Abstract
A simple, reproducible test was used to quantify muscle weakness in mdx mice, an animal model of Duchenne muscular dystrophy. The effect of bedding on wheat kernels and of dietary supplementation ofα-tocopherol on the progression of muscle weakness was investigated in mdx mice. When measured during the first 200 d of life,mdx mice developed muscle weakness, irrespective of bedding and diet. When kept on wood shavings and fed a conventional rodent diet,mdx mice showed progressive muscle weakness over the consecutive 200 d, and eventually showed a significant weight loss during the next 200-d observation period. Progression of muscle weakness and weight loss were almost completely prevented in mdx mice that were kept on wheat kernel bedding. In contrast, only incomplete maintenance of muscle strength and body weight was observed in mdx mice kept on wood shavings and fed theα-tocopherol-supplemented diet. It is concluded from these experiments that a component of wheat kernels other than α-tocopherol is essential to prevent the progression of muscle weakness in mdx mice.
Similar content being viewed by others
Main
DMD is a progressive X-linked myopathy which usually affects boys before the age of 6 y and leads to death by respiratory and/or cardiac failure by the age of 20 y(1). The musculature is affected by a deficiency in dystrophin, a submembranous cytoskeletal protein(1–3). The X chromosomal m uscle dystrophic (mdx) mouse is an animal model of DMD(4, 5). In the mdx strain, a point mutation in exon 23 of the dystrophin gene causes premature termination of the polypeptide chain and eliminates expression of the 427 kD protein in muscle and brain(6, 7). The life span of mdx mice is reduced from approximatley 900 d to about 640 d(8, 9). However, in contrast to the obvious progression of DMD in humans(1), observations on muscle strength development in mdx mice are inconsistent. Although some authors found no evidence of muscle weakness up to the age of 180 d(10), 300 d(11), 550 d(12), or beyond(13), others found various degrees of muscle weakness(14–17), incoordination(4), reduced litter size(14), and problems with grooming and feeding(9, 12) in various age groups ranging from the first(16) to the last(9, 12) month of life.
We obtained phenotypically healthy animals of the strain C57B1/10 with(mdx) and without (controls) the genetic defect from a successful breeding stock and attempted to continue breeding them under the conditions of our institute. In contrast to control animals, which successfully reproduced, no progeny could be obtained for mdx animals. Although the normal animals stayed perfectly healthy, the mdx animals showed a gradual decline in activity, lost weight and hair, failed to adequately groom themselves, and eventually died from starvation and/or respiratory distress. Meticulous analysis of the housing conditions revealed one key difference. In the insitute where the animals had been obtained from, the cages of the animals had been bedded with wheat kernels, whereas we used wood shavings for bedding. Because wheat is a major source of α-tocopherol(18) and α-tocopherol has been discussed as a potential treatment approach to muscular dystrophy(19, 20), we speculated that the mdx animals used the bedding as a dietary source of α-tocopherol and thus maintained their muscular and reproductive integrity. Indeed,α-tocopherol plasma levels in the mdx animals on the wheat kernel bedding were significantly elevated both over mdx animals bedded on wood shavings, as well as over control animals bedded on wheat kernels (see “Results”), suggesting that mdx, but not control animals, ingested their wheat kernel bedding. Based on these observations, we have performed and report here a series of experiments in which we systematically evaluated the effect of wheat kernel bedding and of dietary α-tocopherol supplementation on the phenotypic expression of muscular dystrophy in young, middle-aged, and senescent mdx mice.
METHODS
Animals. mdx mutant and normal control mice(C57B1/10) were investigated in this study(4–7). All animals were held in cages with two to eight animals per cage and a day/night rhythm of 14 h/10 h. Animals received water and diets ad libitum. The animals were randomly assigned to the experimental groups immediately after weaning. Until weaning, dams and litter were bedded on wood shavings covered by a complete layer of wheat kernels. According to assignment to the individual experimental groups, the cage bedding consisted either of wood shavings alone or of wood shavings covered by a complete layer of wheat kernels.
