Abstract
Our aim was to determine the effects of two commonly used sedatives, promethazine and diazepam, on arousal and cardiorespiratory responses to airflow obstruction in sleeping lambs. In eight lambs fitted with obstructable rubber face masks, we recorded electrocortical, electroocular, and electromyographic activities to identify sleep-wake states; intrapleural pressure, heart rate, and percentage O2 saturation (Sao2) were also recorded. In each lamb, arousal and respiratory responses were measured after tidal airflow was obstructed during rapid eye movement (REM) and nonREM sleep. Each lamb was studied, on different days, when unsedated and after being mildly sedated with either promethazine or diazepam. Seven of the lambs were studied while sleeping after being sedated with promethazine (1.6± 0.07 mg/kg, orally with milk) and six were studied after sedation with diazepam (0.31 ± 0.03 mg/kg, intramuscularly). In unsedated lambs, airflow obstruction led to augmentation of respiratory efforts, bradycardia, hypoxemia, and arousal; in REM sleep, arousal was delayed and occurred at lower Sao2 (16 ± 3 s; 75.3 ± 3%) compared with nonREM sleep (8 ± 1 s; 90 ± 1%). Sedation increased the arousal latency in both REM and nonREM sleep and caused arousal to occur at lower Sao2; in some sedated lambs Sao2 fell to less than 30% before arousal. The augmentation of inspiratory and expiratory efforts immediately before arousal was increased after sedation. We conclude that promethazine and diazepam depress arousal responses in sleeping lambs leading to profound hypoxia, and that this may be due to impaired sensitivity to augmented respiratory efforts and other physiologic changes during airflow obstruction.
Similar content being viewed by others
Main
In infants, as in adults, the ability to arouse from sleep soon after the obstruction of respiratory airflow is essential for survival. Total or partial airflow obstruction may be involved in SIDS, as evidenced by the presence of intrathoracic petechiae at autopsy(1, 2). Obstruction of tidal airflow may occur at the level of the external nares, pharynx, or larynx; in all cases, airflow obstruction during sleep results in augmentation of inspiratory efforts and progressive hypoxemia, usually leading to arousal from sleep(3–5). Arousal is considered a necessary response to airflow obstruction to eliminate the cause of the obstruction(6).
The influence of sedating or tranquilizing drugs on the control of breathing and on respiratory defense mechanisms during infancy has received little attention. Phenothiazines, which are widely available as nonprescription (i.e. over the counter) sedatives, have been implicated in SIDS(7, 8) and in the generation of sleep apnea in normal infants(9–11). Phenothiazines have also been shown to exacerbate upper airway obstruction in children, leading to profound hypoventilation(12). Benzodiazepines are also reported to induce respiratory depression in neonates(11). We have previously found that, in awake lambs, diazepam sedation delayed respiratory and upper airway responses to nasal obstruction(13).
Little information is available on the extent of sedative usage during infancy. A recent Australian survey of sleep patterns of normal children up to 38 mo old revealed that sedatives were used in 4% of them(14); the greatest usage (7%) was among 13-18-mo-old infants, whereas 2% of infants younger than 3 mo received medication for sleep. In view of the reported use of sedatives during infancy and their possible effects on the control of respiration and arousal, we considered that a study of the effects of commonly used sedatives on responses to airflow obstruction was warranted. We have chosen to study promethazine, a widely available phenothiazine-based drug, and diazepam, a commonly used benzodiazepine drug. Our aim was to determine the effects of these drugs on arousal and cardiorespiratory responses of normal sleeping lambs during early postnatal development. The doses that we have used were chosen to induce mild sedation and to be similar to those used in humans.
In our study, respiratory airflow was obstructed a number of times during identified sleep states on the same day. As it has been shown that repeated airway obstruction and arousal in sleeping animals may lead to alterations in arousal responses(15, 16), we have also investigated the influence of repeated airflow obstructions and arousal on responses to airflow obstruction.
