Abstract
To determine whether reduced maternofetal transfer of neutral amino acids in growth-restricted fetal rats is due to decreased system A transporter activity, we measured Na+-dependent MeAIB uptake by membrane vesicles from placentas of fetuses growth-restricted due to uterine artery ligation and control placentas (sham ligation). Na+-dependent uptake of methylaminoisobutyric acid (MeAIB) was linear over 15-60 s in vesicles from both ligated and sham-ligated sides of the uterus. Na+-dependent uptake of MeAIB at 30 s did not differ in paired measurements on vesicles from ligated and sham-ligated horns, 0.063 ± 0.004 versus 0.056± 0.005 nmol/mg of vesicle protein. The kinetics of Na+-dependent MeAIB uptake were similar in paired measurements on vesicles from ligated and sham-ligated horns, with overall Km= 4.4 ± 0.5 mM and Vmax = 0.93 ± 0.08 nmol/mg vesicle protein per 30 s. Uptake of tracer was inhibited 85-95% by known substrates for the system A amino acid transporter (alanine ≥ serine > MeAIB > glycine = proline). We conclude that the system A transporter is present in the maternal facing plasma membrane of rat syncytiotrophoblast, but that the activity of this system, per mg of vesicle protein, is unaffected in fetal growth restriction induced by a decrease in maternal placental blood flow in late pregnancy.
Similar content being viewed by others
Main
Elucidation of the mechanisms involved in FGR is an important priority in perinatal medicine, because of the increased morbidity associated with low birth weight(1, 2). Additionally, a better understanding of the physiology of fetal growth is important in the context of adult health, because there seems to be a strong link between low birth weight and the later incidence of hypertension and diabetes(3).
It has been suggested that the primary limitation on fetal growth in FGR is an insufficient supply of amino acids and restraint of protein synthesis(4, 5). Thus a significant reduction inα-amino nitrogen and in most essential amino acids is demonstrable in cord blood obtained from SGA infants(6). Indeed, there is a disproportionate reduction in the supply of amino acids to the SGA fetus compared with glucose and oxygen uptake(7). Amino acid transport across the syncytiotrophoblast of human placenta involves uptake across the maternal facing microvillous membrane and release at the basal membrane(8). We have shown that theVmax for uptake of MeAIB, a nonmetabolizable amino acid, by the Na+-dependent system A amino acid transporter is 63% lower, per mg of protein, in microvillous membrane vesicles prepared from placentas of SGA infants(9). Substrates for the system A transporter are amino acids with short, nonpolar side chains, and it is characterized by measuring uptake of MeAIB(8).
Many features of FGR, such as asymmetric growth and brain sparing, are reproducible in animal models that rely on interference with the maternal blood supply to the placenta. The simplest way to do this in rodents is by uterine artery ligation(10–12). Because decreased placental amino acid transporter activity may be an important aspect of human FGR, it is important to establish whether this is reproducible in animal models. Intervention studies could then be carried out to test possible therapies, thereby reducing the hazards attendant on direct human studies(13). There is evidence that the rodent model is appropriate for the study of amino acid transport in FGR. Thus a significant reduction in maternofetal transfer of AIB has been shown to occur after uterine artery ligation in rats(14) and guinea pigs(15). We hypothesized that this might result from diminished Na+-dependent transport activity analogous to the reduction in system A activity in vesicles from the placentas of SGA infants(9). To address this hypothesis, we have measured the uptake of MeAIB by membrane vesicles prepared by established techniques(16) from placentas of normally grown rat fetuses and of fetuses that were growth restricted as a result of uterine artery ligation. We have also examined the kinetics of Na+-dependent MeAIB uptake, as well as its inhibition by other amino acids, to determine whether the transporter in the rat placenta has characteristics similar to those of the system A transporter in human placenta.