Plasma was obtained from heparinized blood taken from the aorta after laparatomy in pentobarbital-anesthetized mice. Immediately after hemorrhage, the heart was removed and weighed. Then, the following hind limb muscles were carefully dissected on both sides and pooled to assess the hind limb muscle weight: musculus gluteus medius, m. gluteus superficialis, m. pectineus, m. rectus femoris, m. gastrocnemius, m. semimembranosus, m. vastus medialis, m. adductor longus, m. flexor digitorum profundus, and on the right side only the m. tibialis caudalis. All studies have been approved by the Institutional Review Board.
Diets. A conventional standard rodent diet was used for basic nutrition (Ssniff R/M-H, Ssniff Spezialdiäten GmbH, 59494 Soest, Germany). The diet consisted of carbohydrates (50%), crude protein (19%), water (12%), fat (3%), fiber (5.2%), ash (6.7%), mineral salts (3.35%), and trace elements. The diet ingredients were products of wheat (65%), soybean(25%), and sugar (4%) processing, mineral salts, amino acids, vitamins, and trace elements (6%). This standard diet contained α-tocopherol supplementation to a concentration of 120 mg (= 120 IU) all-rac-α-tocopheryl acetate (DL-α-tocopheryl acetate) per kg diet, which is equal to 80.4 mg of α-tocopherol equivalents, that is, to the activity of 80.4 mg of RRR-α-tocopherol(21) per kg of diet. According to the assignment to the individual experimental groups, this basic diet was supplemented with 8000 mg of all-rac-α-tocopheryl acetate per kg (= a total of 8120 IU/kg = 5440.4 mg of α-tocopherol equivalents/kg).
After weaning, three different diet groups were established. In the first group, animals were kept on a bedding of wood shavings, covered by a complete layer of wheat kernels (wheat bedding group). In the second group, animals were bedded on wood shavings (standard diet group). In the third group, animals were bedded on wood shavings and fed with the highlyα-tocopherol-enriched diet (tocopherol diet group). This highlyα-tocopherol-enriched diet produced plasma α-tocopherol concentrations similar to those in mdx mice bedded on wheat kernels(see Table 1).
Biochemical investigations. Pooled right-sided hind limb muscle tissue (m. gluteus medius, m. gluteus superficialis, m. pectineus, m. rectus femoris, m. vastus medialis, and m. adductor longus) was pulverized under liquid nitrogen. One part of the tissue powder was homogenized according to Folch et al.(22). The resulting lipophilic tissue preparations as well as the plasma samples were used for fatty acid determination by capillary gas chromatography and for quantification ofα-tocopherol by HPLC with electrochemical detection(23). The other half of the tissue powder was homogenized according to Rath et al.(24), omitting the last centrifuge step. Free (nonprotein-bound) and total (free and protein-bound) thiobarbituric acid-reactive substances were determined using a modified method of Naidoo and Knapp(25).
For antioxidant enzyme analysis, pooled left-sided hind limb muscles (m. gastrocnemius, m. semimembranosus, m. flexor digitorum profundus) were homogenized with an Ultraturrax in 15 volumes 10 mM potassium phosphate, pH 7.4, containing 30 mM KCl, 10 mM diethylenetriaminepentaacetic acid, and 100 KIU/mL aprotinin. After extraction for 30 min, the homogenates were centrifuged (20 000 × g for 15 min), and the supernatants were used for all assays. GSH-Px (EC 1.11.1.9)(26), CuZnSOD(EC 1.15.1.1), MnSOD (EC 1.15.1.1)(27), and protein(28) were assayed as previously described.
Muscle selenium concentration was determined by hydride generation atomic absorption spectrophotometry after stepwise wet ashing of the right hind limb m. tibialis caudalis(29). Plasma activities of creatine kinase (EC 2.7.3.2) and pyruvate kinase (EC 2.7.1.40) were determined by standard methods.
Quantification of muscle strength and fatiguability. Mice were placed with their forepaws on a sandpaper-covered bar. Normal mice pulled themselves up and climbed onto the bar within less than 2 s (Fig. 1). Depending on the degree of muscle weakness,mdx mice either climbed the bar or failed to pull themselves up and thus kept hanging from the bar. For each test, the climbing trial was repeated 15 times within 3 min. A muscle weakness index was calculated based on the number of failed climbings among the 15 repeat trials per test.