METHODS
Surgical preparation. Eight lambs were anesthetized (2-3% halothane in O2/N2O) and underwent aseptic surgery 1-4 (2.0± 0.4, mean ± SEM) d after term birth for the implantation of sensing devices. Three pairs of stainless steel electrodes (AS 632, Cooner, Chatsworth, CA) were used to determine sleep-wake states(17); one pair was implanted bilaterally over the parietal cortex to record the ECoG, another pair was implanted at the inner and outer canthi of one eye to record the EOG, and a third pair was sewn bilaterally into the nuchal muscles to record the EMGnuch. A catheter was introduced into a carotid artery for blood sampling, and a saline-filled latex balloon (1-2 mL), was introduced into the pleural cavity via a mid-thoracic incision for measurement of changes in Ppl to provide an index of inspiratory and expiratory efforts. Electrode leads and catheters were tunneled s.c., exiting over the mid-thoracic spine. Externally, electrodes and catheters were sutured to the skin and placed in a bag held in place beneath elastic netting around the animal's trunk. After recovery from surgery, lambs were returned to their mothers and were raised by them. Lambs and ewes were kept in a 12 h(7:00-19:00 h) light-dark cycle which was reversed so that they were studied during their hours of darkness; this was done to facilitate lambs sleeping during the study period. Lambs received antibiotics (procaine penicillin, 200 mg; dihydrostreptomycin, 250 mg, intramuscularly) on the day of surgery and for 3 d afterward.
Experimental protocol. At least 1 d of postsurgical recovery(usually 2 d) was allowed before the lambs were studied. Lambs were aged 2-26 d at the time of studies; these were conducted between 10:00 and 17:00 h to reduce possible effects of circadian rhythms. Before each study, body weight was measured and arterial blood sampled to determine blood gas status. Wool was clipped from the snout and a rubber mask (Kruuse, Denmark, catalog no. 271670, 50-mm diameter) was fixed in place with rapid-setting dental molding material (vinyl polysiloxane, Reprosil; Dentsply, York, PA). To effect airflow obstruction, the opening of the face mask was obstructed remotely by inflating a balloon connected via a catheter to an air-filled syringe. Lambs were typically studied over 3-4 h during which time multiple airflow occlusion trials were performed. At the completion of each study, the face mask was easily and painlessly removed.
Lambs were studied while resting in a prone position in a canvas sling in a dimly lit sound-attenuating chamber. A video system was used to observe the lamb's head. The chamber temperature was 22-23 °C, which is within the thermoneutral range for lambs up to 1 mo of age(18). Ppl was monitored by connecting the intrapleural balloon to a saline-filled pressure transducer held at the mid-thoracic level. A pulse oximeter (N200; Nellcor, Pleasanton, CA) was attached to the shaved tail for the continuous measurement of Sao2, and HR. Signals were displayed and recorded using a polygraph and data logging equipment (Maclab, ADI, Australia).
On separate days, and in a random sequence, lambs were either studied unsedated (n = 8 lambs) or after administration of either promethazine syrup (Phenergan, Wyeth, Santa Barbara, CA, given orally with milk, n = 7 lambs) or diazepam (Valium, Roche, Nutley, NJ, intramuscularly, n = 6 lambs). Five of the eight lambs received both drugs (on different days), two received only promethazine, and one received only diazepam. Doses of sedating drugs were based on doses given to human subjects, but were varied to achieve evidence of mild sedation in the conscious lambs. Doses were adjusted to cause a reduction in vigorous motor activity and vocalization; lambs were, however, still able to stand and walk. The mean dose of promethazine was 1.6 ± 0.07 (range 0.7-2.5) mg/kg and that of diazepam was 0.31 ±.03 (range 0.20-0.48) mg/kg. Studies on unsedated lambs were conducted at 9 ± 1 (2-19) d; promethazine was used at 11 ± 1 (3-21) d and diazepam at 15 ± 1 (6-26) d. At least 2 d were allowed between studies in which promethazine was used, owing to the possibility of delayed clearance(9); the mean interval between trials was 3.0 ± 0.4 d. At the start of each study there was no evidence of sedation in any lamb. On each study day, responses to airflow obstruction were recorded repeatedly during identified sleep states(5). No attempt was made to obstruct airflow at a particular phase of the respiratory cycle, and the obstruction was maintained until arousal occurred.
