Abstract
Total lung capacity (TLC), inspiratory capacity (IC), functional residual capacity (FRC), and deflation pressure-volume (P-V) curves were studied in 16 intubated neonates (540-3300 g), 10 with severe respiratory distress syndrome(RDS) and 6 air-ventilated with normal chest radiograms. FRC was measured using washout of a tracer gas (sulfur hexafluoride), and TLC and IC were calculated after inflating the lungs to 30 cm H2O. P-V curves were obtained during expiration from TLC using an interrupter technique, and the steepest slope of the curve, i.e. the maximum compliance(Crs-max), was calculated. In addition, an index of ventilation inhomogeneity (pulmonary clearance delay, PCD) was computed from the shape of the SF6 washout curve. TLC/body weight was less in the RDS group than in the air-ventilated group (median 19 and range 16-43 mL/kg versus 48 and 43-52 mL/kg, respectively; p < 0.01), mainly because of a marked reduction in IC (median 11 and range 8-24 mL/kg versus 29 and 28-40 mL/kg; p < 0.01). The flatter P-V curve in the RDS group was reflected also in a lower Crs-max (median 0.7 and range 0.4-1.7 cm H2O-1 kg-1) than in the air-ventilated group (2.3 and 2.0-3.1 mL cm H2O-1 kg-1, respectively; p < 0.01). Thus, there was no overlap in IC or Crs-max between the groups, suggesting that reductions in these measures may be main characteristics of RDS. On the other hand, no difference in PCD was found, indicating that, in infants with RDS, the tidal volume is distributed fairly homogeneously to the ventilated parts of the lungs.
Similar content being viewed by others
Main
The impaired oxygenation seen in neonates with RDS is usually explained by alveolar collapse, resulting in reduced FRC(1). Jacksonet al.(2) found that premature pigtail monkeys(Macaca nemestrina) in addition had an early decline in IC, indicating that, soon after the onset of RDS, large parts of the lost lung volume are not recruitable. In mechanically ventilated neonates with RDS, FRC is the only lung volume that has been measured(3–5), and estimates of IC or TLC have not been reported(6, 7).
In RDS, the compliance of the lung and respiratory system as measured within the tidal volume is low(5, 8–11). However, these compliance values depend on the lung volume at which they are measured, the size of the tidal volume, and the preceding volume history, and therefore only in a rather indirect way reflect the elastic properties of the respiratory system(12). Ideally, these should be assessed by pressure and volume measurements during deflation of the lungs from TLC after a standardized volume history(13, 14), but this has previously not been done in premature infants.
The goal of this study was to analyze the impairment of lung function in RDS. This was done by measuring TLC and its subdivisions, by studying tracer gas washout, and by recording deflation P-V curves over the whole IC range with an interrupter technique. The method requires that the subjects are intubated and paralyzed, and can therefore not be applied in healthy infants. Instead, we compared findings in infants with early, severe RDS, with those obtained in a group of infants who were mechanically ventilated, but with air only, and who had normal or near-normal chest radiograms.
METHODS
The study was approved by the local Human Investigations Committee, and informed parental consent was obtained. Two groups of mechanically ventilated infants were studied (see Table 1). The air-ventilated group included six infants with normal chest radiograms, ventilated because of birth asphyxia (Table 1). The RDS group included 10 infants with severe RDS. This was defined as: typical chest radiogram, no sign of infection, and the need for an Fio2 of 0.50 or more to maintain a transcutaneous Po2 above 6 kPa. We did not attempt to examine very unstable infants or those with large leaks around the endotracheal tube. All but one (no. 14 of Table 1) subsequently received surfactant treatment. The infants in the two groups were all 4 d of age or less, but mean weight and gestational age were greater in the air-ventilated group (1.9 kg and 33 wk, respectively) than in the RDS group (1.2 kg and 29 wk).