Examination of muscle weakness. Mice were placed with their forepaws on a sandpaper-covered bar. Depending on the degree of muscle strength, they pulled themselves onto the bar or kept hanging from the bar. This trial was repeated 15 times per test within 3 min for each animal. The number of failed trials (= hanging from bar) per 15 repeated trials was recorded.
Muscle fatiguability per experimental group was determined as the slope of a linear regression line through the mean percentage of failed climbings per trial count. A flat curve implies good maintenance of muscle strength from the first to the 15th trial, whereas a steep slope implies a marked loss of muscle strength from the first to the 15th trial. The bar test of muscle strength (Fig. 1) proved to be superior to other established tests of muscle strength in that not only muscle strength per se, but also muscle fatiguability could be evaluated for each animal. Each time the animals were tested for muscle strength, their weight was also recorded. One hundred sixty-six (M/F = 96/70) mdx and 139 (M/F = 73/66) normal mice were tested with a minimum of a 4-wk time lapse between experiments, thus avoiding any training effect.
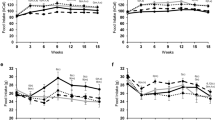
Statistics. Statistical calculations were carried out using the SAS/STAT Software Release 6.03 program. The p values of the nonparametric Mann-Whitney U test were determined using procedure NPAR1WAY option Wilcoxon with data classified in two levels(30). Nonsignificant p values (p> 0.05) are not always shown. Results are expressed as means ± SEM. Least squares linear regression analysis provided slopes of percentage of failed climbings versus trial count for the nine mdx mouse groups investigated (Fig. 2). These slopes are the fatiguability indices, which are given with ± 99% confidence intervals.
Fatiguability of mdx mice in relation to age(days) and diet (▪―▪, standard; ▴-.-.▴,α-tocopherol; [○....○, wheat; same classification as in Table 3). In muscle weakness tests that consisted of 15 consecutive climbing trials per test (see “Methods”), the percentage of failed climbing trials (failures%) increased linearily. Therefore, fatiguability was determined as the slope of a linear regression line (p < 0.0001). The slopes (±99% confidence interval) were at the age of 1-199 d, 2.67 ± 0.06 (▪―▪;r2 = 0.97), 1.23 ± 0.05 (▴-.-.▴;r2 = 0.88), and 1.90 ± 0.06 (○....○,r2 = 0.93), at the age of 200-399 d, 3.40 ± 0.25(▪―▪; r2 = 0.92), 3.26 ± 0.18(▴-.-.▴; r2 = 0.93), and 2.77 ± 0.17(○....○; r2 = 0.92), and at the age of 400-599 d, 3.60 ± 0.20 (▪―▪; r2 = 0.94), 3.11± 0.13 (▴-.-.▴; r2 = 0.94), and 3.50± 0.10 (○....○; r2 = 0.97).
RESULTS
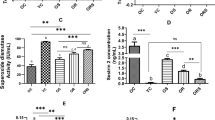
Characterization of mdx mice by pertinent pathognomonic markers. Dystrophin deficiency in muscle of mdx mice on standard diet was confirmed by immunofluorescence and Western blot (not shown). Mean plasma activities of creatine kinase (mdx: 4078 ± 630 U/L,n = 46, age = 201 ± 15 d; controls: 507 ± 66 U/L,n = 44, age = 205 ± 13 d; p < 0.0001) and of pyruvate kinase (mdx: 6635 ± 680 U/L, n = 12; controls; 69 ± 4 U/L, n = 12; p < 0.0001) were elevated in mdx mice. Mean wet weight of heart (mdx: 154± 3 mg, n = 36, age = 223 ± 18 d; controls: 134± 3 mg, n = 36, age = 213 ± 13 d; p < 0.0001) and pooled hind limb muscles (mdx: 1689 ± 59 mg,n = 36; controls: 1147 ± 38 mg, n = 34; p< 0.0001) were increased in mdx mice.