METHODS
Uterine artery ligation. The studies were performed according to protocols licensed under the U.K. Animals (Scientific Procedures) Act, 1986. Forty-seven Sprague-Dawley rats with time-dated pregnancies (copulation plug found on d 1) were used for this study. On d 17 of gestation they were anesthetized with 2% halothane in N2O and O2 (50% each by volume), and the uterine artery supplying one horn was ligated at the level of the cervix with silk thread(11). We alternated the ligation procedure between left and right horns and performed sham ligation of the contralateral artery by drawing a silk thread beneath the vessel.
On d 21 of gestation the rats were again anesthetized with halothane in N2O and O2 and the uterus and its contents were inspected. Four rats were excluded at this stage, three due to extensive bleeding within the uterus and one because it had only a single living fetus. We noted the number of dead fetuses and the weights of the surviving fetuses and harvested the placentas from the remaining 43 rats. The placentas were trimmed of fetal membranes, blotted, weighed, and kept on ice until pooled. The fetuses were decapitated and the rats killed by dislocation of the neck and exsanguination.
To ensure a sufficient yield of protein for our studies, we were compelled to pool placentas from several rats. Care was taken to ensure that these were at the same stage of gestation, as indicated by the weights of the fetuses in the control horn. We established 14 pools of placentas, derived from the paired ligated and sham-ligated horns, respectively, of 34 rats (two to six rats in each pool). These seven pairs were used for comparisons of the initial rates and kinetics of MeAIB uptake. The placentas of all surviving fetuses on the ligated side were included, irrespective of the degree of growth restriction achieved. However, in 13 rats a single fetus at the cervical end of the ligated horn was supplied by vessels below the ligature; these fetuses weighed 4.14 ± 0.21 g, not significantly different from fetuses in the control horn, and were excluded from the study.
Preparation of plasma membrane vesicles. Plasma membrane vesicles were prepared from the placentas by a homogenization and MgCl2 precipitation technique, as described in detail elsewhere(16). This yielded a final pellet that was resuspended in the intravesicular buffer (5 mM Tris, 5 mM HEPES, 290 mM sucrose, pH 7.4). This suspension was passed 15-20 times through a 25-G syringe needle to vesiculate the membranes. The vesicle suspension was stored at 4°C, and all analyses were performed within 48 h of vesicle preparation.
The protein content of the homogenate and vesicle suspension was measured by the Lowry method(17), using BSA as a standard. Alkaline phosphatase (EC 3.1.3.1) activity, as a marker of the maternal facing plasma membrane of syncytiotrophoblast layer II(16), was measured at pH 9.8 using p-nitrophenylphosphate as a substrate(18).
Determination of MeAIB uptakes. Uptake of MeAIB by placental plasma membrane vesicles in the presence or absence of an inwardly directed Na+-gradient was determined as formerly described(19). Briefly, uptake was initiated by the addition of 20 μL of vesicle suspension to 20 μL of extravesicular buffer (5 mM Tris, 5 mM HEPES, 145 mM NaCl or KCl, and 0.33 mM [14C]MeAIB, specific activity 56.3 mCi/mmol). At timed intervals uptake was stopped by the addition of 2 mL of ice-cold KRP (130 mM NaCl, 10 mM Na2HPO4, 4.2 mM KCl, 1.2 mM MgSO4, and 0.75 mM CaCl2, pH 7.4)(19). Two milliliters of the resultant mixture were applied to a 0.45-μm Millipore filter, which had been soaked in ice-cold KRP buffer, and the filter was washed with 10 mL of ice-cold KRP buffer. The filters were then dissolved in 2 mL of 2-ethoxyethanol, 12 mL of scintillation fluid were added (Optiphase Hisafe II, Pharmacia, Milton Keynes, UK), and the vials were counted in a liquid scintillation counter (Packard 2000CA).
In four experiments the time course of MeAIB uptake over 15-60 s was studied, to determine linearity; all subsequent uptake measurements were made at 30 s. We also measured MeAIB uptake at equilibrium (24 h) and used these data to calculate the mean total intravesicular volume of each preparation.
To determine the kinetics of the transport system, we measured Na+-dependent uptake of [14C]MeAIB in the presence of increasing concentrations (1-20 mM) of cold MeAIB. Uptakes were also measured in the presence of 20 mM concentrations of four amino acids known to be transported by the system A transporter of human placenta(19).