At the time of the study all infants were intubated nasally with uncuffed Portex endotracheal tubes (size 2.5-3.0) and ventilated with a Servo 900C ventilator (Siemens Elema, Stockholm, Sweden) in pressure-controlled mode, with settings decided by the neonatologist in charge. Normoventilation was strived for, and this resulted in tidal volumes of 4.3-11.7 mL/kg (median 7.5 mL/kg for the air-ventilated group and 5.7 mL/kg for the RDS group). Fio2 was set to achieve a transcutaneous Po2 of 6-8 kPa. The rate was 45-80 breaths/min and insufflation time 33-50% of the breathing cycle. During measurement of FRC and dynamic compliance, an end-inspiratory pause of 5% of the breathing cycle was added. Peak inspiratory ventilator pressure was higher (p < 0.01) in the RDS group [26 ± 3 cm H2O (mean ± SD)] than in the air-ventilated group (14 ± 3 cm H2O), as was the PEEP setting [4.0 ± 0.4 cm H2Oversus 2.6 ± 0.8 cm H2O (p < 0.01)]. The infants were monitored continuously using varying combinations of ECG, direct arterial blood pressure recording, pulse oximetry, and transcutaneous Po2. Phenobarbitone and morphine were used for sedation, and all infants were given a muscle relaxant (pancuronium 0.1 mg/kg or atracurium 0.5 mg/kg) before measurement to abolish spontaneous respiratory efforts. Gentle digital compression was always applied over the trachea during the P-V maneuvers, and also during FRC measurements if a leak around the endotracheal tube was detected.
All infants tolerated the measurements well. During the P-V maneuvers, a transient decrease in blood pressure was sometimes observed. There were no incidents of hypoxia or bradycardia; in fact, transcutaneous Po2 usually increased during the repeated inflations.
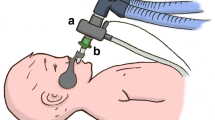
The recording of P-V curves(15, 16) always preceded FRC measurements. Before and after obtaining each P-V diagram, the lungs were manually inflated with 10 large breaths to counteract atelectasis and standardize the volume history. P-V measurements were done as follows: the equipment shown in Figure 1 was attached to the endotracheal tube, and the lungs were inflated by a syringe, attempting to reach an airway pressure just above 30 cm H2O, which was maintained for about 2 s. During the following passive deflation to zero airway pressure, the expired flow was interrupted for 80 ms every 160 ms by a fast, computer-controlled, electromagnetic valve (Harting 08300320120, Siemens Elema, Stockholm, Sweden), so that exhalation occurred in small portions. Airway flow was measured by a heated pneumotachograph (Fleisch no. 00, Lausanne, Switzerland; linearity flow range = 0-100 mL/s) connected to a differential pressure transducer (MP 45-1-871; < ±2.5 cm H2O; Validyne, Northridge, CA), and the expired volume between the interruptions was calculated by integration of the flow signal with a computer (PDP 11/23, Digital Equipment Corp., Marlboro, MA). Airway pressure was measured by a pressure transducer (SCX01DN, SenSym, Rugby, UK) at the end of each occlusion and was assumed to equal alveolar pressure. Static P-V curves were drawn by an X-Y-writer (HP 7575A). Measurements were discarded if the curves showed signs of spontaneous breathing. The first few interrupter cycles occurred while the investigator's finger prevented the lungs from emptying(Fig. 1), and a constant pressure during these cycles assured that no leak was present. Twenty to sixty data points were recorded during each deflation, which took 3-10 s. Two to four technically acceptable recordings were achieved in each case. Because an increase in the amplitude and steepness of the P-V curve was often seen between the first and the final measurement in the RDS patients, the last two P-V curves were taken to represent the infant.
IC was defined as the volume expired between 30 and 0 cm H2O of airway pressure. Because of the short deflation time, volume changes due to oxygen uptake were disregarded. In three infants a maximum airway pressure of only 26-27 cm H2O was reached. In these infants (marked with footnoted in Table 1) IC was calculated by extrapolation, assuming that the uppermost part of the P-V curve could be approximated by the equation(17):
The difference between actual IC, and IC extrapolated from 26-30 cm H2O, in the 13 infants in whom a pressure of 30 cm H2O was reached, was -3.6 to +0.7 mL (median -0.1 mL).
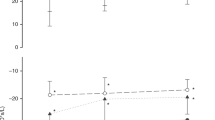
Crs-max was calculated as the slope of the steepest segment of the P-V curve. In many infants the P-V curves had no clearly defined inflection point, but rather contained a nearly linear segment (seeFig. 2). In these cases, the pressure and volume at which Crs-max occurred were defined as the mid-point of the linear portion of the curve. TLC was defined as FRC at used PEEP (measured as explained below) plus the exhaled volume from 30 cm H2O to PEEP, i.e. it was assumed that FRC during PEEP ventilation coincided with the volume at the corresponding pressure during the P-V maneuver. Specific compliance was calculated as Crs-max/TLC. Deflation stability (V10) was defined as the fraction of TLC still present after deflation to 10 cm H2O(2). The volumes at airway pressure 0, 2.5, 5, 15, 20, 25, and 30 cm H2O were also calculated and were used to construct mean P-V curves in each group (Fig. 2).
