Abstract
The production of IL-10 by human neonatal blood mononuclear leukocytes(BML) stimulated with lipopolysaccharide (LPS), tumor necrosis factor-α(TNF-α), antibodies to CD3, or phorbol 12-myristate 13-acetate (PMA) was measured. The production of IL-10 by neonatal BML cultured with LPS or TNF-α was ≈20 and ≈15%, respectively, of adult BML. The combination of human recombinant TNF-α and LPS failed to augment IL-10 production in neonatal BML. The decreased production of IL-10 by neonatal leukocytes was not due to an autocrine feedback mechanism because only low concentrations of IL-10 were found in newborn sera. A connection with TNF-α could not be ruled out, because TNF-α production by LPS-stimulated newborn BML and the expression of TNF-α receptors on newborn monocytes were reduced. Mean ± SD of concentrations of IL-10 in supernatants from adult and neonatal BML after stimulation with antibodies to human CD3 for 48 or 72 h were 914 ± 386 and 178 ± 176 pg/mL, respectively (p < 0.0001). In experiments with enriched populations of neonatal T cells, the addition of PMA failed to augment IL-10 production. This suggested that newborn T cells may be in a different state of activation than adult T cells Thus, IL-10 production in neonatal monocytes and T cells is reduced and this study suggests that the reduction may be secondary in part to regulatory processes involving TNF-α and its receptors.
Similar content being viewed by others
Main
Human infants display an increased susceptibility to infections in the first few months of life, due largely to delays in the development of the immune system including neutrophil functions(1), complement components(2), macrophage activation by interferon-γ(3), usage of Ig-variable region genes(4), IgG antibody formation to T cell-independent immunogens(4, 5), the production of secretory IgA(6, 7), and the formation of T cells that display the memory phenotype, CD45RO(8–11), Also, BML from human newborns produce lower amounts of the cytokines lymphotoxin(12), granulocyte/macrophage colony-stimulating factor(13), granulocyte colony-stimulating factor(14), IL-3(14), IL-4(15), interferon-γ(15, 16), and possibly IL-6(17–19) and TNF-α(12, 20).
Despite in vitro deficiencies in production of certain proinflammatory cytokines by neonatal blood leukocytes, increased plasma concentrations of TNF-α, IL-1β, and IL-6 occur in septic neonates(21), which contribute to their increased morbidity and mortality. In addition, in two disorders restricted to newborns, bronchopulmonary dysplasia(22) and NEC(23), high blood concentrations of proinflammatory cytokines TNF-α and platelet-activating factor occur(24, 25). Furthermore, increases of a third proinflammatory cytokine, IL-8, occur in tracheal aspirates from infants with respiratory distress syndrome and bronchopulmonary dysplasia(26).
These observations raised the possibility of developmental delays in the production of antiinflammatory cytokines. IL-10 was a likely candidate for the following reasons. 1) It inhibits the production of several proinflammatory cytokines including IL-1, IL-8, and TNF-α(27–36).2) This cytokine limits the participation of T helper-1 cells in delayed hypersensitivity(29, 30, 36).3) IL-10 promotes the expression of T helper-2 cell responses that enhance the synthesis of Igs, such as IgA, that protect without provoking inflammation(35–40).4) There is also in vivo evidence that IL-10 protects against inflammation. Lethal experimental endotoxemia is prevented by parenteral administration of IL-10, and the protection is abrogated by injecting antibodies to IL-10(41). Moreover, the protection due to IL-10 correlates with a decreased production of TNF-α(41). In addition, it has been suggested that the early production of IL-10 in septic adults might control the proinflammatory cytokines associated with shock found in these infected patients(42).
A second animal model that highlights the antiinflammatory properties of IL-10 is the homozygous IL-10 null-gene mouse. These animals develop a lethal enterocolitis, which is initiated and perpetuated by enteric microflora and which resembles NEC(43). The findings suggest the enterocolitis is due to an overproduction of proinflammatory cytokines. In that respect, administration of IL-10 improves the enterocolitis in this genetically engineered disease(43).
A final reason to hypothesize a developmental delay in the production of IL-10 was our recent discovery of IL-10 in human milk(44). Because of the inverse relationship between previously discovered defense factors in human milk and the production of those factors by the recipient infant(45), one would predict that the production of IL-10 by the newborn infant would be much less than that of older individuals. Hence, the current study was designed to test whether the production of IL-10 by neonatal blood monocytes and T cells is delayed.
METHODS
Human research assurances-subject selection. The use of human subjects was approved by the Institutional Review Board for research with human subjects at the University of Texas Medical Branch at Galveston. The subjects were healthy adult volunteers between the ages of 18 and 45 y and healthy full-term infants with uncomplicated antenatal and early neonatal periods. Infants with suspected sepsis or other diseases or whose mothers had a recent acute or a chronic systemic illness during pregnancy, labor, or the immediate postpartum period were excluded.
Collection/preparation of BML. BML were harvested from heparinized adult venous blood or umbilical cord venous blood by Ficoll-hypaque density gradient centrifugation. The cells were resuspended in RPMI 1640 and counted in a hemocytometer.
T cell enrichment. T cells were enriched by depleting monocytes and NK cells from certain preparations of BML. Isolated BML were resuspended at a concentration of 1.0 × 106 cells/mL of D-PBSE/CMF containing 0.5% γ-globulin to prevent nonspecific binding, and the cell suspensions were incubated for 15 min at room temperature. Then MAbs against human CD14 and CD56 were added (Becton-Dickinson, Mountain View, CA) at a concentration of 0.25 μg of antibody/106 cells, and the mixture was incubated at room temperature for 20 min. The samples were then washed and counted. Samples in which there were less than 5 × 106 cells were discarded.
