when the respective primary antibody was omitted or replaced with nonimmune rabbit or mouse serum. Control mismatched incubations revealed no cross-reactivity between the two detection systems.
RESULTS
Fig. 1 illustrates the effect of increasing single-stranded cDNA concentrations (50-1000 ng) on PCR products for 9-kD CaBP and VDR in a duodenum sample from a 17-wk-old fetus. Under the conditions described, no transcripts for 24-OHase could be evidenced. Note that GAPDH amplification already plateaued at 250 ng of cDNA and that signals were observed for neither blank reactions. A concentration of 500 ng of cDNA was chosen for subsequent PCR amplifications. Fig. 2 shows the abundance of amplified labeled cDNAs for 9-kD CaBP, VDR, 24-OHase, and GAPDH in the different segments of the intestinal tract studied. It can readily be seen that, whereas VDR transcripts are expressed throughout the intestinal tract, those for the 9-kD CaBP are present mainly in the duodenum and the jejunum and to a lesser extent in the ileum and the colon. As before, transcripts for 24-OHase could not be revealed in any of the intestine segments despite prolonged exposition of the gels or increased number of PCR cycles. Fig. 3 represents the densitometric analysis of the gels when results are compared with GAPDH. It is evident that, whereas VDR is well expressed from duodenum to colon, 9-kD CaBP transcripts are well expressed, albeit to a lesser extent, in the duodenum and the jejunum. Their abundance is even lower in the ileum and the colon.
Representative effect of the concentration of single-stranded cDNA, obtained by reverse transcriptase, on PCR product amounts in a duodenum sample from a 17-wk-old fetus. Increasing amounts of the first-strand cDNA were added to the labeled PCR amplification buffer. The reaction was carried out on a Perkin-Elmer thermocycler model 9600 as described in “Methods.” A, 1000 ng; B, 500 ng;C, 250 ng; D, 100 ng; E, 50 ng; F, blank reaction consisting of the PCR reaction mixture and water in place of the cDNA.
Distribution of transcripts encoding for 9-kD CaBP, VDR, 24-OHase, and GAPDH along a representative 18-wk-old fetal intestine explant from duodenum (duod) through jejunum (jejun), ileum, and colon. First-strand cDNA was added to the labeled PCR amplification buffer, and the reaction was carried out on a Perkin-Elmer thermocycler model 9600 as described in “Methods.” Primers sets were derived from published sequences(16, 20–23) and insofar as possible, located on different exons. Approximately the same number of base pairs were chosen for each transcript so as to obtain similar reaction rates. In this example, films were overexposed to ensure that poorly expressed genes could be detected.
Relative 9-kD CaBP (open bars) and VDR mRNA(hatched bars) levels. Labeled PCR amplification products were electrophoresed on polyacrylamide gel, exposed to sensitized Kodak XAR-5 films, and quantified in a laser scanning densitometer. Data from the densitometric scanning were normalized for the GAPDH signal. The relative levels of 9-kD CaBP and of VDR are indicated as the relative OD. Bar graph shows the mean ± SEM of three experiments performed in triplicate.
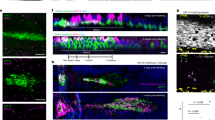
Fig. 4 illustrates the cellular localization of 9-kD CaBP along the crypt-villus axis of the developing human small intestine and colon from 10 wk of gestation onward. Immunofluorescence staining was exclusively observed in the epithelial cells of both segments, the subepithelial layers being always negative. The 9-kD CaBP distribution along the crypt-villus axis appeared as a gradient, increasing from the developing crypt to the tip of the villus in the duodenum (Fig. 4A), jejunum (Fig. 4B), and ileum (Fig. 4C). Staining of 9-kD CaBP was detected mainly in the cytoplasm of absorptive cells of the upper third or half of the villus. Furthermore, the terminal region of the supranuclear cytoplasm exhibited a bright immunoreactivity all over the crypt-villus axis (Fig. 4). Staining was observed neither in cell nuclei nor in goblet cells. A similar pattern was seen in adult ileal mucosa (Fig. 4D). A comparative study was carried out in the colonic mucosa. At 10 wk of gestation, when the epithelium is still stratified and the villus structure absent, a bright immunoreactivity for 9-kD CaBP was observed in the luminal upper cell layer (Fig. 4E). With the development of the crypt-villus axis, similar cellular localization and gradient for 9-kD CaBP expression were noted along the axis as the small intestine. Again, the numerous goblet cells present in the colonic epithelium were negative(Figs. 4F and5C). Fig. 5 illustrates the double-labeling immunofluorescence for sucrase-isomaltase and for 9-kD CaBP in the jejunum and the colon. In both segments, the staining for sucrase-isomaltase delineates the brush border (Fig. 5,B and D), and the bright 9-kD CaBP immunoreactivity observed in the terminal region of the supranuclear cytoplasm (Fig. 5,A and C) exhibits a close relationship with the brush-border region.