P-V curves, normalized to body weight and to TLC, from each of the 16 neonates. The curve with the steepest slope (greatest maximal compliance) was taken to represent the individual. Mean P-V curves of the two groups were obtained from the last two P-V curves of each infants (see text).Error bars indicate SEM.
After rearranging the equipment and reconnecting the infant to the ventilator, the flowmeter used during P-V measurements was used to measure Cdyn as tidal volume divided by the difference between end-inspiratory pause pressure and PEEP. Inspection of the flow curves revealed that flow had virtually ceased at end-expiration, i.e. Cdyn was obtained between points of zero airway flow. (Flow usually ceased also during end-inspiration-even before the end-inspiratory pause.)
FRC was measured with a computerized multiple breath washin-washout technique, using SF6 as tracer gas(18). The method allows repeated measurements (each washin-washout sequence takes 1-2 min), without interfering with ventilator settings or the inspired oxygen concentration. Briefly, the system included a ventilator (Servo 900C), inspiratory and expiratory valves (360P0121, Neptune Research, Maplewood, NJ) placed in the patient circuit close to the Y-piece, and a mainstream SF6 infrared transducer-analyzer(19) with its cuvette placed just downstream the expiratory valve. Pressures(end-inspiratory pressure and PEEP) were measured via a pressure port in the Y-piece by the ventilator pressure transducer, which was moved into close contact with the Y-piece. In addition inspiratory and expiratory flow was measured with heated pneumotachographs (Fleisch no. 00) connected to differential pressure transducers (MP 45-1-871, Validyne Engineering Corp., Northbridge, CA). For an FRC measurement to be accepted the difference between inspired and expired tidal volumes had to be less than 10%. SF6 was washed in at a rate proportional to inspiratory flow, until a stable alveolar concentration of approximately 1.5% was achieved. Signals representing flows and SF6 concentration were fed into the computer, which calculated FRC as the volume of SF6 washed out divided by the alveolar concentration at the end of washin. The apparatus volume between the tip of the SF6 dispensing catheter and the cuvette windows (1.5 mL) was subtracted.
Clinically used PEEP varied between 2 and 4 cm H2O, and FRC at this end-expiratory pressure (FRCp) was measured at least twice in each infant and the mean taken. FRC at ZEEP (FRCz) was measured in 11 infants (5 in the group with severe RDS and all in the air-ventilated group) by switching to ZEEP 5-10 breaths before the start of washout. In two infants, FRCz was measured only once. Five infants were judged too unstable for measurements during ZEEP (indicated by footnote d inTable 1). In these, FRCp was used to estimate FRCz, assuming that FRCp - FRCz equaled the volume change between the corresponding pressures in the P-V curve. The regression equation between calculated and measured FRCz in the 11 infants where FRCz was measured was: calculated FRCz = -1.4 mL + 1.09 × actual FRCz (r = 0.91). The difference between measured and calculated FRCz was -5 to +7 mL (median 1 mL).
Inhomogeneity of ventilation was assessed by calculating the PCD from the SF6 washout curves as described by Fowler et al.(20), but with the modification suggested by Bouhuyset al.(21), i.e. end-tidal concentration was used instead of mean expired concentration. PCD compares the average time a tracer gas molecule remains in the lungs (T1)-which is identical to the average time of clearing the lungs-with the average time (T2) a tracer gas molecule would remain in a uniformly ventilated ideal lung with the same FRC, series dead space, and ventilated with the same tidal volume. PCD was defined as: 100 × (T1 - T2)/T1. An interactive computer program was used(22). This approximated the curve as the sum of exponentially decaying functions: the computer plotted the washout curve semilogarithmically, and the user indicated how lines should be drawn to get the exponential curve components. The “peel-off” method was used,i.e. the slowest component was first identified and subtracted, and the plot was then redrawn to identify the remaining component(s). In all infants, an adequate approximation of the curve was achieved with two components. In normal infants, previously reported PCD values using N2 washout(8) were below 30%, in RDS 7-40%, and in chronic lung disease 90-350%. This agrees with values calculated from SF6 washout in healthy anesthetized children (25 ± 8%)(22) and in mechanically ventilated neonates with no or mild-to-moderate lung disease (43 ± 22%)(18).
Calibration. The flow signals were calibrated daily with oxygen in air, corresponding to the infant's Fio2, using a 50-mL syringe. Airway pressures were calibrated against a water manometer. The SF6 analyzer was stable(19) and was only intermittently calibrated with a precise reference gas. All volumes were converted to body temperature pressure saturation by multiplying with 1.09.