The depletions were accomplished with an AIS MicroCEL-Lector goat anti-mouse T-25 cell culture flask system (AIS, Santa Clara, CA). The flasks were coated with 10 mL of D-PBSE/CMF for 1 h before use. The cells, suspended in 4 mL of D-PBSE/CMF with 0.5% γ-globulin, were incubated in the MicroCELLector T-25 flask for 1 h. Nonadherant cells were then gently removed, and viability was determined by trypan blue exclusion. Samples with less than 95% cell viability were discarded. Immunofluorescence/flow cytometry was used to determine the percentages of T lymphocytes, NK cells, and monocytes in the BML preparations, both before and after depletion. Samples in which there was less than 90% depletion of CD14 and CD56 cells were discarded. Acceptable samples were then used for the anti-CD3 and PMA stimulation experiments.
Characterization of blood mononuclear populations. The percentages of T cells and monocytes in the BML preparations were determined by immunofluorescence/flow cytometry(46). Cells were incubated either with isotype-specific polyclonal antibodies or with MAb(Becton-Dickinson, Franklin Lakes, NJ) to CD45 (common leukocyte antigen), CD14 (monocyte/macrophage marker), CD3 (pan-mature T cell marker), CD4 and CD8(markers of T cell subpopulations), or CD16 and CD56 (NK cell markers). The concentrations of the antibodies ranged from 0.5 to 5 μg/mL. The antibodies were conjugated to FITC, phycoerythrin, or peridinin chlorophyll protein. The preparations were then analyzed by multicolor flow cytometry using an instrument equipped with a 15-mW argon-ion laser tuned to 488 nm(Becton-Dickinson FACScan). In analysis of single-color and two-color flow cytometric data, an electronic gate was set on the cell population based on the forward-angle versus the right-angle scatter. Then quadrant markers were set using matched isotype controls. Becton-Dickinson CaliBRITE beads were run before analysis to monitor instrument performance and to set detector levels for the forward and right-angle light scatter and the fluorescence 1 and 2 channels. To compensate for spectral overlap of fluorescent dyes, the detectors were optimized. Three-color flow cytometry was performed as previously described(11, 46).
Stimulation of BML. BML were cultured either with LPS(Escherichia coli 0111:B4, Difco Laboratories, Detroit, MI), human recombinant TNF-α (Sigma Chemical Co., St. Louis, MO)(47), TNF-α and/or LPS, murine MAb to human CD3(OKT-3) prepared in our laboratory, PMA (Sigma Chemical Co.), or anti-CD3 and PMA.
The LPS stimulation experiments were as follows. Adult or newborn BML in RPMI 1640 and 10% FCS were plated into 96-well cell culture plates (2 × 105 cells/well) with or without LPS (concentrations, 250, 500, or 1000 ng/mL). Cell viability was determined by trypan blue exclusion; experiments in which trypan blue exclusion was <95% were discarded. Each sample was run in duplicate. The plates were incubated at 37 °C with 7% CO2 for 24 h. The supernatants were then harvested and stored at -60 °C until IL-10 was quantified.
The TNF-α experiments were the same as those described for LPS preparations. Concentrations of TNF-α used were 1.25, 2.5, 5.0, or 10.0 ng/mL. In some experiments BML were stimulated with both agents.
Anti-CD3 experiments were carried out in duplicate. The optimal dose of anti-CD3 (0.025 μg/mL) was determined by dose response experiments (data not shown). Mononuclear leukocytes from adult or umbilical cord venous blood or aliquots of those cells enriched for T cells were suspended in a medium containing RPMI 1640 and 10% FCS and were plated into 96-well plates (2× 105 cells/well) previously coated with or without anti-CD3. The plates were incubated at 37 °C in 7% CO2 for 48 or 72 h. Supernatants were harvested and stored at -60 °C until IL-10 was quantified.
In the anti-CD3-stimulated preparations, cell viability was checked by trypan blue exclusion and by quantifying the incorporation of[3H]thymidine. Trypan blue exclusion was consistently >95% in all samples. After 48 or 72 h in culture, the cells were incubated with[3H]thymidine (Amersham Corp., Arlington Heights, IL; 0.3 mCi/well) for 4 h and harvested. The degree of [3H]thymidine incorporation was determined by scintillation spectroscopy (model LS 3801, Beckman Instruments, Inc., Fullerton, CA). Experiments in which the increase in the incorporation of [3H]thymidine was less than 4-fold were discarded, because cell viability may not have been optimal in such preparations.
The PMA experiments were performed on BML that were and were not depleted of monocytes and NK cells. The conditions of the experiments were similar to those described for anti-CD3-stimulated cells. Some preparations were stimulated with PMA (2.0 ng/mL) alone, whereas others were stimulated with a combination of anti-CD3 and PMA.
IL-10 production by BML. IL-10 production by BML was quantified by a sandwich ELISA method, which used capture rat MAb to human recombinant IL-10 (IgG1 isotype clone JES 3-9D7; Pharmigen, San Diego, CA) and detector-biotinylated rat MAb (IgG2 isotype clone JES-12G8, Pharmigen, San Diego, CA). Streptavidin-horseradish peroxidase (Sigma Chemical Co.) was used as the second step conjugate (1:400 dilution). Plates coated with 3μg/mL rat anti-human IL-10, followed by 100 μL of the specimen and the addition of 2 μg/mL biotinylated antibody produced optimal results. Reactions were carried out in microtiter plates (Immunol-2 plates; Dynatech, Chantilly, VA), and the reaction product was detected with O- phenylenediamine dihydrochloride (Sigma Chemical Co.). Absorbance(492-540 nm) was used as the end point determination. Standard plots for converting absorbance to concentrations of IL-10 were constructed by measuring known concentrations of human recombinant IL-10 (R&D Systems, Inc., Minneapolis, MN) and plotting the log of the resulting readings against the log of known concentrations of human recombinant IL-10. Concentrations of 80-1200 pg/mL of human recombinant IL-10 were detected with this method. The correlation between the concentration of standards and the log of the absorbance was high (r values between 0.98 and 0.99).