Expression and distribution of 9-kD CaBP. Representative indirect immunofluorescence micrographs of cryosections of human duodenum (A), jejunum (B), and ileum (C) at 18 wk of gestation; adult ileum (D) and colon at 10 (E) and 18 (F) wk of gestation. Cryosections were stained with rabbit antibody against human intestinal 9-kD CaBP. An increasing gradient of 9-kD CaBP immunoreactivity was observed from the developing crypt to the tip of the villus. No staining was noted either in cell nuclei or in goblet cells. Magnification: A and F, ×136; B, C, andE, ×85; D, ×68.
Relationship between the cellular localization of brush-border sucrase and 9-kD CaBP. Representative micrographs of cryosections of human jejunum (A and B) and colon (C andD) at 18 wk of gestation, stained by double immunofluorescence for detection of 9-kD CaBP (A and C) and sucrase (B and D). Magnification: ×136.
DISCUSSION
We have previously shown the presence of VDR in different segments of human fetal intestine(6) and recently demonstrated that calcitriol biphasically affected intestinal cell proliferation and differentiation(7). Data on the expression of 9-kD CaBP and VDR in human fetal intestinal cells are scarce and in fact, except for the report of Brun et al.(28) on 9-kD CaBP, are not available. We have now established, using reverse transcription-PCR with primers prepared from previously published sequences, that various segments of the human fetal intestinal tract contain messages encoding for 9-kD CaBP and VDR. We have also shown that both entities are coexpressed in the same segments of the intestine, although their relative abundance differ from duodenum to colon. Whereas VDR transcript levels are high in all parts of the intestine studied, those of 9-kD CaBP steadily decrease from duodenum to colon. Ménard(1) has stated that the morphogenesis of the colonic villi is similar to that of the small intestine but occurs 4-5 wk later. We may therefore argue that, at 17 wk of gestation, the developmental stage of the colon is equivalent to that of a 12-wk duodenum or jejunum. On the other hand, we have shown that calcitriol enhanced cell proliferation of 12-wk-old fetal small intestine and cell differentiation in older specimens(7). Therefore the close relation between the expression of 9-kD CaBP and VDR in duodenum reflects a differentiated status. In the colon, the high expression of VDR with a concomitant low expression of 9-kD CaPB may therefore be partially explained on the basis that VDR acts on proliferative genes. This hypothesis is based on extending to the human fetal intestine reports which show that, in the parathyroid or the osteoblast, calcitriol-VDR complexes interact with specific upstream gene promoter regions, designated as a vitamin D-responsive elements, retinoid receptors, and specific nuclear accessory factors to either stimulate or inhibit the transcription of genes involved in cell differentiation or proliferation(29–31). Previous sets of experiments, in which we have observed that calcitriol increased[3H]thymidine incorporation into colon specimens from 16- and 17-wk-old fetuses, without affecting either lactase or sucrase activities (our unpublished data), gives support to this theory. No transcripts for 24-OHase could be elicited in the explants studied even upon prolonged gel exposure or increased PCR cycles beyond 30 cycles (data not shown). However, Lemayet al.(32) have shown that mRNA for 24-OHase was undetectable in the intestine of hypocalcemic vitamin D-depleted rats and that chronic treatment with calcitriol failed in inducing its expression. It may therefore be that the human fetal intestine, having a constant delivery of the hormone, is much in the same situation, thus explaining our negative observation.
Since 28-kD CaBP was first discovered in chick small intestine(11), many studies attempted to establish its cellular location. Our observation that 9-kD CaBP is located in the cytoplasm of human absorptive cells is in agreement with previous results obtained in the pig, rat, and chick small intestine(33, 34). Although several studies indicated the presence of CaBP in both goblet and absorptive cells(35, 36), the thorough investigation of Taylor(37) clearly demonstrated that the localization of CaBP outside the absorptive cells resulted from an artifactual diffusion and redistribution. In the present study, the goblet cells from the human fetal small intestine and colon were always negative, in agreement with studies done in human adult(38), rat(39), and chick(33) intestine. To our knowledge, this is the first report on the presence and of the location of 9-kD CaBP in the developing human gut. The increasing gradient of 9-kD CaBP staining from crypt to villus is consistent with quantitative data illustrating an increasing amount of 9-kD CaBP along the crypt-villus axis in human jejunum(25).