Determination of sleep states and arousal. The awake state was recognized by a low voltage ECoG, open eyes, tonic EMGnuch activity, and the presence of body movements. REM sleep was recognized by a low voltage ECoG and the absence of tonic EMGnuch activity. The eyes were closed, but the EOG indicated the presence of REM; occasional twitches of the ear, face, and limb movements were present, and respiration was irregular. Arousal from REM sleep was recognized by the reappearance of sustained EMGnuch activity (often with eye movements and eye opening) with a continued low voltage ECoG(Fig. 1). NonREM sleep was identified by high voltage ECoG activity, tonic EMGnuch activity, closed eyes, and no REM; there were no body movements, except for occasional startles. Arousal from nonREM sleep was identified by a sudden reduction in the voltage of the ECoG with continued or increased EMGnuch activity and appearance of eye movements(Fig. 1). Recognition of arousal was aided by video observations.
Polygraph recordings from an unsedated 15-d-old lamb showing responses to airflow obstruction in REM and nonREM sleep. The periods of obstruction are indicated by the horizontal bars and arousal is indicated by arrows; the duration of the obstruction periods was 39 s (REM sleep) and 15 s (nonREM sleep). Tracings, from above, are: ECoG, EOG, Nuchal EMG, time average of bilateral EMG of the dorsal neck (nuchal) muscles in arbitrary units; Sao2, percent saturation of Hb with oxygen; and Ppl. The chart speed was reduced near the end of each recording.
Data analysis. During the 30-s preobstruction (control) period, we measured Sao2, HR, Ppl fluctuations, and fR. During periods of airflow obstruction we measured the interval between the onset of obstruction and arousal (arousal latency), the number of breaths between the onset of obstruction and arousal, Sao2 and HR at the time of arousal, and minimal and maximal Ppl generated during the last two inspiratory and expiratory efforts before arousal. Using a two-way (repeated factor) analysis of variance, data were analyzed to determine whether respiratory and arousal responses in REM and nonREM sleep were altered by sedation with promethazine or diazepam; factors were drug (i.e. diazepam and promethazine) and sleep state (i.e. REM and nonREM sleep). If a significant difference(p < 0.05) was found between variables in sedated and unsedated lambs, a least significant difference test was used to determine the difference between individual means. The results are expressed as mean± SEM. Only significant differences are reported. The influence of repeated airflow obstruction on arousal responses during the same study session was analyzed in data from five lambs (3-23 d old) using a pairedt test. Only those study sessions that had at least five obstructions in each sleep state were analyzed. We tested whether respiratory and arousal responses to the first two obstructions in REM and nonREM sleep during each study session were different from responses to the last two obstruction trials.
Experimental procedures were approved by the Monash University Committee for Ethics in Animal Experimentation.
RESULTS
The birth weight of lambs (4.1 ± 0.2 kg) indicates that they were normally grown as fetuses, and their rate of postnatal weight gain (0.21± 0.02 kg/day) indicates that they grew normally after birth. Arterial pH and gas tensions at the time of experimentation were: pH, 7.40 ± 0.01, Pco2, 41.4 ± 0.8 mm Hg, Po2, 102.1 ± 2.2 mm Hg.
Effects of sedation on cardiorespiratory variables during the preobstruction period in REM and nonREM sleep. Both drugs reduced the latency for sleep onset after the lambs had been prepared for study and were allowed to sleep; unsedated lambs commenced sleep in 48 ± 10 min, whereas after diazepam and promethazine administration lambs commenced sleep after 15 ± 6 min and 16 ± 5 min, respectively (both p< 0.05). Cardiorespiratory variables during REM and nonREM sleep in unsedated and sedated sleeping lambs are shown in Table 1. Breathing frequency was not significantly affected by sleep states or sedation. Sao2 was lower in REM sleep than in nonREM sleep; this difference was maintained after diazepam sedation, but eliminated after promethazine sedation. In both unsedated and sedated lambs, HR was lower in REM sleep than in nonREM sleep. Lambs sedated with promethazine had a higher HR than unsedated lambs in both sleep states; this effect was not observed after administration of diazepam. In lambs treated with promethazine, inspiratory deflections in Ppl during REM sleep were smaller than in nonREM sleep, both before and after sedation. This difference was not observed in the diazepam-treated lambs, although the same trend was evident.