IL-10 concentrations in adult or umbilical cord blood sera were quantified by an enzyme immunoassay that detects very low concentrations of the cytokine(range, ≈3-550 pg/mL) (Endogen, Inc., Cambridge, MA). In the assay, the detector-antibody was coupled to biotin and the biotin to streptavidin-horseradish peroxidase. Final reactions were developed with 3,3′,5,5′-tetramethylbenzidine (TMB liquid substrate system, Sigma Chemical Co.). Absorbance (450 nm) was used as the end-point determination. There was a linear relationship between the concentrations of recombinant human IL-10 used to standarize the test and absorbance (r > 0.9).
TNF-α production by BML. The concentrations of TNF-α in supernatants from cultured BML stimulated with LPS (1.0 μg/mL) for 6 and 12 or 24 h were quantified by an enzyme immunoassay designed to detect very low concentrations of the naturally occurring TNF-α trimers (R&D Systems, personal communication). In this assay, the detector-antibody was coupled to horseradish peroxidase. Final reactions were developed with chromogen (tetramethylbenzidine) and hydrogen peroxide, and stopped with 2 N sulfuric acid. Absorbance (450 nm) was determined at the end point of the reaction. The range of detected concentrations was ≈16-1000 pg/mL. There was a linear relationship between the concentrations of recombinant human TNF-α standards and absorbance(r > 0.9). The assumption in interpreting the data were that the recombinant cytokine was in its trimeric form because of spontaneous combination of monomers (R&D Systems, personal communication).
TNF-R on BML. TNF-R on BML were detected by two-color flow cytometry using the previously described instrument and flow cytometry procedures except for the following. The detector for TNF-R was a biotinylated human recombinant TNF-α that binds to the 75- and 55-kD receptors(R&D Systems)(48). Ten μL (1.0 ng) of the recombinant cytokine were added to 25 μL (≈2 × 105 cells) of a suspension of BML and incubated at 4 °C for 60 min. Then 10 μL of avidin-FITC were added, and the mixture was incubated in the dark at 4 °C for 30 min. The cells were then incubated with fluorochrome-labeled monoclonal antibodies to either CD14 or CD3 to detect monocytes or T cells, respectively, in the two-color analysis.
A negative control was used in all experiments. A heterologous protein, soybean trypsin inhibitor biotinylated to the same degree as for TNF-α, was added to a separate aliquot of each sample to determine nonspecific background. The information was used to set electronic gates for flow cytometry studies of the binding of labeled TNF-α. The specificity of binding was tested by adding goat polyclonal IgG antibodies against human TNF-α to selected aliquots of cells mixed with labeled TNF-α. The rest of the procedure was carried out as described. In those experiments, much less than 5% of cell-bound labeled TNF-α was detected. In addition, according to the manufacturer (R&D Systems), very similar blocking effects were obtained by using an excess of unconjugated recombinant human TNF-α.
Statistical analyses. Unless otherwise specified, data were expressed as the mean (X) ± SD. Groups of data were evaluated by analysis of variance. Differences in individual means were considered to be statistically significant by analysis of variance if p values were<0.05. These data were also analyzed by t test and were considered to be significantly different if p values were <0.05. For small sample sizes, a nonparametric analysis (Kruskal-Wallis rank test) was also performed. In addition, the relationships between the production of IL-10 by LPS-stimulated cells and the numbers of monocytes and T cell subpopulations in the preparations were examined by linear regression.
RESULTS
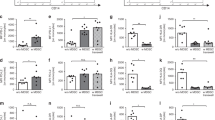
Stimulation of BML with LPS. The production of IL-10 was less by umbilical cord than adult BML after LPS stimulation (Fig. 1). As previously reported(32), the concentrations of IL-10 in supernatants collected from cultured adult BML varied according to the amount of LPS (Fig. 1). Significantly less IL-10(X ± SD) was detected in supernatants from newborn BML than adult BML cultured with LPS (Fig. 1). Differences in the production of IL-10 were not, however, due to dissimilarities in the relative frequencies of monocytes in the adult (n = 22) and newborn(n = 30) blood (X ± SD, 15 ± 8% and 12± 8%, respectively; p > 0.1).
IL-10 production by adult or newborn BML (n = 9-22 and 10-30, respectively, depending upon the concentration of the stimulus) stimulated or not stimulated with LPS. Data are expressed as mean± SD of IL-10 concentrations in supernatants from 24 h cultures. IL-10 production by neonatal BML increased after stimulation (p < 0.01), but the production was much less than that found in stimulated adult BML (*p < 0.005) by analysis of variance.
As shown by linear regression analysis, opposite relationships were found between the production of IL-10 by newborn or adult BML stimulated with LPS and the numbers of monocytes in the preparations (i.e. CD14+ cells) (Fig. 2). There was a direct relationship between the number of newborn monocytes and the concentration of IL-10 produced by these cultured cells, regardless of the concentration of LPS that was used(r = 0.81; p = 0.02 with 500 ng/mL of LPS) (Fig. 2). In contrast, an inverse relationship was found between the number of adult monocytes and the concentration of IL-10 when the cells were stimulated with a 500 ng/mL dose of LPS (r = -0.69;p = 0.03). The inverse relationship found with adult cells disappeared, however, with higher (1000 ng/mL) (r = -0.07;p = 0.83) or lower concentrations (250 ng/mL) (r = -0.47;p = 0.2) of LPS.