The presence of a strong immunofluorescence staining in the terminal region of the apical cytoplasm of enterocytes and colonocytes strongly suggests an accumulation of 9-kD CaBP at the terminal web-brush border level. This hypothesis is supported by the double-labeling immunofluorescence experiments for sucrase-isomaltase(17, 26) and 9-kD CaBP, showing that the protein is closely associated with the brush border region. The gradient of cytosolic staining which was maximal at the level of the terminal web-brush border junction is consistent with previous studies on rat duodenum(39, 40), as well as with quantitative evaluations of the immunocytochemical protein A-gold labeling in chick duodenum(33) and human adult jejunum(38). The presence of 9-kD CaBP staining in the apical cytoplasm along the entire crypt-villus axis epithelium from 10 wk of gestation and up, as well as in an adult ileum specimen shown in the present study, corroborates the observations made by Staun et al.(38), but contrasts with the reported absence of CaBP in crypt cells of chick and rat intestines(33, 40) assessed by immunocytochemical techniques. However, the amount of intestinal CaBP has been shown to increase steadily from crypt to villus(25, 40) in vitamin D-repleted animals and individuals. Furthermore, both crypt and villus cells possess receptors for calcitriol(41), and the appearance of the crypt-villus CaBP gradient was reported to be dependent upon the vitamin D status(42). Whether the presence of CaBP in crypt cells in human fetal small intestine and colon depends on the vitamin D status of the fetuses is impossible to say. However, we do know that the pattern of appearance of calcitriol receptors in the human is quite different from that of rodents. Indeed, as opposed to both rats(43, 44) and rabbits(45), in which intestinal calcitriol receptors appear only at the time of weaning, human fetal intestine(6) not only possesses such receptors but also responds to the hormone in terms of cellular proliferation and differention(7). Therefore this report warrants caution in extrapolating, to human, data observed in other species.
Whether calcitriol is able to modulate 9-kD CaBP and induce 24-OHase synthesis in human small intestinal and colonic cells remains to be established. However, based on the present observations and on the fact that calcitriol promotes human fetal proliferation and differentiation(7), the presence of transcripts for VDR and 9-kD CaBP in intestinal enterocytes and colonocytes opens interesting possibilities as for their role in the in utero human gut development and the control of colorectal cancers, as suggested by Lointier et al.(46).
Abbreviations
- CaBP:
-
calcium-binding protein
- VDR:
-
calcitriol receptor
- 24-OHase:
-
calcidiol-24-hydroxylase
- GAPDH:
-
glyceraldehyde-3-phosphate dehydrogenase
- PCR:
-
polymerase chain reaction
References
Ménard D 1989 Growth-promoting factors and the development of the human gut. In: Lebenthal E (ed) Human Gastrointestinal Development. Raven Press, New York, pp 123–150.
Eisman JA, MacIntyre I, Martin TJ, Frampton RJ 1980 Normal and malignant breast tissue is a target organ for 1,25-dihydroxy vitamin D3. Clin Endocrinol 13: 267–272.
Colston K, Colston MJ, Fedman D 1981 1,25-Dihydroxyvitamin D3 and malignant. Endocrinology 108: 1083–1086.
Frampton RJ, Osmond SA, Eisman JA 1983 Inhibition of human cancer cell growth by 1,25-dihydroxyvitamin D3 metabolites. Cancer Res 43: 4443–4447.
Manolagas SC 1987 Vitamin D and its relevance to cancer. Anticancer Res 7: 625–638.
Delvin EE, Richard P, Pothier P, Ménard D 1990 Presence and characteristics of calcitriol receptors in human fetal gut. FEBS Lett 262: 55–57.
Ménard D, Lévy E, Delvin EE 1995 Effect of calcitriol on human fetal cell differentiation and proliferation. Biol Neonate 68: 157–162.
Arab N, Ghishan F 1989 Vitamin D-regulated, ATP-dependent calcium transport by intestinal Golgi vesicles during maturation in the rat. Pediatr Res 26: 58–62.
Bronner F 1990 Intestinal calcium transport: the cellular pathway. Miner Electrolyte Metab 16: 94–100.
Timmermans JAH, Kaune R, Bindels RJM, van Os CH 1991 Quantification of Ca2+-ATPases in porcine duodenum. Effects of 1,25-(OH)2 D3 deficiency. Biochim Biophys Acta 1065: 177–184.