Radioisotopes were obtained from DuPont (Stevenage, Hertfordshire, UK) and chemicals from Sigma Chemical Co. (Poole, Dorset, UK) or BDH (Liverpool, UK).
Statistical analysis. Data are shown as means ± SEM. The significance of differences between means was examined by a paired t test. To study the kinetics of MeAIB uptake, we plotted uptake at 30 s against the total MeAIB concentration in the extravesicular buffer and fitted the data to the Michaelis Menten equation by nonlinear regression (P-Fit, Biosoft, Cambridge, UK).
RESULTS
Effect of uterine artery ligation. Fetal outcome after confirmed unilateral uterine artery ligation in 43 rats and sham ligation of the contralateral artery is shown in Table 1. In nine rats, uterine artery ligation resulted in the death of all fetuses in that horn, but placentas from the contralateral horn were in some cases used for supplementary studies on the inhibition of MeAIB uptake by other amino acids. Comparisons of the initial rates and kinetics of MeAIB uptake, however, were all based on seven pools of placentas derived from the paired ligated and sham-ligated horns, respectively, of 34 rats (two to six rats in each pool). The mean fetal weights in the seven pools from ligated and sham-ligated horns, respectively, were 3.17 ± 0.13 and 4.02 ± 0.09 g (p< 0.001); the corresponding placental weights were 0.43 ± 0.03 and 0.50 ± 0.04 g (p < 0.01).
Characterization of plasma membrane vesicles. The yield of vesicle protein from each gram of placenta was similar in pools of vesicles prepared from ligated and sham-ligated sides of the uterus(Table 2). There was a significant difference in alkaline phosphatase enrichment between paired pools of vesicles, with slightly higher enrichments in vesicles made from placentas on the ligated side of the uterus(p < 0.05; Table 2). The corresponding intravesicular volumes, calculated from MeAIB uptake at equilibrium, did not differ significantly although the variance was comparatively large(Table 2).
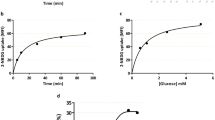
Effect of uterine artery ligation on MeAIB uptake. Time courses over 15-60 s for MeAIB uptake in the presence or absence of an inwardly directed Na+ gradient, as well as the difference between the two(Na+-dependent uptake), are shown in Fig. 1. Linear regression analysis of the Na+-dependent MeAIB uptake over this period gave a good fit for both groups (for vesicles from the ligated horn,r = 0.96, n = 4, p < 0.05 and for the vesicles from the sham-ligated horn, r = 0.99, n = 4,p < 0.01). Therefore, we proceeded to measure uptakes at 30 s and took these to be representative of the initial rate.
Time course of MeAIB uptake into plasma membrane vesicles from placentas of growth restricted (A, uterine artery ligated) and normally grown (B, uterine artery sham ligated) rat fetuses in the presence of a Na+ gradient (▪) and a K+ gradient (▴). Values are mean ± SEM (n = 4 pools of vesicles in each group). Also shown is the Na+-dependent MeAIB uptake(♦), calculated as the difference between the uptake in the presence of a Na+ gradient and that in the presence of a K+ gradient, mean± SEM; the dashed line shows the linear regression fit to this data.
Na+-dependent MeAIB uptake at 30 s did not differ in paired measurements on vesicles from ligated and sham-ligated horns, 0.063 ± 0.004 versus 0.056 ± 0.005 nmol/mg vesicle protein per 30 s(n = 7 pools in each group).
Kinetics of sodium dependent MeAIB uptake. Uptake kinetics were measured in each of four paired pools of vesicles from ligated and sham ligated horns. The mean Km> and Vmax, derived assuming Michaelis-Menten kinetics, were similar in ligated and sham-ligated pools (Km = 4.4 ± 0.8 versus 4.3 ± 1.0 mM; Vmax = 0.96 ± 0.15versus 0.84 ± 0.13 nmol/mg of vesicle protein/30 s). We therefore pooled the eight sets of measurements and meaned theKm and Vmax values derived from them to obtain the data shown in Fig. 2.