The relationship between IL-10 production by BML stimulated with LPS (500 ng/mL) from adults (○) (n = 9) or newborn infants (•) (n = 11) and the numbers of blood monocytes determined by flow cytometry. The numbers of neonatal monocytes and IL-10 produced by neonatal cells were directly related (r = 0.81;p = 0.02). In contrast, an inverse relationship was found with adult BML stimulated with 500 ng/mL of LPS (r = -0.69; p = 0.03). In the equation y = ax + b, y is the vertical axis; x, the horizontal axis; a, the slope; and b, the intercept on the y axis. In the newborn,a = 0.004 and b = 3.7. In the adult, a = -0.002 and b = 22.4.
Linear regression analyses were carried out to examine possible relationships between the production of IL-10 in the LPS stimulation experiments and the numbers of CD4+ or CD8+ T cells in the preparations. No relationship was found (data not shown).
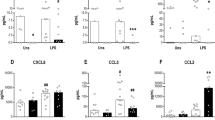
TNF-α production by BML. Because TNF-α induces IL-10 production(47), we quantified TNF-α production by adult and umbilical cord BML stimulated with LPS for 6, 12, or 24 h. It has previously been shown that maximal production of TNF-α occurs 6-8 h after stimulation(49). We found that adult BML (n = 6) cultured for 6 h produced almost twice as much TNF-α as the newborns (n = 7) (1803 ± 557 and 940 ± 285, respectively, p < 0.01) (Fig. 3). A similar relationship was found at 12 and 24 h (data not shown).
Stimulation of BML with TNF-α. We next examined the production of IL-10 by adult and umbilical cord BML stimulated with different doses of recombinant human TNF-α. The production of IL-10 by umbilical cord BML increased after TNF-α stimulation (2.5 ng/mL or more), but the increment was significantly less than that found for adult BML (Fig. 4). There was twice as much IL-10 detected in supernatants from newborn BML stimulated with TNF-α at doses of 2.5 ng/mL or more, compared with unstimulated preparations. However, adult BML cultured with TNF-α at concentrations of 1.25, 2.5, 5, and 10 ng/mL showed an increment in the amount of IL-10 detected in the supernatants, ranging from 2 to 10 times as much as released by baseline unstimulated cells (Fig. 4). Thus, there was a direct relationship between the dose of TNF-α used to stimulate adult BML and the concentrations of IL-10 produced by these cultured cells (r = 0.52; p < 0.001; df = 47).
The effect of TNF-α upon the production of IL-10 by newborn BML. Adult or newborn BML (n = 2-10 and 13-18, respectively, depending upon the concentration of the stimulus) were or were not stimulated with TNF-α. Data are expressed as mean ± SD of IL-10 concentrations in supernatants from 24 h cultures. IL-10 production by neonatal BML increased after stimulation, but the increase was not significant. Also, the production from stimulated newborn BML was much less than that found in TNF-α stimulated adult leukocytes(*p < 0.001).
Addition of TNF-α at concentrations of 2.5 ng/mL or more to adult BML also stimulated with 1.0 μg/mL LPS was found to augment production of IL-10 by 1.3-1.5 times over that of cells stimulated with LPS alone, as previously reported(47). However, in contrast, addition of TNF-α at any of the concentrations used on umbilical cord BML also stimulated with 1.0 μg/mL of LPS failed to augment production of IL-10 over that of cells stimulated with LPS alone (data not shown).
Differences in the production of IL-10 did not correlate with differences in the relative frequencies of monocytes or T lymphocytes in the adult and newborn blood. Nor was there any relationship between the production of IL-10 in the TNF-α stimulation experiments and the number of CD14+ cells in the preparations.
TNF-R on monocytes and T cells. We investigated whether the poor production of IL-10 by newborn BML stimulated with recombinant human TNF-α correlated with a decreased expression of TNF-R on these monocytes and T cells. By analyzing group data by the t test and Kruskal-Wallis rank order method, the numbers of CD3+ cells that expressed TNF-R (X ± SD, 32 ± 20% and mean rank 7 for newborns; X ± SD, 26 ± 7%, mean rank 6 for adults) and the number of receptors (mean channel fluorescence intensity, MCFI) per CD3+ T cell (X ± SD, 30 ± 17, mean rank 7 for newborns; X ± SD, 21 ± 8, mean rank 5 for adults) were similar (p > 0.3). Individual histograms of two-color analysis revealed that the number of TNF-R positive monocytes and the degree of expression of the receptor on monocytes (MCFI) were less in newborns (Fig. 5). This was borne out in Kruskal-Wallis nonparametric rank analysis (p < 0.05) (Fig. 6).
Stimulation of blood T cells with anti-CD3. There was no evidence of production of IL-10 by umbilical cord BML stimulated in vitro with antibodies to human CD3 for 24-72 h. Because the maximal production of IL-10 by anti-CD3-stimulated adult cells occurred after 48 h of culture, only those data from adult and newborn mononuclear leukocytes are presented. The X ± SD of the concentrations of IL-10 in supernatants collected from cultured adult (n = 17) and newborn(n = 17) BML after stimulation with antibodies to human CD3 for 48 or 72 h were 914 ± 386 and 178 ± 176 pg/mL, respectively (Fig. 7) (p < 0.0001). The use of higher(0.05 μg/mL) or lower (0.0125 μg/mL) concentrations of antibodies to CD3 produced similar results (data not shown). Differences in the production of IL-10 by adult and newborn cells could not be attributed to discrepancies in the relative frequencies of CD3+CD4+ (X ± SD, 45 ± 8% and 37 ± 14%, respectively; p > 0.1) or CD3+CD8+ T cells in the blood (X ± SD, 23± 7%, and 16 ± 7%, respectively; p > 0.05).