Biochemical markers of lipid peroxidation and antioxidant enzyme activities in hind limb muscle. In hind limb muscles of mdx (age= 161 ± 13 d) and control (age = 176 ± 13 d) mice on standard diet the content of poly-cis-unsaturated fatty acids (mdx: 39.35 ± 1.22 wt% of total fatty acids, n = 32; controls: 37.70 ± 0.93 wt%, n = 33); and the content of both free thiobarbituric acid-reactive substances (mdx: 7.66 ± 1.04 nmol/g wet wt, n = 24; controls: 6.50 ± 0.75 nmol/g wet wt,n = 23) and total thiobarbituric acid-reactive substances(mdx: 29.53 ± 1.57 nmol/g wet wt, n = 23; controls: 26.86 ± 2.12 nmol/g wet wt, n = 21) were normal.
Activities of CuZnSOD were similar in mdx (190.3 ± 8.4 U/mg of protein, n = 24) and control (204.8 ± 9.3 U/mg of protein, n = 24) mice, and MnSOD activity was slightly decreased(mdx: 5.17 ± 0.63 U/mg of protein, n = 24; controls: 7.14 ± 0.61 U/mg of protein, n = 24; p< 0.05) in mdx mice. The activity of the selenoprotein GSH-Px was increased in mdx mice over controls (mdx: 195.3 ± 14.5 U/mg of protein, n = 24; controls: 120.0 ± 7.7 U/mg of protein, n = 24; p < 0.0005), as was the concentration of selenium (mdx: 187.2 ± 8.9 ng/g wet wt, n = 12; controls: 160.1 ± 6.8 ng/g wet wt, n = 12; p < 0.05).
Effect of wheat kernel bedding and dietary vitamin E onα-tocopherol levels in plasma and hind limb muscle. mdx mice had significantly higher α-tocopherol plasma levels when bedded on wheat kernels than mdx animals on wood shavings and control animals on wheat bedding (Table 1). This suggests that mdx, but not normal animals, ingested the wheat kernel bedding. However, α-tocopherol levels in the muscle were not affected by the ingestion of wheat kernel bedding (Table 2). A comparable increase in α-tocopherol levels in both plasma (Table 1) and muscle (Table 2) was observed in mdx and normal mice fed an α-tocopherol-supplemented diet.
Effect of wheat kernel bedding and dietary vitamin E on muscle strength, fatiguability, and body weight. We have developed a simple test to quantify both muscle strength and fatiguability in mdx and normal mice (see “Methods”). Healthy, strong mice quickly pulled themselves onto a bar on which they were placed with their forepaws, whereas weak mice failed to pull themselves up and instead remained hanging from the bar with their forepaws (Fig. 1). Control mice only very rarely failed to pull themselves onto the bar, irrespective of age, bedding, and dietary conditions (data not shown).
mdx mice of all age groups, irrespective of the bedding and dietary conditions, were significantly weaker than normal mice(Table 3). Yet, the degree of muscular weakness was dependent on age and bedding/dietary conditions. Young mdx mice, up to d 199 of age, had a similar muscle strength under all experimental conditions. Later, muscle strength declined with time in animals bedded on wood shavings, with no significant difference between conventional standard and α-tocopherol-supplemented diets (Table 3). In contrast, the progression of muscle weakness was entirely prevented in animals on a wheat kernel bedding and conventional diet (Fig. 3; Table 3).
Effect of wheat kernel ingestion on the progression of muscle weakness in mdx mice. Bedding of wheat kernels protected mdx mouse on the left from progression of muscle weakness. In comparison, the mdx mouse on the right was bedded on wood shavings and showed flaccid paralysis with dragging legs and hair loss.
The above-mentioned climbing trials were performed 15 consecutive times within 3 min. Normal animals showed no decline of muscle strength from the first to the 15th trial, irrespective of age, bedding, or dietary conditions(data not shown). In contrast, mdx mice of all age and experimental groups showed various degrees of fatiguability. When plotted on a scale (Fig. 2), the percentage of failed climbing trials resulted in positive slopes, ranging from a minimum of 1.23 ± 0.05 in young mice on the α-tocopherol diet (= low fatiguability) to a maximum of 3.60 ± 0.20 in old mice on conventional standard diet (= high fatiguability). Generally, fatiguability increased over time in all experimental groups and was not significantly affected by the bedding or diet, except in the youngest age group, where the least fatiguability was observed in the α-tocopherol diet groups (Fig. 2).