Statistics. Measurements in the RDS group and the air-ventilated group were compared with the Mann-Whitney U test.p values < 0.05 were considered to indicate statistical significance. Values are presented as mean ± SD when not otherwise indicated.
RESULTS
Results in the 16 infants are shown in Table 1, and the differences between the RDS group and air-ventilated groups are summarized in Table 2.
The coefficient of variation (range with median in parentheses) of repeated measurements was 0-20% (6%) for FRCp, 0-13% (6%) for FRCz, 0-15%(5%) for IC, and 0-14% (5%) for Crs-max. The most marked intraindividual variation was usually seen in infants with RDS. Crs-max increased between the first and last measurement in seven infants in the RDS group, and in six of these the increase was more than 10% of the initial value. The major part of the increase occurred between the first and second P-V maneuver and was thought to represent recruitment of lung volume after deep inflation. Therefore the last two P-V curves in each infant were used when calculating IC, TLC, and Crs-max. In the air-ventilated group, the first and last P-V curves were similar.
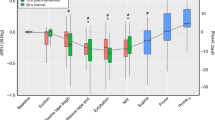
The shape of the P-V curves varied most between individuals in the RDS group. FRCz, IC, and TLC, normalized to body weight, were significantly less in the RDS group than in the air-ventilated group(Table 2, Figs. 2 and 3). However, FRCz/weight in the two groups overlapped, whereas IC/weight did not(Fig. 3). Crs-max and Cdyn were less in the RDS group, a relation that remained after the data had been normalized to weight (Table 2). When the compliance values were normalized to volume (TLC), only Crs-max was significantly less in the RDS group (Table 2). The steepest slope of the P-V curve occurred at a higher alveolar pressure in the RDS group (range 2-8, median 6 cm H2O) than in the air-ventilated group (range 3-6, median 5 cm H2O).
FRCz/TLC was greater (p < 0.05) in the RDS group (46± 10%) than in the air-ventilated group (35 ± 8%)(Table 2, Fig. 2). PCD in the group with RDS was not significantly different from PCD in the air-ventilated group (53 ± 24 versus 41 ± 32%).
Values for deflation stability, V10(Tables 1 and 2), were not significantly different in the RDS and air-ventilated groups.
DISCUSSION
This study confirms that FRC and compliance are low in mechanically ventilated neonates with severe RDS(3, 5). In addition we found that TLC was reduced, mainly due to a markedly reduced IC. Thus, the P-V curves in RDS had smaller amplitude and were less steep(implying a lower Crs-max) than those of air-ventilated infants with normal chest radiograms.
IC and Crs-max discriminated distinctly between infants with severe RDS and those ventilated with air (Fig. 3). These measures can be obtained without knowledge of absolute lung volumes; only the readily available equipment in Fig. 1 is needed. The computerized interrupter technique has previously been used in animal RDS models(15, 16) and in a modified version to obtain P-V diagrams in anesthetized infants and children(23). The occlusion period of 0.08 s was chosen to obtain a high resolution of the P-V curve (20-60 data points), while still achieving an accurate pressure plateau at the end of each occlusion, indicating equilibration with the alveolar pressure(15, 24). The short-term muscle relaxation that is necessary for performing the P-V maneuver is not harmful if there is a reasonable fit between the tracheal tube and the trachea, as was the case in the present study. However, if the tube is too small, there is the risk that relaxation of muscles in the larynx and pharynx will produce an increased leak around the tracheal tube and, especially in the sickest neonates with stiff lungs, difficulty in achieving adequate alveolar pressures during mechanical ventilation.