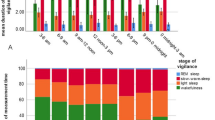
Responses to airflow obstruction in eight unsedated lambs. In both REM and nonREM sleep, airflow obstruction resulted in reductions in Sao2, HR, and fR and increases in respiratory efforts before arousal (Figs. 1–3). In the absence of sedation, arousal latencies were greater during REM sleep (16 ± 3 s) than in nonREM sleep (8 ± 1 s, p < 0.05), and there were greater falls in Sao2 in REM (20 ± 3%) than in nonREM sleep (8 ± 1%, p < 0.01). At the time of arousal, Sao2 was 75± 3% (range 38-95%) in REM sleep and 90 ± 1% (range 59-98%) in nonREM sleep (p < 0.01). We measured the two lowest Sao2 values at arousal for each lamb; mean values were 61 ± 5% in REM sleep and 80 ± 3% in nonREM sleep.
Arousal and cardiorespiratory responses of six lambs(aged 15 ± 1 d, range 6-26 d), under control, unsedated conditions(CON) and after sedation with diazepam (DIAZ). See text for details. Levels of significance are indicated as in Fig. 2.
HR at arousal was lower in REM sleep (151 ± 7 beats/min) than in nonREM sleep (181 ± 8 beats/min, p < 0.01), but the reductions in HR that occurred during airflow obstruction were not different between sleep states. The number of obstructed respiratory efforts before arousal was greater in REM sleep (8 ± 2) than in nonREM sleep (4± 1, p < 0.05), whereas the magnitudes of maximal inspiratory and expiratory efforts immediately preceding arousal did not differ between sleep states (Figs. 2 and3).
Effect of promethazine on respiratory and arousal responses to airflow obstruction. We compared responses to airflow obstruction in seven lambs during REM and nonREM sleep after promethazine sedation with those of the same lambs sleeping in the absence of sedation (Fig. 2). After administration of promethazine, arousal latencies in both REM (20± 4 s) and nonREM sleep (13 ± 2 s) were greater than in unsedated lambs (REM, 16 ± 4 s, p < 0.01; nonREM, 8± 2 s, p < 0.01). In accordance with the longer arousal latencies in sedated lambs there were greater falls in Sao2 in both REM(27 ± 4%) and nonREM sleep (15 ± 1%) than in unsedated lambs(REM, 20 ± 4%, p < 0.01; nonREM, 8 ± 1%,p < 0.01). At the time of arousal, Sao2 had fallen to 69± 4% (range 24-96%) in REM sleep and 82 ± 1% (range 35-97%) in nonREM sleep in sedated lambs; these values were lower than those in the same lambs when unsedated (REM, 75 ± 4%, p < 0.01; nonREM, 90± 1%, p < 0.01). The two lowest Sao2 values at arousal for each lamb were 51 ± 7% in REM sleep and 66 ± 6% in nonREM sleep.
In sedated lambs, bradycardias before arousal were greater in both REM (32± 8 beats/min) and nonREM sleep (29 ± 6 beats/min) than in unsedated lambs (REM, 16 ± 6 beats/min, p < 0.05; nonREM, 17 ± 3 beats/min, p < 0.05). In REM sleep the maximal inspiratory efforts immediately before arousal were greater in sedated lambs(5.7 ± 1.6 mm Hg) than in unsedated lambs (4.3 ± 1.4 mm Hg,p < 0.01) but there was no difference in nonREM sleep. After promethazine, the maximal expiratory efforts before arousal were greater in both REM (3.2 ± 0.9 mm Hg) and nonREM sleep (4.1 ± 0.4 mm Hg) than in unsedated lambs (REM, 2.4 ± 0.6 mm Hg, p < 0.05; nonREM 2.7 ± 0.6 mm Hg, p < 0.05).