Wasserman RH, Taylor AN 1966 Vitamin D3-induced calcium-binding protein in chick intestinal mucosa. Science 152: 791–793.
Bruns ME, Boass A, Toverud S 1987 Regulation by dietary calcium of vitamin D-dependent calcium-binding protein and calcium transport in the small intestine of lactating rats. Endocrinology 121: 278–283.
Christakos S, Gill R, Lee S, Li H 1992 Molecular aspects of the calbindins. J Nutr 122: 678–682.
Staun M, Sjoström H, Noren O 1986 Calcium-binding protein from human small intestine. Purification and characterization of a 10,000 molecular weight protein. Eur J Clin Invest 16: 468–472.
Howard A, Legon S, Spurr NK, Walters JRF 1992 Molecular cloning and chromosomal assignment of human calbindin-D9K. Biochem Biophys Res Commun 185: 663–669.
Jeung E-B, Krisinger J, Dann JL, Leung PCK 1992 Molecular cloning of the full-length cDNA encoding the human calbindin-D9k. FEBS Lett 307: 224–228.
Beaulieu JF, Weiser MM, Herra L, Quaroni A 1990 Detection and characterization of sucrase-isomaltase in adult human colon and in colonic polyps. Gastroenterology 98: 1467–1477.
Chomczynski P, Sacchi N 1987 Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal Biochem 162: 156–159.
Jeung E-B, Leung P, Krisinger J 1994 The human calbindin-D 9k gene. Complete structure and implications on steroid hormone regulation. J Mol Biol 235: 1231–1238.
Baker AR, McDonnell DP, Hughes M, Crisp TM, Mangelsdorf DJ, Haussler MR, Pike WJ, Shine J, O'Malley BW 1988 Cloning and expression of full-length cDNA encoding human vitamin D receptor. Proc Natl Acad Sci USA 85: 3294–3298.
Malloy PJ, Weisman Y, Feldman D 1994 Hereditary 1α,25-dihydroxyvitamin D-resistant rickets resulting from a mutation in the vitamin D receptor deoxyribnucleic acid-binding domain. J Clin Endocrinol Metab 78: 313–316.
Chen K-S, Prahl JM, DeLuca HF 1993 Isolation and expression of human 1,25-dihydroxyvitamin D3 24-hydroxylase cDNA. Proc Natl Acad Sci USA 90: 4543–4547.
Ercoloani L, Florence B, Denard M, Alexander M 1988 Isolation and complete sequence of functional human glyceraldehyde-3-phosphate dehydrogenase gene. J Biol Chem 263: 15335–15341.
Maniatis T, Fritsch EF, Sambrook J 1989 Molecular Cloning: A Laboratory Manual, 2nd Ed. Cold Spring Harbor Laboratory, Cold Spring Harbor, NY
Staun M 1987 The crypt-villus distribution of the 10,000Mr intestinal calcium-binding protein in human jejunum. Clin Chim Acta 167: 31–36.
Ménard D, Beaulieu JF 1994 Human intestinal brush-border membrane hydrolases. In: Bkaily G (ed) Membrane Physiopathology. Kluwer Academic Publishers, Norwell, MA, pp 319–341.
Beaulieu JF, Nichols B, Quaroni A 1989 Postranslational regulation of sucrase-isomaltase expression in intestinal crypt and villus cells. J Biol Chem 264: 20000–20011.
Brun P, Dupret JM, Thomasset M, Mathieu H 1987 Vitamin D calcium-binding proteins (CaBPs) in human fetuses: comparative distribution of 9k-CaBP mRNA and 28k-CaBP during development. Pediatr Res 21: 362–367.
Demay MB, Kiernan MS, DeLuca HF, Kronenberg HM 1992 Sequences in the human parathyroid hormone gene that bind the 1,25-dihydroxyvitamin D3 receptor and mediate transcriptional repression in response to 1,25-dihydroxyvitamin D3 . Proc Natl Acad Sci USA 89: 8097–8101.
Terpening CM, Haussler CA, Jurutka PW, Galligan MA, Komm BS, Haussler MR 1991 The vitamin D-responsive element in the rat bone Gla protein gene is an imperfect direct repeat that cooperates with other cis-elements in 1,25-dihydroxyvitamin D3-mediated transcriptional activation. Mol Endocrinol 5: 373–385.
Ross TK, Moss VE, Prahl JM, DeLuca HF 1992 A nuclear protein essential for binding of rat 1,25-dihydroxyvitamin D3 receptor to its response elements. Proc Natl Acad Sci USA 89: 256–260.