IL-10 production by adult (n = 17) or newborn(n = 17) BML not stimulated or stimulated with antibodies to CD3(anti-CD3). Data are expressed as mean ± SD of IL-10 concentrations in supernatants from 48-72-h cultures. There were no significant differences between unstimulated neonatal and adult mononuclear leukocytes (p> 0.9) or between unstimulated and anti-CD3 stimulated neonatal mononuclear leukocytes (p > 0.3), whereas significant differences were found between stimulated adult and newborn BML (*p < 0.0001 by t test).
Experiments with enriched T cells. Out of the above 17 samples, T cell-enriched aliquots from six adult and five newborn samples were used for the following experiments. In these experiments, unstimulated T cell-enriched adult specimens produced significantly more IL-10 from culture supernatants than the unstimulated, unfractionated adult cells (829 ± 596 and 186± 154 pg/mL, respectively; p = 0.04). However, there was no significant difference in spontaneous production of IL-10 from neonatal T cell-enriched or unfractionated preparations (53 ± 23 and 121 ± 64 pg/mL, respectively; p = 0.1). Again, there was substantially more IL-10 produced by anti-CD3-stimulated adult T cells than by anti-CD3-stimulated newborn specimens (1707 ± 1123 and 195 ± 113 pg/mL, respectively; p = 0.01) (Fig. 8A).
Il-10 production by adult (○) or newborn (•) BML that either were not or were enriched for T cells and stimulated with either anti-CD3 (panel A), PMA (panel B), or both agents(panel C). In contrast to adults, there was no augmentation of IL-10 production in newborn cells after any of the stimuli, regardless of whether the preparations were or were not enriched for T cells.
PMA was also used as a stimulant because anti-CD3 stimulation alone may not have been enough to optimally activate newborn leukocytes(50). A similar relationship was seen when enriched T cells from adults and from newborns were stimulated with PMA. The x± SD were 1244 ± 667 and 166 ± 81 pg/mL, respectively;p = 0.05) (Fig. 8B). Moreover, a combination of PMA and anti-CD3 failed to augment IL-10 production by newborn T cells (139± 80 pg/mL in newborns versus 1231 ± 654 pg/mL in adults; p = 0.005) (Fig. 8C).
All adult T cell-enriched preparations showed a statistically significant increase in IL-10 production compared with the unfractionated adult specimens (Fig. 8A-C). Whereas, there was no significant difference between unfractionated and T cell-enriched preparation in newborns stimulated with anti-CD3, PMA, or a combination of the two stimuli (Fig. 8A-C).
Concentrations of IL-10 in adult and umbilical cord blood. Because of the paucity of production of IL-10 by stimulated newborn BML, we tested whether or not the low production of IL-10 by these cells was due to autoregulatory effect of IL-10(32). Therefore IL-10 was quantified in 13 specimens of serum from umbilical cord blood and eight specimens of adult venous blood. The concentrations of IL-10 in the adult and umbilical cord blood specimens were not statistically different (X± SD, 8 ± 6 and 5 ± 2 pg/mL, respectively).
DISCUSSION
These experiments indicate that the production of IL-10 by stimulated T cells and monocytes from newborn infants is impaired. Although neonatal monocytes were found to produce more IL-10 than neonatal T cells, the production of IL-10 by LPS-stimulated cells was much less than their counterparts in adults. Because the differences were not due to smaller numbers of monocytes in the neonatal blood specimens, other possibilities were considered. These included 1) a quantitative decrease in the expression of the mRNA for IL-10, 2) a posttranscriptional instability(51), and 3) an alteration in the translation step for protein production.
We also considered that the decreased production of IL-10 by stimulated neonatal cells may also have been due in part to an altered interaction between T cells and monocytes or a decreased production of cytokines such as TNF-α that enhance the production of IL-10(47). In addition, although the production of IL-10 in the LPS-stimulated preparations of neonatal leukocytes was directly related to the numbers of monocytes in the preparations, the low production of IL-10 by LPS-stimulated neonatal blood leukocytes may be due in part to an impaired ability of neonatal blood monocytes to be activated by certain cytokines(3).
We further examined these possibilities by quantifying TNF-α production by LPS-stimulated newborn blood monocytes. We confirmed earlier reports where a decreased production of TNF-α by newborn monocytes was demonstrated by bioassays(12, 20). Inasmuch as TNF-α enhances the production of IL-10(47), a decreased production of TNF-α by newborn monocytes may contribute to the poor elaboration of IL-10 by them. IL-10 production by neonatal cells did not, however, increase significantly after exposure to exogenous TNF-α. We therefore measured the expression of TNF-R on monocytes. It has been reported that the expression of TNF-R by newborn monocytes is similar to adults(52), but group data were not reported. In our study, the number of blood monocytes that expressed receptors to TNF-α and the degree of expression of those receptors by neonatal monocytes were significantly reduced. We used biotinylated human recombinant TNF-α, whereas Zola et al.(52) used antibodies to the 55- and 75-kD receptors. It is, thus, unclear whether one or both of these receptors is decreased on newborn monocytes.
T cells predominantly express the 75-kD TNF-R(52, 53), whereas adult monocytes express both receptors(52–54). This, in context with our findings, suggests that there may be different patterns for the development of receptors to TNF-α on neonatal monocytes and T cells. An alternative is that newborn cells were activated and consequently shed their TNF-R(55). Although an increased expression of TNF-R on adult monocytes has been demonstrated in vitro over time(54), there has also been reports of an increase in serum-soluble TNF-R, especially the 55-kD moiety, during pregnancy and in newborns(56, 57). The origin of the soluble receptors in newborn sera was not ascertained. Because of the different functions attributed to each receptor(58), it will be important to determine whether either TNF-R on monocytes is developmentally delayed or more readily shed. Regardless of the cause, the investigation suggests that reduced IL-10 production in neonatal monocytes may be secondary in part to regulatory processes involving TNF-α and one or more of its receptors.