Effect of diazepam sedation on respiratory and arousal responses to airflow obstruction. Responses to airflow obstruction in six lambs during REM and nonREM sleep in diazepam-sedated lambs were compared with those of the same lambs when unsedated (Fig. 3). After diazepam sedation, arousal latencies in both REM (30 ± 8 s) and nonREM sleep (23± 6 s) were greater than in the absence of sedation (REM, 18 ± 4 s, p < 0.05; nonREM, 9 ± 2 s, p < 0.05). In REM sleep in the presence of diazepam the reduction in Sao2 (32± 7%) tended to be greater than in the absence of sedation (21 ± 5%, p < 0.2); the mean Sao2 at arousal in REM sleep was 65± 7% (range, 22-90%) after diazepam and 76 ± 4% (range, 38-95%) in its absence (p < 0.2). In accordance with the longer arousal latencies in nonREM sleep induced by diazepam, there were greater falls in Sao2 in sedated (22 ± 4%) than unsedated lambs (7 ± 1%,p < 0.05); Sao2 at arousal was 76 ± 4% (range, 30-96%) in the presence of diazepam and 91 ± 1% (59-98%) in its absence(p < 0.05). The two lowest Sao2 values at arousal for each lamb were 50 ± 9% in REM sleep and 49 ± 7% in nonREM sleep.
After diazepam administration, the bradycardia during airflow obstruction was greater in both REM (40 ± 9 beats/min) and nonREM sleep (35± 6 beats/min) than in unsedated lambs (REM, 16 ± 6 beats/min,p < 0.05; nonREM 17 ± 3 beats/min, p < 0.05) The inspiratory efforts immediately preceding arousal were greater in sedated lambs in both REM (8.3 ± 1.2 mm Hg) and nonREM sleep (8.5 ± 1.7 mm Hg) than in unsedated lambs (REM, 4.9 ± 1.4 mm Hg, p < 0.05; nonREM, 4.8 ± 1.1 mm Hg, p < 0.05). During diazepam sedation, expiratory efforts before arousal from nonREM sleep were greater(5.6 ± 1.1 mm Hg) than in unsedated lambs (2.6 ± 0.7 mm Hg,p < 0.05), but there was no difference in REM sleep(Fig. 3).
Influence of repeated upper airway obstruction on arousal responses. We compared respiratory and arousal responses at the beginning and end of a series of episodes of airflow obstruction. No significant changes in cardiorespiratory parameters or arousal latencies were found in unsedated or sedated lambs. The greatest changes were seen in REM sleep after diazepam administration, but these did not reach significance; repeated airflow obstructions resulted in the mean arousal latency tending to increase from 27± 10 s to 36 ± 9 s (p = 0.1) and the mean Sao2 at arousal tending to decrease from 67 ± 10% to 56 ± 6%(p = 0.06).
DISCUSSION
The major finding of this study was that mild sedation of lambs with promethazine or diazepam caused a marked increase in the latency of arousal from sleep in response to airflow obstruction. With both drugs, this effect was seen in REM and nonREM sleep. Consequently, during periods of airflow obstruction, lambs became profoundly desaturated before arousal, more so than in the absence of sedation; in some sedated lambs, Sao2 fell to less than 30% before arousal occurred. Sedation of sleeping neonates is likely to impair arousal responses other than those induced by airway obstruction; indeed, a recent study has shown that promethazine depresses arousal and swallowing responses in newborn piglets after infusion of liquids into the pharynx(19). These findings are consistent with data indicating that the sedation of infants increases the duration of spontaneous apnea during sleep(9–11) and may increase the risk of SIDS(7, 8).
Although it is difficult to quantify the degree of sedation induced by the drugs used in our study, it was considered to be mild in that lambs were still able to stand, walk, and vocalize after drug administration. After sedation, lambs began to sleep earlier than when unsedated and apparently slept for a greater proportion of the study period. Lambs appeared to be behaviorally normal before the onset of successive trials; however, although we allowed, on average, 3 d between trials, it is possible that circulating concentrations of sedatives or their metabolites had not returned to zero.
Airflow obstruction induces a wide range of physiologic changes, many of which have been shown to be capable of causing arousal from sleep. These include hypoxia, via stimulation of peripheral chemoreceptors(20), hypercapnia(21), and hypertension via stimulation of carotid baroreceptors(22, 23). Although we did not measure arterial pressure or Pco2, it is likely that each of these increased during periods of airflow obstruction in our lambs. Bradycardia was particularly marked during airflow obstruction, with HR falling by up to 40 beats/min in REM sleep; however, we know of no information on the ability of bradycardiaper se to induce arousal.