Lemay J, Demers C, Hendy GN, Delvin EE, Gascon-Barré M 1995 Expression of the 1,25-dihydroxyvitamin D3-24-hydroxylase gene in rat intestine: response to calcium, vitamin D3 and calcitriol administration in vivo. J Bone Miner Res 10: 1148–1157.
Thorens B, Roth J, Norman AW, Perrelet A, Orci L 1982 Immunocytochemical localization of the vitamin D-dependent calcium-binding protein in chick duodenum. J Cell Biol 94: 115–122.
Arnold BM, Kovacs K, Murray T 1976 Cellular localization of intestinal calcium-binding protein in pig duodenum. Digestion 14: 77–84.
Lippiello L, Wasserman RH 1975 Fluorescent antibody localization of the vitamin D-dependent calcium-binding protein in the oviduct of the laying hen. J Histochem Cytochem 23: 111–116.
Piazolo P, Hotz J, Hehnike K, Franz HE, Schleyer M 1975 Calcium-binding protein in the duodenal mucosa of uremic patients and normal subjects. Kidney Int 8: 110–118.
Taylor AN 1981 Immunocytochemical localization of the vitamin-D induced calcium-binding protein: relocalization of antigens during frozen section processing. J Histochem Cytochem 29: 65–73.
Staun M, Früs S, Dabelsteen E, Hansen GH 1989 Immunomicroscopic localization of the 10,500 molecular weight calcium-binding protein in the human enterocyte. APMIS 97: 901–907.
Marche P, Cassier P, Mathieu H 1980 Intestinal calcium-binding protein. A protein indicator of enterocyte maturation associated with the terminal web. Cell Tissue Res 212: 63–72.
Marche P, Le Guern C, Cassier P 1979 Immunocytochemical localization of a calcium-binding protein in the rat duodenum. Cell Tissue Res 197: 69–77.
Stumpf WE, Sar M, Reid FA, Tanaka Y, DeLuca HF 1979 Target cells for 1,25-dihydroxyvitamin D3 in intestinal tract, stomach, kidney, pituitary and parathyroid. Science 206: 1188–1190.
Shinki T, Takahashi N, Kawate N, Suda T 1982 The possible role of calcium-binding protein in duced by 1,25-dihydroxyvitamin D3 in the intestinal calcium transport mechanism. Endocrinology 111: 1545–1551.
Halloran BP, DeLuca HF 1981 Appearance of the intestinal cytosolic receptor of 1,25-dihydroxyvitamin D3 during the neonatal development in the rat. J Biol Chem 256: 7338–7342.
Huang YC, Lee S, Stoltz R, Gabrielides C, Pansini-Porta A, Bruns ME, Bruns DE, Miffins TE, Pike WJ, Christakos S 1989 Effects of hormones and development on the expression of the rar 1,25-dihydroxyvitamin D3 receptor gene. Comparison with calbindin gene expression. J Biol Chem 264: 17454–17461.
Duncan WE, Walsh PG, Kowalski MA, Haddad JG 1984 Ontogenesis of the rabbit intestinal receptor for 1,25-dihydroxyvitamin D3-evidence for increased receptor content during late suckling and lactating periods. Comp Biochem Physiol 78A: 333–336.
Lointier P, Meggouh F, Dechelotte P, Pezet D, Ferrier Ch, Chipponi J, Saez S 1991 1,25-Dihydroxyvitamin D3 receptors and human colon adenocarcinoma. Br J Surg 78: 435–439.
Acknowledgements
The authors thank Dr. Michael Staun from the Medical Department (Gastroenterology) Rigshospitalet, Copenhagen, Denmark, for his generous gift of the MAb against human calcium-binding protein, and Dr. John Krisinger from the Department of Obstetrics and Gynecology, University of British Columbia, Vancouver, Canada, for kindly providing the full-length cDNA encoding the human 9-kD calbindin. We gratefully acknowledge the expert technical contribution of Lina Corriveau in setting up the organ cultures.
Author information
Authors and Affiliations
Additional information
Supported by the Medical Research Council of Canada (MT 11709 to E.E.D.) and le Centre de recherche de l'Hôpital Sainte-Justine.
Rights and permissions
About this article
Cite this article
Delvin, E., Lopez, V., Levy, E. et al. Developmental Expression of Calcitriol Receptors, 9-Kilodalton Calcium-Binding Protein, and Calcidiol 24-Hydroxylase in Human Intestine. Pediatr Res 40, 664–670 (1996). https://doi.org/10.1203/00006450-199611000-00004
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1203/00006450-199611000-00004