Body weights of mdx mice in the different experimental groups followed to a certain extent the course of muscle weakness(Table 3). Bedding and diets did not affect body weights in young mdx mice. Older mdx mice, however, lost a significant amount of body weight compared with middle aged mdx mice. This drop was almost completely prevented by wheat kernel bedding, but was only incompletely counteracted by the α-tocopherol diet(Table 3).
DISCUSSION
Motivated by an incidental observation made during an attempt to breed mdx mice, we established a protective effect of wheat kernel bedding on progressive muscular weakness and weight loss in mdx mice. In contrast, we found that bedding on wood shavings and feeding of a standard diet or a α-tocopherol-supplemented diet did not prevent the progression of muscle weakness and weight loss with age.
The observation that mdx mice developed muscular weakness is in agreement with histopathologic observations on hind limb muscles of mdx mice raised on conventional diet without wheat kernel bedding(C. Pastoret, personal communication)(8, 9). Likewise, Muntoni et al.(16) observed muscular weakness at an early age of mdx mice raised on conventional diet without wheat kernel bedding (F. Muntoni, personal communication), and Makiejus et al.(15) found muscular weakness in mdx mice older than 140 d. To our knowledge, muscle fatiguability in mdx mice, and its increase over time, has not been described previously in mdx mice. We confirmed studies that have shown an increase in hind limb muscle weight due to hypertrophy of regenerated fibers(8, 9).
mdx mice have deficits in mental retention(31) and in passive avoidance behavior(32). This cognitive dysfunction may be related to a lack of dystrophin at postsynaptic membranes, which is present in normal mice(33), and may possibly interfere with the evaluation of muscle strength in mdx mice. However, brain dysfunction as a consequence of dystrophin deficiency is not progressive in DMD patients(1, 3). Therefore, the progressive weakness of mdx mice up to an age beyond 200 d most likely is not related to cognitive dysfunction. Moreover, mdx mice showed fatiguability, indicating that muscle strength and not cognitive dysfunction was evaluated by the tests.
Progression of muscular weakness in mdx mice was almost completely prevented by wheat kernel bedding. Increased α-tocopherol plasma levels in mdx mice, but not in normal mice on wheat kernel bedding, suggest that only mdx mice feel the urge to supplement their diet by some component in the wheat kernel bedding. Wheat is a rich source of α-tocopherol(18). Shortly after its discovery, it was observed that α-tocopherol-deficient rats show clumsy walking and climbing, flaccid paralysis with dragging legs, and hair loss(34, 35). Inasmuch as we saw these very same features in the mdx mice that we had placed on a bedding of wood shavings, and because bedding on wheat kernels appeared to prevent this clinical picture (Fig. 3), we assumed thatα-tocopherol could be the key preventive agent in wheat kernels. In a series of experiments we found that supplementation of α-tocopherol resulted in similar plasma α-tocopherol levels as bedding on wheat kernels. However, the results with α-tocopherol-supplemented diet were disappointing: no significant protection from progression of muscle weakness was observed in middle-aged mice. There was only a reduction of fatiguability in the youngest age group of mdx mice.
Beside α-tocopherol, wheat kernels contain a vast array of other biologically active constituents, such as γ-tocopherol. tocotrienols(18), or wheat germ agglutinin, to name only a few. Wheat germ agglutinin is a lectin which has been shown to bind dystrophin via the dystrophin-glycoprotein complex with high affinity(3, 36, 37). To what extent wheat germ agglutinin or other components of wheat kernels confer the beneficial effects on muscular strength in mdx mice is a matter of speculation at this point, but will be addressed in future studies. In any case, a simple high caloric effect due to additional wheat kernel ingestion is unlikely, because high fat diet does not influence the muscle degeneration in mdx mice(38).
It should be noted that wheat products make up roughly 65% of a conventional rodent diet, such as the one used in our study. However, the crucial protective components may be located within the seed coat and aleurone layer and may thus be lost during diet preparation, i.e. during the process of pelleting, which exposes the diet to heat, oxidative, and mechanical stress.