Inhibition of sodium dependent MeAIB uptake. The effects of 20 mM cold MeAIB and four other amino acids on the initial rate of uptake of[14C]MeAIB were similar in vesicles derived from ligated and sham ligated sides of the uterus and have therefore been pooled. [14C]MeAIB uptake at 30 s was reduced to 5 ± 1% (n = 13) of the control value in the presence of alanine, to 6 ± 1% (n = 5) by serine, 11 ± 1% (n = 13) by MeAIB, 15 ± 2%(n = 12) by glycine, and 15 ± 1% (n = 5) by proline(n = number of observations). Thus in terms of inhibition, the order of effectiveness of these amino acids was alanine ≥ serine > MeAIB > glycine = proline.
DISCUSSION
This study has demonstrated, first, that Na+-dependent MeAIB uptake by plasma membrane vesicles from rat placenta utilizes a transporter with characteristics closely similar to the human placental system A transporter(9, 19). Second, when FGR was induced in rats by uterine artery ligation, there was no demonstrable effect on Na+-dependent vesicular uptake of MeAIB, per mg of protein, which is in contrast to our previous finding that this uptake is decreased in vesicles prepared from the placentas of SGA infants(9).
Effect of uterine artery ligation on fetal growth. FGR by uterine artery ligation was first described by Wigglesworth(12) and has been used to study placental transport of nonmetabolizable amino acids in the intact rat(14) and guinea pig(15). As shown previously(12), we found the most severe growth restriction to occur in fetuses close to the ligature, whereas those at the tubal end of the horn are less affected. Overall, we achieved a 25% decrease in fetal weight. In contrast to an earlier report(14), we also saw a significant 17% reduction in placental weight.
Characterization of plasma membrane vesicles. Alkaline phosphatase is localized mainly to the maternal facing plasma membrane of syncytiotrophoblast layer II of the hemotrichorial rat placenta(16). Earlier we demonstrated that vesicles prepared by the present procedure are derived mainly from this plasma membrane and that they are oriented predominantly right side out, i.e. with the extracellular face outermost(16).
Enrichment of alkaline phosphatase activity in vesicles compared with homogenate was 11-13-fold, as we have reported previously for the rat(16). Although enrichments are higher in microvillous membrane vesicles prepared from human placenta by an identical procedure(20), our values for the rat are markedly higher than the 6-fold enrichment of alkaline phosphatase obtained in the rat by Alonso de la Torre et al.(21).
There is no ready explanation for the slightly greater values for alkaline phosphatase enrichment in vesicles derived from the ligated side of the uterus. Intravesicular volumes were comparable between pools from ligated and sham-ligated horns and similar to those previously observed (J. D. Glazier, unpublished data).
Characteristics of the MeAIB transporter. Others have reported that plasma membrane vesicles from rat placenta contain two transport systems for the neutral amino acid L-alanine, of which one is Na+-dependent(22). We have refined and extended that study by measuring the Na+-dependent uptake of MeAIB, a specific substrate for the system A transporter in human placenta(19). The kinetics of MeAIB uptake in rat vesicles closely resemble those of the system A transporter of human placenta, measured under identical conditions in this laboratory(9); values for rat and human, respectively, are Km = 4.42 and 5.35 mM, Vmax = 0.93 and 0.64 nmol/mg protein per 30 s.
Additional evidence for functional homology between the MeAIB transporter of rat placenta and the system A transporter of human placenta was acquired by examining the effects of amino acids at 20 mM concentration on uptake of MeAIB. Their effectiveness in inhibiting uptake was alanine ≥ serine > MeAIB > glycine = proline, the same order as observed for inhibition of the system A transporter in microvillous membrane vesicles from human placenta(19).