It is not clear which of the numerous physiologic changes during airflow obstruction led to arousal. Increased respiratory efforts may contribute to the arousal response because measurements of inspiratory efforts in unsedated sleeping lambs in this and a related study(5) and in a study of adult humans(24) have indicated that arousal occurs when obstructed respiratory efforts reach a critical level. A similar conclusion was reached in relation to the initiation of oral breathing in lambs in response to airway obstruction(25). It is likely that airway receptors responding to changes in luminal pressure mediate these responses(26); such receptors may be located in the upper(27) or lower(25) airways. In our sedated lambs, the magnitude of both inspiratory and expiratory efforts before arousal were increased in comparison to values in the same lambs when unsedated, indicating that the threshold for arousal by this stimulus may have been raised centrally.
Both promethazine and diazepam, as well as other sedatives or anesthetics, are known to depress respiration(28), which could have contributed to the delayed arousal responses in our study. At the doses used, neither promethazine nor diazepam depressed respiration under control conditions (i.e. in the absence or airway obstruction), as indicated by the lack of an effect on fR, Ppl fluctuations, or Sao2; furthermore, the influence of sleep states on these measures of respiration was largely unaffected by sedation. It is possible, however, that sedation depressed the augmentation of respiratory efforts in response to airway obstruction, as sedation and anesthesia have been shown to reduce the inspiratory activity of the diaphragm and its innervation in response to respiratory challenges(29). If arousal is indeed stimulated by obstructed respiratory efforts, as suggested above, an impairment of the augmentation of respiratory efforts by sedation, in combination with a more generalized depression of central arousal mechanisms, could be responsible for delayed arousal in sedated animals.
As in previous studies of sleeping, unsedated lambs(5, 30, 31) and piglets(4, 32), we found that arousal was more delayed and occurred after a greater fall in Sao2 in REM sleep than in nonREM sleep. These differences were apparent in the promethazine studies, both before and after sedation. In the diazepam studies the state-related differences were less marked. The reason for the differences between the two studies is not known, but it could relate to the large variations in responses between trials. A feature of responses to airflow obstruction in all lambs was the large variability in arousal latencies and associated cardiorespiratory responses in both REM and nonREM sleep. This variability could have been due to inhomogeneities in sleep states or to differences in the phase of the respiratory cycle, and hence lung volume, at which airflow obstruction occurred.
In human infants, arousal responses to auditory stimuli(33) and respiratory responses to airway obstruction(34) have been reported to be affected by postnatal age. In a recent study of responses to airflow obstruction in unsedated sleeping lambs, we observed that, with increasing age, lambs became less arousable in REM sleep(5); a similar observation was made when arousal was stimulated in lambs by tracheal obstruction(35). In the present study, we did not observe an effect of postnatal age on the influence of sedatives on responses to airflow obstruction in either sleep state. Therefore, we do not consider that differences in the ages of unsedated and sedated lambs could have contributed to the differences observed.
In this study we wished to replicate as closely as possible the situation in the human infant, in which sedatives would most likely be administered in a bolus dose. Inherent in the design of the present experiments is that the circulating levels, and probably the brain tissue concentrations, of the sedating drugs would have been declining during the study period, owing to their metabolism or excretion. This may have caused the effects on arousal responses to change with time. The only changes that we observed in responses over time were trends for a small increase in arousal latency and for a decrease in Sao2 at arousal after diazepam administration; these nonsignificant effects were restricted to REM sleep. A potential limitation of our study design, which involved repeatedly arousing a sleeping animal, is that repeated arousal caused by upper airway obstruction(15) or hypoxemia(16, 36) can lead to impaired arousal responses in sleeping lambs, particularly in REM sleep(15, 36). Thus it is possible that the reduced effects of diminishing drug concentrations may have been partially offset by the opposite effects of sleep fragmentation and repeated hypoxia.