Our observation that α-tocopherol, one of the most plentiful and powerful chain-breaking lipophilic antioxidants(39), did not significantly preserve muscle strength suggests that progression of muscular weakness in mdx mice is not related to lipid peroxidation. Indeed, as already shown by others(40) we did not find any differences between control and mdx muscle in the potential substrates of lipid peroxidation(39, 41), such as poly-cis-unsaturated fatty acids or degradation products of lipid hydroperoxides (thiobarbituric acid-reactive substances). Only the increase of GSH-Px activity that has been found in DMD(42) and mdx(43) (our study) muscle tissues may indicate elevated production of oxyradicals(39, 41). However, there is no activation of the antioxidant CuZnSOD and MnSOD in DMD(42) and mdx(43)(our study) muscle. GSH-Px, but not superoxide dismutases, are activated by calpain(44), and calpain activity is increased in DMD(45) and mdx(46) muscle tissue. Skeletal muscle calpain in turn is inactivated by NO(47). Neuronal-type NO synthase is associated with dystrophin and is deficient in the sarcolemma of DMD and mdx muscle tissues and in cytosol of DMD muscle cells(37). Because No is also a messenger molecule for myoblast fusion(48), it is not surprising that incomplete myoblast fusion has been described in muscle tissue from DMD patients(49). Thus, increased activity of calpain in DMD(45) and mdx(46) muscle tissues may be caused by NO deficiency and activate GSH-Px. Preliminary data from our laboratory suggests an inhibitory effect of wheat kernel components on GSH-Px activity in mdx muscle tissue (wheat bedding: 101.8± 12.4 U/mg of protein, n = 6; standard diet: 210.0 ± 12.7 U/mg of protein, n = 12; p < 0.002). Whether the inhibition of GSH-Px by wheat kernel ingestion is related to the protective effect on muscular dystrophy deserves further investigation.
Abbreviations
- CuZnSOD:
-
copper-zinc superoxide dismutase
- DMD:
-
Duchenne muscular dystrophy
- GSH-Px:
-
glutathione peroxidase
- mdx, :
-
X chromosomal muscle dystrophic mouse
- MnSOD:
-
manganese superoxide dismutase
- NO:
-
nitric oxide
- wt:
-
weight
References
Engel AG, Yamamoto M, Fischbeck KH 1994 Dystrophinopathies. In: Engel AG, Franzini-Armstrong C (eds) Myology, 2nd Ed, Vol II. McGraw-Hill, New York, pp 1133–1187
Hoffman EP, Brown RH Jr, Kunkel LM 1987 Dystrophin: the protein product of the Duchenne muscular dystrophy locus. Cell 51: 919–928
Ahn AH, Kunkel LM 1993 The structural and functional diversity of dystrophin. Nat Genet 3: 283–291
Bulfield G, Siller WG, Wight PAL, Moore KJ 1984 Xchromosome-linked dystrophy (mdx) in the mouse. Proc Natl Acad Sci USA 81: 1189–1192
McArdle A, Edwards RH, Jackson MJ 1995 How does dystrophin deficiency lead to muscle degeneration? Evidence from the mdx mouse. Neuromuscul Disord 5: 445–456
Sicinski P, Gang Y, Ryder-Cook AS, Barnard EA, Darlison MG, Barnard PJ 1989 The molecular basis of muscular dystrophy in the mdx mouse: a point mutation. Science 244: 1578–1580
Cox GA, Phelps SF, Chapman VM, Chamberlain JS 1993 New mdx mutation disrupts expression of muscle and non muscle isoform of dystrophin. Nat Genet 4: 87–93
Pastoret C, Sebille A 1993 Further aspects of muscular dystrophy in mdx mice. Neuromuscul Disord 3: 471–475
Pastoret C, Sebille A 1995 Mdx mice show progressive weakness and muscle deterioration with age. J Neurol Sci 129: 97–105
Tanabe Y, Esaki K, Nomura T 1986 Skeletal muscle pathology in X chromosome-linked muscular dystrophy (mdx) mouse. Acta Neuropathol 69: 91–95