Effect of uterine artery ligation on MeAIB uptake. Based on our studies of system A activity in placentas from SGA babies(9), and reports of a diminished transport capacity for AIB in placentas of growth-restricted rats(14), we hypothesized that system A activity would be reduced in plasma membrane vesicles from placentas of rat fetuses whose growth had been curtailed by uterine artery ligation. Contrary to our expectations, however, we found no difference in the rate of MeAIB uptake in paired measurements on vesicles from ligated and sham-ligated horns. The affinity and capacity of the MeAIB transporter were also similar in ligated and sham ligated pools.
To ensure a sufficient yield of protein for our studies, we were compelled to pool placentas from several rats. However, care was taken to ensure that these were at the same stage of gestation, as indicated by the weights of the fetuses in the control horns. More importantly, we pooled the placentas of all surviving fetuses on the ligated side, irrespective of the degree of growth restriction achieved. It cannot entirely be excluded that a different outcome would have resulted from selection of the smallest fetuses. However, we followed a procedure similar to that described by Nitzan et al.(14), who were able to show a large reduction in the rate of accumulation of AIB by rat fetuses.
Factors that determine placental transport capacity include the rate at which a substance is presented (essentially a function of placental blood flow), the surface area of membrane available for transfer, and the density of transporter protein in the membrane. Because the effect of uterine artery ligation is essentially to reduce maternal placental blood flow(23), this might in itself suffice to explain the reduction in AIB transfer reported for the intact rat(14). Alternatively, the analogy between rat and human may not be complete. Despite the apparent similarity between the maternal facing plasma membrane of syncytiotrophoblast layer II of the rat placenta and the microvillous membrane of human syncytiotrophoblast(16), MeAIB transport across the former need not be rate limiting. System A activity is present in the basal membrane of human syncytiotrophoblast(24) and could conceivably be present on other plasma membranes of the rat placenta.
There can be little doubt that a diminished amino acid supply is a critical feature of human FGR(6, 13) that is reproducible in some animal models(5). However, the reduced system A activity that we have observed in placentas from SGA pregnancies(9) may reflect intrinsic placental pathology(25) that is not reproduced after reduction of maternal placental blood flow in the uterine artery ligation model.
Abbreviations
- AIB:
-
aminoisobutyric acid
- FGR:
-
fetal growth restriction
- KRP:
-
Krebs Ringer phosphate buffer
- MeAIB:
-
methylaminoisobutyric acid
- SGA:
-
small for gestational age
References
Blair E, Stanley F 1990 Intrauterine growth and spastic cerebral palsy. I. Association with birth weight for gestational age. Am J Obstet Gynecol 162: 229–237
Dunn HG 1986 Sequelae of Low Birthweight: The Vancouver Study. Blackwell Scientific Publications, Oxford
Barker DJ, Gluckman PD, Godfrey KM, Harding JE, Owens JA, Robinson JS 1993 Fetal nutrition and cardiovascular disease in adult life. Lancet 341: 938–941
Carter AM 1993 Restriction of placental and fetal growth in the guinea-pig. Placenta 14: 125–135
Owens JA, Owens PC, Robinson JS 1989 Experimental fetal growth retardation: metabolic and endocrine aspects. In: Gluckman PD, Johnston BM, Nathanielsz PW (eds) Advances in Fetal Physiology: Reviews in Honor of G.C. Liggins. Perinatology Press, Ithaca, NY, pp 263–286
Cetin I, Corbetta C, Sereni LP, Marconi AM, Bozzetti P, Pardi G, Battaglia FC 1990 Umbilical amino acid concentrations in normal and growth-retarded fetuses sampled in utero by cordocentesis. Am J Obstet Gynecol 162: 253–261