The regulation of IL-10 production by neonatal mononuclear leukocytes was also addressed by linear regression analyses of relationships between numbers of monocytes and the production of IL-10. A direct relationship between IL-10 production by LPS-stimulated neonatal leukocytes and numbers of monocytes was found. In contrast, an inverse relationship was found between IL-10 production by adult leukocytes and the number of monocytes when cells were stimulated with a 500 ng/mL dose of LPS. This finding is consistent with an inhibitor produced by adult BML. The inhibitor may be interferon-γ, because such an effect occurs with human recombinant interferon-γ(59), and because there is a developmental delay in the production of that agent by human newborn infants(16).
The production of IL-10 by neonatal T cells stimulated with well characterized T cell activators, i.e. antibodies to CD3, and/or the addition of PMA, a protein kinase C stimulator, was exceptionally low. This was not due to differences in numbers or viability of CD4+ or CD8+ T cells or of monocytes. Furthermore, IL-10 production was not enhanced by enriching the numbers of T cells in the preparations. These findings may be due to a different state of activation of blood T cells in newborn infants. In that respect, T cells that produce IL-10 are principally CD45RO+(50), and very few blood CD45RO+ T cells are found in newborn infants(8–11).
The less effective production of IL-10 by blood T cells and monocytes from newborns suggests why newborns are more susceptible to inflammatory-mediated diseases. In that respect, the risk of NEC appears to be lessened by feedings of human milk(60), which contains significant concentrations of IL-10(44). That might partly explain how human milk protects against NEC. Also, bronchoalveolar fluids of neonates with respiratory distress syndrome lack IL-10(61). Thus, the ability of neonates to produce cytokines, the quantities of these agents in human milk, their delivery to mucosal surfaces in the infant via milk, and their role in neonatal diseases should be further investigated.
Abbreviations
- BML:
-
blood mononuclear leukocytes
- D-PBSE/CMF:
-
Dulbecco's PBS/calcium and magnesium-free
- LPS:
-
lipopolysaccharide
- NEC:
-
necrotizing enterocolitis
- PMA:
-
phorbol 12-myristate 13-acetate
- TNF-α:
-
tumor necrosis factor-α
- TNF-R:
-
tumor necrosis factor-α receptor
- NK:
-
natural killer
References
Hill HR 1987 Biochemical, structural, and functional abnormalities of polymorphonuclear leukocytes in the neonate. Pediatr Res 22: 375–382
Wilson CB, Lewis DB, Penix LA 1996 The physiologic immunodeficiency of immaturity. In: Stiehm ER (ed) Immunologic Disorders in Infants and Children. WB Saunders, Philadelphia, pp 253–295
Marodi L, Kaposzata R, Campbell DE, Polin RA, Csongor J, Johnston RB Jr 1994 Candidacidal mechanisms in the human neonate: impaired IFN-γ activation of macrophages in newborn infants. J Immunol 153: 5643–5649
Adderson EE, Johnston JM, Shackerford PG, Carroll WL 1992 Development of the human antibody repertoire. Pediatr Res 32: 257–263
Peltola H, Kayhty H, Virtanen M, Makela PH 1984 Prevention of Haemophilus influenzae type b bacterial infections with the capsular polyaccharide vaccine. N Engl J Med 310: 1561–1565
Burgio GR, Hanson LÅ, Ugazio AG (eds) 1987 Immunology of the Neonate. Springer, Vienna
Rognum TO, Thrane PS, Stoltenberg L, Vege Å, Brandstzaeg P 1992 Development of intestinal mucosal immunity in fetal life and the first postnatal months. Pediatr Res 32: 145–149
Hayward AR, Groothius J 1991 Development of T cells with memory phenotype in infancy. Adv Exp Biol Med 310: 71–76
Maccario R, Chirico G, Mingrat G, Arico M, Lanfranchi A, Montagna D, Moretta A, Rondini G 1993 Expression of CD45RO antigen on the surface of resting and activated neonatal T lymphocyte subsets. Biol Neonate 64: 346–353
Bofill M, Akbar AN, Salmon M, Robinson M, Burford G, Janossy G 1994 Immature CD45RAlowROlow T cells in human cord blood. J Immunol 152: 5613–5623
Chheda S, Palkowetz KH, Rassin DK, Goldman AS 1996 Deficient quantitative expression of CD45 isoforms on CD4+ and CD8+ T-cell subpopulations and subsets of CD45RAlowCD45ROlow T cells in newborn blood. Biol Neonate 69: 128–132
English BK, Burchett SK, English JD, Amman A, Wara DW, Wilson CB 1988 Production of lymphotoxin and tumor necrosis factor by human neonatal mononuclear cells. Pediatr Res 24: 717–722
Cairo MS, Suen Y, Knoppel E, van de Ven C, Nguyen A, Sender L 1991 Decreased stimulated GM-CSF expression and GM-CSF gene expression but normal numbers of GM-CSF receptors in human term newborns as compared with adults. Pediatr Res 30: 362–367
Cairo MS, Suen Y, Knoppel E, Dana R, Park L, van de Ven C, Sender L 1992 Decreased G-CSF and IL-3 production and gene expression from mononuclear cells of newborn infants. Pediatr Res 31: 574–578
Lewis DB, Yu CC, Meyer J, English BK, Kahn SJ, Wilson CB 1991 Cellular and molecular mechanisms for reduced interleukin 4 and interferon-γ production by neonatal T cells. J Clin Invest 87: 194–202