Coulton GR, Morgan JE, Partridge TA, Sloper JC 1988 The mdx mouse skeletal muscle myopathy: I. A histological, morphometric and biochemical investigation. Neuropathol Appl Neurobiol 14: 53–70
Lefaucheur JP, Pastoret C, Sebille A 1995 Phenotype of dystrophinopathy in old MDX mice. Anat Rec 242: 70–76
Carnwath JW, Shotton DM 1987 Muscular dystrophy in the mdx mouse: histopathology of the soleus and extensor digitorum longus muscles. J Neurol Sci 80: 39–54
Torres LFB, Duchen LW 1987 The mutant mdx: inherited myopathy in the mouse. Brain 110: 269–299
Makiejus RV, Patel VK, Krishna G, Dierdorf SF, Bonsett C 1991 Effect of adenylosuccinate on the strength of dystrophin lacking muscles of mdx mice. Biochem Arch 7: 95–103
Muntoni F, Mateddu A, Marchei F, Clerk A, Serra G 1993 Muscular weakness in the mdx mouse. J Neurol Sci 120: 71–77
Carter GT, Wineinger MA, Walsh SA, Horasek SJ, Abresch RT, Fowler WM Jr 1995 Effect of voluntary wheel-running exercise on muscles of the mdx mouse. Neuromuscul Disord 5: 323–332
Sheppard AJ, Pennington AT, Weihrauch JL 1993 Analysis and distribution of vitamin E in vegetable oils and foods. In: Packer L, Fuchs J (eds) Vitamin E in Health and Disease. Marcel Decker, New York, pp 9–31
Bicknell F 1940 Vitamin E in the treatment of muscular dystrophies and nervous diseases. Lancet 1: 10–13
Fenichel GM, Brooke MH, Griggs RC, Mendell JR, Miller JP, Moxley RT III, Park JH, Provine MA, Florence J, Kaiser KK, King WM, Pandya S, Robison J, Signore L 1988 Clinical investigation in Duchenne muscular dystrophy: penicillamine and vitamin E. Muscle Nerve 11: 1164–1168
Sies H 1993 Efficacy of Vitamin E in the Human. VERIS, LaGrange, II
Folch J, Lees M, Sloane Stanley GH 1957 A simple method for the isolation and purification of total lipids from animal tissue. J Biol Chem 226: 497–509
Kontush A, Hübner C, Finckh B, Kohlschütter A, Beisiegel U 1994 Low density lipoprotein oxidizability by copper correlates to its initial ubiquinol-10 and polyunsaturated fatty acid content. FEBS Lett 341: 69–73
Rath M, Niendorf A, Redlin T, Dietel M, Krebber HJ, Beisiegel U 1989 Detection and quantification of lipoprotein (a) in the arterial wall of 107 coronary bypass patients. Arteriosclerosis 9: 579–592
Naidoo R, Knapp ML 1992 Studies of lipid peroxidation products in cerebrospinal fluid and serum in multiple sclerosis and other conditions. CLin Chem 38: 2449–2454
Marklund SL, Westman NG, Roos G, Carlsson J 1984 Radiation resistance and the CuZn superoxide dismutase, Mn superoxide dismutase, catalase and glutathione peroxidase activities of seven human cell-lines. Radiat Res 100: 115–123
Marklund SL 1985 Direct assay of superoxide dismutase with potassium superoxide. In: Greenwald RA (ed) Handbook of Methods for Oxygen Radical Research. CRC Press, Boca Raton, FL, pp 249–255
Bradford MM 1976 A rapid and sensitive method for the quantitation of protein utilizing the principle of protein-dye binding. Ann Biochem 72: 248–254
Lombeck I, Menzel H 1988 Selenium in neonates and children. In: Favier A (ed) Selenium in Medicine and Biology. Walter de Gruyter, Berlin, pp 197–206
SAS Institute 1988 SAS/STAT User's Guide, Release 6.03. SAS Institute, Inc, Cary, NC
Vaillend C, Rendon A, Misslin R, Ungerer A 1995 Influence of dystrophin-gene mutation on mdx mouse behavior. 1. Retention deficits at long delays in spontaneous alternation and bar-pressing tasks. Behav Genet 25: 569–579