Cetin I, Marconi AM, Corbetta C, Perugino G, Ronzoni S, Battaglia FC, Pardi G 1994 The relative uptakes of amino acids, glucose and oxygen across the umbilical circulation in normal and intrauterine growth-retarded (IUGR) pregnancies. Society for Gynecologic Investigation, Chicago, P159(abstr)
Morriss FH, Boyd RDH, Mahendran D 1994 Placental transport. In: Knobil E, Neill JD (eds) The Physiology of Reproduction. Raven Press, New York, pp 813–861
Mahendran D, Donnai P, Glazier JD, D'Souza SW, Boyd RDH, Sibley CP 1993 Amino acid (system A) transporter activity in microvillous membrane vesicles from the placentas of appropriate and small for gestational age babies. Pediatr Res 34: 661–665
Detmer A, Carter AM 1992 Factors influencing the outcome of ligating the uterine artery and vein in a guinea pig model of intrauterine growth retardation. Scand J Lab Anim Sci 19: 9–16
Kaminsky S, D'Souza SW, Massey RF, Smart JL, Sibley CP 1991 Effects of maternal undernutrition and uterine artery ligation on placental lipase activities in the rat. Biol Neonate 60: 201–206
Wigglesworth JS 1964 Experimental growth retardation in the fetal rat. J Pathol Bacteriol 88: 1–13
Harding JE, Owens JA, Robinson JS 1992 Should we try to supplement the growth retarded fetus? A cautionary tale. Br J Obstet Gynaecol 99: 707–710
Nitzan M, Orloff S, Schulman JD 1979 Placental transfer of analogs of glucose and amino acids in experimental intrauterine growth retardation. Pediatr Res 13: 100–103
Jansson T, Persson E 1990 Placental transfer of glucose and amino acids in intrauterine growth retardation: studies with substrate analogs in the awake guinea pig. Pediatr Res 35: 376–381
Glazier JD, Jones CPJ, Sibley CP 1988 Preparation of plasma membrane vesicles from the rat placenta at term and measurement of Na+ uptake. Placenta 11: 451–463
Lowry OH, Rosebrough NJ, Farr AL, Randall RJ 1951 Protein measurement with the Folin phenol reagent. J Biol Chem 193: 265–275
McComb RB, Bowers GN 1972 Study of optimum buffer conditions for measuring alkaline phosphatase activity in human serum. Clin Chem 18: 97–104
Johnson LW, Smith CH 1988 Neutral amino acid transport systems of microvillous membrane of human placenta. Am J Physiol 254:C773–C780
Glazier JD, Jones CPJ, Sibley CP 1988 Purification and Na+ uptake by human placental microvillous membrane vesicles prepared by three different methods. Biochim Biophys Acta 945: 127–134
Alonso de la Torre SR, Serrano MA, Alvarado F, Medina JM 1991 Carrier-mediated L-lactate transport in brush-border membrane vesicles from rat placenta during late gestation. Biochem J 278: 535–541
Alonso-Torre SR, Serrano MA, Medina JM, Alvarado F 1992 Heterogeneity of L-alanine transport systems in brush-border membrane vesicles from rat placenta during late gestation. Biochem J 288: 47–53
Gilbert M, Leturque A 1982 Fetal weight and its relationship to placental blood flow and placental weight in experimental intrauterine growth retardation in the rat. J Dev Physiol 4: 237–246
Hoeltzli SD, Smith CH 1989 Alanine transport systems in isolated basal plasma membrane of human placenta. Am J Physiol 256:C630–C637
Jackson MR, Walsh AJ, Morrow RJ, Mullen JBM, Lye SJ, Knox Ritchie JW 1995 Reduced placental villous tree elaboration in small-for-gestational-age pregnancies: Relationship with umbilical artery Doppler waveforms. Am J Obstet Gynecol 172: 518–525
Acknowledgements
The authors thank Professor Robert Boyd for his helpful comments on the manuscript.
Author information
Authors and Affiliations
Additional information
Supported by Action Research Endowment Fund (J.D.G. C.P.S.) and the Danish Medical Research Council (A.M.C.).
Rights and permissions
About this article
Cite this article
Glazier, J., Sibley, C. & Carter, A. Effect of Fetal Growth Restriction on System A Amino Acid Transporter Activity in the Maternal Facing Plasma Membrane of Rat Syncytiotrophoblast. Pediatr Res 40, 325–329 (1996). https://doi.org/10.1203/00006450-199608000-00022
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1203/00006450-199608000-00022