Our study has shown that mild sedation in sleeping young animals can profoundly impair arousal responses when respiratory airflow is impeded. In human infants, airflow obstruction could arise externally, as a result of compression of the nares or covering of the face with a material that impedes airflow; it could also occur internally, within the upper respiratory tract. Whatever the site of obstruction, it is likely that the probability of survival would be enhanced by arousal from sleep, leading to movement away from an external obstruction, or increased upper airway dilator muscle activity to overcome obstruction within the upper airway. In this regard, it is of relevance that the activation of upper airway muscles that are considered important for the maintenance of airway patency can be impaired by sedation(29, 37, 38). Any delay in arousal and the reestabishment of a patent airway, such as that induced by sedation, may lead to levels of hypoxia or asphyxia which result in tissue damage in the CNS or to death. Our findings lend support to recommendations that sedation in infancy should be avoided unless surveillance procedures are in place.
Abbreviations
- SIDS:
-
sudden infant death syndrome
- ECoG:
-
electrocorticogram
- EOG:
-
electrooculogram
- EMGnuch:
-
nuchal electromyogram
- Ppl:
-
intrapleural pressure
- Sao2:
-
arterial O2 saturation
- HR:
-
heart rate
- REM:
-
rapid eye movements
- fR:
-
frequency of respiration
References
Stewart S, Fawcett J 1985 Interstitial haemosiderin in the lungs of sudden infant death syndrome: a histological hallmark of“near-miss” episodes?. J Pathol 145: 53–58
Kleemann WJ, Wiechern V, Schuck M, Troger HD 1985 Intrathoracic and subconjunctival petechiae in sudden infant death syndrome(SIDS). Forensic Sci Int 72: 49–54
Fewell JE 1987 The effect of short-term sleep fragmentation produced by intense auditory stimuli on the arousal response to upper airway obstruction in lambs. J Dev Physiol 9: 409–417
Barrington KJ, Buckner PS 1991 Tracheal occlusion in the newborn piglet. Effects of sleep state and occlusion timing on arousal and respiratory responses. J Dev Physiol 16: 217–221
Harding R, Jakubowska AE, McCrabb GJ 1995 Postnatal development of responses to airflow obstruction. Clin Exp Pharmacol Physiol 22: 537–543
Remmers JE 1984 Obstructive sleep apnea. Am Rev Respir Dis 130: 153–155
Kahn A, Blum D 1982 Phenothiazines and sudden infant death syndrome. Pediatrics 70: 75–78
Degouffe M, Rice J 1982 Possible involvement of promethazine in a sudden unexpected infant death. Can Soc Forensic Sci 15: 166–168
Kahn A, Hasaerts D, Blum D 1985 Phenothiazine-induced sleep apneas in normal infants. Pediatrics 75: 844–847
Buck ML, Blumer JL 1991 Phenothiazine-associated apnea in two siblings. DICP Ann Pharmacother 25: 244–247
Boutroy M 1994 Drug-induced apnea. Biol Neonate 65: 252–257
Kravath RE, Pollack CP, Borowiecki B 1977 Hypoventilation during sleep in children who have lymphoid airway obstruction treated by nasopharyngeal tube and T and A. Pediatrics 59: 865–871
Wood GA, Harding R 1989 The effects of pentobarbitone, diazepam and alcohol on oral breathing in neonatal and mature sheep. Respir Physiol 75: 89–104
Armstrong KL, Quinn RA, Dadds MR 1994 The sleep patterns of normal children Med J A. ust 161: 202–205
Fewell JE, Williams BJ, Szabo JS, Taylor BJ Influence of repeated upper airway obstruction on the arousal and cardiopulmonary response to upper airway obstruction in lambs. Pediatr Res 1988; 23: 191–195
Fewell JE, Konduri GG 1989 Influence of repeated exposure to rapidly developing hypoxaemia on the arousal and cardiopulmonary response to rapidly developing hypoxaemia in lambs. J Dev Physiol 11: 77–82
Harding R, Johnson P, McClelland ME 1980 Respiratory function of the larynx in developing sheep and the influence of sleep state. Respir Physiol 40: 165–179
Symonds ME, Andrews DC, Johnson P 1989 The control of thermoregulation in the developing lamb during slow wave sleep. J Dev Physiol 11: 289–298