Wilson CB, Westfall J, Johnson L, Lewis DB, Dower SK, Alpert AR 1986 Decreased production of interferon-γ by human neonatal cells: intrinsic and regulatory deficiencies. J Clin Invest 77: 860–867
Yachie A, Takano N, Yokwi T, Kato K, Kasahara Y, Miyawaki T, Taniguchi N 1990 The capabilities of neonatal leukocytes to produce interleukin-6 on stimulation. Assessed by whole blood culture. Pediatr Res 27: 227–223
Yachie A, Takano N, Ohta K, Uehara T, Fujita S, Miyawaki T, Taniguchi N 1992 Defective production of interleukin-6 in very small premature infants in response to bacterial pathogens. Infect Immun 60: 749–753
Schibler KR, Liechty KW, White WL, Rothstein G, Christensen RD 1992 Defective production of interleukin-6 by monocytes: a possible mechanism underlying several host defense deficiencies of neonates. Pediatr Res 31: 18–21
Weatherstone KB, Rich EA 1989 Tumor necrosis factor/cachectin and interleukin-1 secretion by cord blood monocytes from premature and term neonates. Pediatr Res 25: 342–346
de Bont ESJM, Martens A, van Raan J, Samson G, Fetter WPF, Okken A, de Leij HFM 1993 Tumor necrosis factor-α, interleukin-1β, and interleukin-6 plasma levels in neonatal sepsis. Pediatr Res 33: 380–383
Aerde JEE 1991 Acute respiratory failure and bronchopulmonary dysplasia. In: Hay WW (ed) Neonatal Nutrition and Metabolism. Mosby Year Book, St. Louis, pp 467–506
Kliegman RM, Walker WA, Yolken RH 1993 Necrotizing enterocolitis: research agenda for a disease of unknown etiology and pathogenesis. Pediatr Res 34: 701–708
Sharma R, Prem RR, Salisbury S 1994 Interleukin-1β, interleukin-2, and tumor necrosis factor-α levels in infants with necrotizing enterocolitis (NEC). Pediatr Res 35: 225A( abstr)
Gaylord M, Smith Z, Lorch V, Blank M, Snyder F 1994 Elevated platelet activating factor levels in the first week of life are associated with the severity of bronchopulmonary dysplasia in very low birthweight infants. Pediatr Res 35: 333A( abstr)
Nijinimbam CG, Cole CH, Frantz ID III 1994 Interleukin-1 receptor antagonist (IL-1ra) and interleukin-8 (IL-8) levels in tracheal aspirates of infants with respiratory distress syndrome (RDS) and bronchopulmonary dysplasia (BPD). Pediatr Res 35: 347A( abstr)
Vieira P, de Waal Malefyt R, Dang M, Johnson KE, Kastelein R, Moore KW 1991 Isolation and expression of human cytokine synthesis inhibitory factor (CSIF/IL-10) cDNA clones: homology to Epstein-Barr virus open reading frame BCRF1. Proc Natl Acad Sci USA 88: 1172–1176
de Waal Malefijt R, Yssel RH, Ronocarlo H, Spits H, de Vries JE 1992 Interleukin 10. Curr Opin Immunol 4: 314–320
Howard M, O'Garra H, Ishida R, de Waal Malefyt R, de Vries JE 1992 Biological properties of IL-10. J Clin Immunol 4: 239–247
Fiorentino DF, Zlotnik A, Mosmann TR, Howard M, O'Garra A 1991 IL-10 inhibits cytokine production by activated macrophages. J Immunol 147: 3815–3822
Fiorentino DF, Zlotnik A, Viera P, Mosmann TR, Howard M, Moore KW, O'Garra A 1991 IL-10 acts on the antigen-presenting cell to inhibit cytokine production by Th1 cells. J Immunol 146: 3444–3451
de Waal Malefijt R, Abrams J, Bennett B, Figdor CG, de Vries JE 1991 Interleukin 10 (IL-10) inhibits cytokine synthesis by human monocytes: an autoregulatory role of IL-10 produced by monocytes. J Exp Med 174: 1209–1220
Wu-J AU, Cunha FQ, Liew FY, Weiser WY 1993 IL-10 inhibits the synthesis of migration inhibitory factor and migration inhibitory factor-mediated macrophage activation. J Immunol 151: 4325–4332
Cassatella MA, Meda L, Bonora S, Ceska M, Constantin G 1993 Interleukin 10 (IL-10) inhibits the release of proinflammatory cytokines from human polymorphonuclear leukocytes: evidence for an autocrine role of tumor necrosis factor and IL-1β in mediating the production of IL-8 triggered by lipopolysaccharide. J Exp Med 178: 2207–2211
D Andrea A, Aste-Amezaga M, Valiante NM, Ma X, Kubin M, Trinchieri G 1993 Interleukin 10 (IL-10) inhibits human lymphocyte interferon gamma-production by suppressing natural killer cell stimulatory factor/IL-12 synthesis in accessory cells. J Exp Med 178: 1041–1048
de Waal Malefyt R, Yssel H, de Vries JE 1993 Direct effects of IL-10 on subsets of human CD4+ T cell clones and resting T cells: specific inhibition of IL-2 production and proliferation. J Immunol 150: 4754–4765
Briere F, Bridon JM, Servet C, Rousset F, Zurawski G, Banchereau J 1993 IL-10 and IL-13 as B cell growth and differentiation factors. Nouv Rev Fr Hematol 35: 233–235
Armitage RJ, Macduff BM, Spriggs MK, Fanslow WC 1993 Human B cell proliferation and Ig secretion induced by recombinant CD40 ligand are modulated by soluble cytokines. J Immunol 150: 3671–3680
Rousset F, Garcia E, Defrance T, Peronne C, Vezzio N, Hsu DH, Kastelein R, Moore KW, Banchereau J 1992 Interleukin 10 is a potent growth and differentiation factor for activated human B lymphocytes. Proc Natl Acad Sci USA 89: 1890–1893
Defrance T, Vanbervliet B, Briere F, Durand I, Rousset F, Banchereau J 1992 Interleukin 10 and transforming growth factor beta cooperate to induce anti-CD40-activated naive human B cells to secrete immunoglobulin A. J Exp Med 175: 671–682