Muntoni F, Mateddu A, Serra G 1991 Passive avoidance behaviour deficit in the mdx mouse. Neuromuscul Disord 1: 121–123
Lidov HGW, Byers TJ, Watkins SC, Kunkel LM 1990 Localization of dystrophin to postsynaptic regions of central nervous system cortical neurons. Nature 348: 725–728
Evans HM, Burr GO 1928 Development of paralysis in the suckling young of mothers deprived of vitamin E. J Biol Chem 76: 273–297
Verzar F 1939 Der Kreatin-Stoffwechsel bei der Muskeldystrophie durch E-Vitamin-Mangel und seine Beeinflussung durch Tocopherol. Z Vitaminforschung 9: 242–251
Ervasti JM, Ohlendieck K, Kahl SD, Gaver MG, Campbell KP 1990 Deficiency of a glycoprotein component of the dystrophin complex in dystrophic muscle. Nature 345: 315–319
Brenman JE, Chao DS, Xia H, Aldape K, Bredt DS 1995 Nitric oxide synthase complexed with dystrophin and absent from skeletal muscle sarcolemma in Duchenne muscular dystrophy. Cell 82: 743–752
Mokhtarian A, Lefaucheur JP, Even PC, Sebille A 1995 Effects of treadmill exercise and high-fat feeding on muscle degeneration in mdx mice at the time of weaning. Clin Sci 89: 447–452
Halliwell B, Gutteridge JMC 1989 Free Radicals in Biology and Medicine, 2nd Ed. Clarendon Press, Oxford
Foxley A, Edwards RHT, Jackson MJ 1991 Enhanced lipid peroxidation in Duchenne dystrophy muscle may be secondary to muscle damage. Biochem Soc Trans 19( suppl): 180S.
Brown, RH 1995 Free radicals, programmed cell death and muscular dystrophy. Curr Opin Neurol 8: 373–378
Austin L, de Niese M, McGregor A, Arthur H, Gurusinghe A, Gould MK 1992 Potential oxyradical damage and energy status in individual muscle fibres from degenerating muscle diseases. Neuromuscul Disord 2: 27–33
Asayama K, Hayashibe H, Dobashi K, Kato K 1989 Lipid peroxide and antioxidant enzymes in muscle and nonmuscle of dystrophic mouse. Muscle Nerve 12: 742–748
Johnson P, Hammer JL 1994 Effects of calpain on antioxidant enzyme activities. Free Radic Res 21: 27–33
Nagy B, Samaha FJ 1986 Membrane defects in Duchenne dystrophy: protease affecting sarcoplasmic reticulum. Ann Neurol 20: 50–56
Spencer MJ, Croall DE, Tidball JG 1995 Calpains are activated in necrotic fibers from mdx dystrophic mice. J Biol Chem 270: 10909–10914
Michetti M, Salamino F, Melloni E, Pontremoli S 1995 Reversible inactivation of calpain isoforms by nitric oxide. Biochem Biophys Res Commun 207: 1009–1014
Lee KH, Back MY, Moon KY, Song WK, Chung CH, Ha DB, Kang M-S 1994 Nitric oxide as a messenger molecule for myoblast fusion. J Biol Chem 269: 14371–14374
Schmalbruch H 1984 Regenerated muscle fibers in Duchenne muscular dystrophy: a serial section study. Neurology 34: 60–65
Acknowledgements
We thank Dr. D. Koeltgen (Munich) for providing the mouse strains and Prof. Dr. K. Meßmer and Dr. F. Krombach(Munich) for the use of the animal housing facilities. The excellent technical assistance of B. Sehringer-Mansur and I. Wernicke (Hamburg) is gratefully acknowledged.
Author information
Authors and Affiliations
Additional information
Supported in part by the parents' action group Helft dem muskelkranken Kind, Hamburg, and in part by a grant from the Deutsche Forschungsgemeinschaft(Vo 392/2-3).
Rights and permissions
About this article
Cite this article
Hübner, C., Lehr, HA., Bodlaj, R. et al. Wheat Kernel Ingestion Protects from Progression of Muscle Weakness in mdx Mice, an Animal Model of Duchenne Muscular Dystrophy. Pediatr Res 40, 444–449 (1996). https://doi.org/10.1203/00006450-199609000-00013
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1203/00006450-199609000-00013