Wright SJ, McKelvey GM, Wood AKW, Jeffery HE 1995 The effects of sedation on airway protection and oesophageal motility in neonatal piglets. Proc Aust Physiol Pharmacol Soc 26: 171P( abstr)
Fewell JE, Taylor BJ, Kondo CS, Dascalu V, Filyk SC 1990 Influence of carotid denervation on the arousal and cardiopulmonary responses to upper airway obstruction in lambs. Pediatr Res 28: 374–378
Fewell JE, Baker SB 1989 Arousal and cardiopulmonary responses to hyperoxic hypercapnia in lambs. J Dev Physiol 12: 21–26
Fewell JE, Johnson P 1984 Acute increases in blood pressure cause arousal from sleep in lambs. Brain Res 311: 259–265
Horne RSC, De Preu ND, Berger PJ, Walker AM 1991 Arousal responses to hypertension in lambs: effect of sinoaortic denervation. Am J Physiol 260:H1283–H1289
Gleeson K, Zwillich CW, White DP 1990 The influence of increasing ventilatory effort on arousal from sleep. Am Rev Respir Dis 142: 295–300
Harding R, Hooper SB, Wood GA 1991 Initiation of oral breathing in lambs in response to airway obstruction: mechanisms. J Appl Physiol 71: 1574–1580
Puddy A, Giesbrecht G, Sanii R, Vounas M 1992 Mechanisms of detection of resistive loads in conscious humans. J Appl Physiol 72: 2267–2270
Basner RC, Ringler J, Garpestad E, Schwartzstein RM, Sparrow D, Weinberger SE, Lilly J, Weiss JW 1992 Upper airway anesthesia delays arousal from airway occlusion induced during human NREM sleep. J Appl Physiol 73: 642–648
Catchlove RFH, Kafer ER 1971 The effects of diazepam on respiration in patients with obstructive pulmonary disease. Anesthesiology 34: 14–18
Hwang J, St.John WM, Bartlett D 1983 Respiratory-related hypoglossal nerve activity: influences of anesthetics. J Appl Physiol 55: 785 792
Henderson-Smart DJ, Read DJC 1976 Depression of respiratory muscles and defective responses to nasal obstruction during active sleep in the newborn. Aust Paediatr J 12: 261–266
Issa FQ, McNamara SG, Sullivan CE 1987 Arousal responses to airway occlusion in sleeping dogs: comparison of nasal and tracheal occlusions. J Appl Physiol 62: 1832–1836
Galland BC, Wehner NS, Bolton DPG, Taylor BJ 1996 Arousal responses of the newborn piglet to airways obstruction and rebreathing during normothermia and hyperthermia. Reprod Fertil Dev 8: 365–371
Newman NM, Trinder JA, Phillips KA, Jordan K, Cruickshank J 1989 Arousal deficit: mechanism of the sudden infant death syndrome?. Aust Paediatr J 25: 196–201
Frantz ID, Adler SM, Abrams IF, Thach BT 1976 Respiratory response to airway occlusion in infants: sleep state and maturation. J Appl Physiol 41: 634–638
Davidson TL, Fewell JE 1995 Arousal response from sleep to tracheal obstruction in lambs during postnatal maturation. Pediatr Res 36: 501–505
Johnston RV, Grant DA, Wilkinson MH, Walker AM 1995 Arousal and respiratory responses to repetitive hypoxia during sleep in newborn lambs. Proc Aust SIDS Conf A26( abstr)
Nishino T, Shirahata M, Yonezawa T, Honda Y 1984 Comparison of changes in the hypoglossal and the phrenic nerve activity in response to increasing depth of anesthesia in cats. Anesthesiology 60: 19–24
Bonora M, St. John WM, Bledsoe TA 1985 Differential elevation by protryptyline and depression by diazepam of upper airway respiratory motor activity. Am Rev Respir Dis 131: 41–45
Acknowledgements
The authors acknowledge the excellent technical assistance of Kerryn Westcott.
Author information
Authors and Affiliations
Additional information
Supported by the Queensland Sudden Infant Death Foundation and the National Health and Medical Research Council of Australia.
Rights and permissions
About this article
Cite this article
Jakubowska, A., McCrabb, G. & Harding, R. Influence of Sedation on Arousal and Cardiorespiratory Responses to Airflow Obstruction in Sleeping Lambs. Pediatr Res 40, 564–570 (1996). https://doi.org/10.1203/00006450-199610000-00009
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1203/00006450-199610000-00009