Howard M, Muchamuel T, Andrrade S, Menon S 1993 Interleukin 10 protects mice from lethal endotoxemia. J Exp Med 177: 1205–1208
Marchant A, Deviere J, Byl B, De Groote D, Vincent J-L, Goldman M 1994 Interleukin-10 production during septicaemia. Lancet 343: 707–708
Kühn R, Löher J, Rennick D, Rajewsky K, Müller W 1993 Interleukin-10-deficient mice develop chronic enterocolitis. Cell 75: 263–274
Garofalo R, Chheda S, Mei F, Palkowetz KH, Rudloff HE, Schmalstieg FC, Rassin DK, Goldman AS 1995 Interleukin-10 in human milk. Pediatr Res 37: 444–449
Goldman AS 1993 The immune system of human milk: antimicrobial, antiinflammatory, and immunomodulating properties. Pediatr Infect Dis J 12: 664–672
Brooks EG, Schmalstieg FC, Wirt DP, Rosenblatt HM, Adkins LT, Lookingbill DP, Rudloff HE, Rakusan TA, Goldman AS 1990 A novel X-linked combined immunodeficiency disease. J Clin Invest 86: 1623–1631
Wanidworanun C, Strober W 1993 Predominant role of tumor necrosis factor-α in human monocyte IL-10 synthesis. J Immunol 151: 6853–6861
Brockhaus M, Schoenfeld H-J, Schlaeger E-J, Hunziker W, Lesslauer W, Loetscher H 1990 Identification of two types of tumour necrosis factor receptors on human cell lines by monoclonal antibodies. Proc Natl Acad Sci USA 87: 3127–3131
Burchett SK, Weaver WM, Westall JA, Laren A, Kronheim S, Wilson CB 1988 Regulation of tumour necrosis factor/cachectin and interleukin-1 secretion in human mononuclear phagocytes. J Immunol 140: 3473–3481
Yssel H, de Waal Malefyt R, Roncarlo M-G, Abrams JS, Lahesmaa R, Spits H, De Vries J 1992 IL-10 is produced by subsets of human CD4+ T cell clones and peripheral blood T cells. J Immunol 149: 2378–2384
Lee SM, Knoppel E, van de Ven C, Cairo MS 1993 Transcriptional rates of granulocyte-macrophage colony-stimulating factor, granulocyte colony-stimulating factor, interleukin-3, and macrophage colony-stimulating factor genes in activated cord versus adult mononuclear cells: alteration in cytokine expression may be secondary to posttranscriptional instability. Pediatr Res 34: 560–564
Zola H, Fusco M, Macardle PJ, Fliego L, Roberton D 1995 Expression of cytokine receptors by human cord blood lymphocytes: comparison with adult blood lymphocytes. Pediatr Res 38: 397–403
Agostini C, Zambello R, Cerutti A, Enthammer C, Facco M, Milani A, Sancetta R, Garbisa S, Semenzato G 1995 Expression of TNF receptors by T cells and membrane TNF-α by alveolar macrophages suggests a role for TNF-α in the regulation of the local immune responses in the lungs of HIV-1-infected patients. J Immunol 154: 2928–2938
Leeuwenberg JFM, Dentener MA, Buurman WA 1994 Lipopolysaccharide LPS-mediated soluble TNF receptor release and TNF receptor expression by monocytes. J Immunol 152: 5070–5076
Porteu F, Nathan C 1990 Shedding of tumour necrosis factor receptors by activated human neutrophils. J Exp Med 172: 599–607
Austgulen R, Liabakk N-B, Lein E, Espevik T 1993 Increased levels of soluble tumour necrosis factor-α receptors in serum from pregnant women and in serum and urine samples from newborns. Pediatr Res 33: 82–86
Austgulen R, Johnsen H, Kjollesdal AM, Liabakk N-B, Espevik T 1993 Soluble receptors for tumour necrosis factor: occurrence in association with normal delivery at term. Obstet Gynecol 82: 343–347
Tartaglia L, Goeddel D 1992 Two TNF receptors. Immunol Today 13: 151–153
Chomarat P, Rissoan M-C, Banchereau J, Miossec P 1993 Interferon γ inhibits interleukin 10 production by monocytes. J Exp Med 177: 523–527
Lucas A, Cole TJ 1990 Breast milk and neonatal necrotising enterocolitis. Lancet 336: 1519–1525
Jones CA, Cavabvab R, Kwong YC, Stotts CL, de Lemos RA Decreased IL-10 expression in the lungs of preterm infants with hyaline membrane disease 1995. Pediatr Res 37: 2002A( abstr)
Acknowledgements
Antibodies to human CD3 were kindly provided by Dr. Edward G. Brooks. The encouragement of Dr. Frank C. Schmalstieg, technical advice of Dr. Gang Ye, and the secretarial support of Susan C. Kovacevich and Freda Purnell are appreciated.
Author information
Authors and Affiliations
Additional information
Supported in part by grants from the Wyeth/Ayerst Nutritional Research and the Wyeth Pediatric Neonatology Research Fund.
Rights and permissions
About this article
Cite this article
Chheda, S., Palkowetz, K., Garofalo, R. et al. Decreased Interleukin-10 Production by Neonatal Monocytes and T Cells: Relationship to Decreased Production and Expression of Tumor Necrosis Factor-α and Its Receptors. Pediatr Res 40, 475–483 (1996). https://doi.org/10.1203/00006450-199609000-00018
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1203/00006450-199609000-00018
This article is cited by
-
Differential mucosal IL‐10‐induced immunoregulation of innate immune responses occurs in influenza infected infants/toddlers and adults
Immunology & Cell Biology (2017)
-
Newborn susceptibility to infection vs. disease depends on complex in vivo interactions of host and pathogen
Seminars in Immunopathology (2017)