The FRC measurement system has been found to yield good agreement with actual volumes in a neonatal size lung model and with helium washout in a rabbit model(18). There are only few studies in equally small mechanically ventilated neonates with which to compare our values for FRC(3, 5), although there are several studies of FRC measurements in spontaneously breathing neonates that have recovered from RDS(25–28) and in older, mechanically ventilated infants(29–31). Richardson et al.(3) studied FRC by nitrogen washout in a group of neonates with RDS (1.0-2.6 kg), ventilated at a PEEP of 4.4 ± 1.2 cm H2O. FRC/body weight was 3-33 mL/kg (mean 15 mL/kg), whereas we found an FRC of 9-24 mL/kg (mean 14 mL/kg) at 4.0 ± 0.4 cm H2O of PEEP in the RDS group and 14-25 mL/kg (mean 21 mL/kg) at 2.6 ± 0.8 cm H2O of PEEP in the air-ventilated group. Although the RDS group had significantly smaller FRC than the air-ventilated group, there was a marked overlap (Table 1, Figs. 2 and 3). In fact, two of the infants in the RDS group had FRC values, normalized to body weight, corresponding to the upper range of the air-ventilated group(Fig. 3). In contrast, IC showed no overlap, suggesting that a small IC may be more characteristic of RDS than a small FRC. Assuming that the air-ventilated infants had nearly normal lungs, the lower limit for“normal” IC/weight was found to be 28 mL/kg. The small IC of RDS infants in our study is consistent with the low crying vital capacity found in spontaneously breathing neonates with RDS(32) and with findings by Jackson et al.(2) in premature pigtail monkeys with RDS. Using rapid freezing of inflated lungs, Jacksonet al.(33) showed that the lung volume reduction in monkey RDS was caused by proteinaceous alveolar edema rather than alveolar collapse. Although we found a small increase in IC between the two first inflations, our findings still indicate that, in the early stages of human RDS, the major part of the lost lung volume is not recruitable. FRCz constituted a greater part of TLC in the neonates with severe RDS than in the air-ventilated infants (Table 2, Fig. 2) probably because, in the former, a greater part of the measured lung volume consisted of conducting airways.
The low dynamic compliance in the RDS group confirms previous studies(5, 9, 10, 34). However, as far as we know, this is the first study in neonates with severe RDS in which the elastic properties of the respiratory system have been measured from TLC after a standardized volume history. We think that this gives a clearer picture than using Cdyn as the only measure of compliance. Cdyn varies with changes in tidal volume, PEEP level and volume history. Even at moderate PEEP and peak pressures, the tidal volume ventilation will take place partly on the flattened, upper part of the P-V curve, explaining why Cdyn was only about one-third of Crs-max-a measure of the steepest slope of the P-V curve. Because chest wall compliance is high in neonates(35), the P-V curves in our study probably mainly reflected the elastic properties of the lungs.
The upper part of the TLC-normalized P-V curve in the RDS group was similar to that in the air-ventilated group-consistent with the findings thatV10, i.e., the fraction of TLC still present at an airway pressure of 10 cm H2O, was almost equal in the two groups. This agrees with findings by Jackson et al.(2) in the monkey model of RDS. At lower pressures, the TLC normalized P-V curves of the infants with RDS were less steep than those in the air-ventilated group(Fig. 2), and both TLC-normalized and weight-normalized Crs-max (reflecting the slope of the P-V curve at 2.5-7.5 cm H2O of inflation pressure) were less in the RDS-group (Table 2). In fact, there was no overlap in Crs-max/weight between the two groups (0.4-1.7 mL cm H2O-1 kg-1 in RDS groupversus 2.0-3.1 mL cm H2O-1 kg-1 in the air-ventilated group). This suggests that Crs-max is a better measure of the severity of RDS than V10. It is also easier to measure.
Because both IC and Crs-max, normalized to body weight, clearly discriminated between neonates with RDS and air-ventilated infants, the present method for obtaining P-V curves may be useful when assessing infants with suspected RDS. In addition, preliminary results(36) suggest that it may be valuable when evaluating the effect of surfactant treatment.
In premature lambs with early RDS we previously found a markedly uneven ventilation, i.e. a high PCD, that improved after tracheal surfactant instillation(15). Therefore, it was unexpected that the PCD was only slightly higher in the RDS infants than in those ventilated with air. However, ventilation distribution in very early and later phases of RDS need not be the same. The lambs were studied at 20-80 min after delivery, whereas the neonates with RDS were studied between 6 h and 2 d of age. In fact, within the RDS group, there was a tendency toward higher PCD in the youngest neonates.
In conclusion, TLC is markedly reduced in neonates with severe RDS. This is caused mainly by a reduction in inspiratory capacity. The P-V curve has a reduced maximum slope when normalized to TLC (specific compliance) or to weight.

Abbreviations
- Cdyn:
-
dynamic compliance of the respiratory system
- Crs-max:
-
maximum static deflation compliance of the respiratory system
- Fio2:
-
fraction of inspired oxygen
- FRC:
-
functional residual capacity
- FRCp:
-
functional residual capacity at PEEP
- FRCz:
-
functional residual capacity at ZEEP
- IC:
-
inspiratory capacity
- PCD:
-
pulmonary clearance delay
- PEEP:
-
positive end-expiratory pressure
- P-V:
-
pressure-volume
- RDS:
-
respiratory distress syndrome
- TLC:
-
total lung capacity
- V 10 :
-
the fraction of TLC still present after deflation to a pressure of 10 cm H2O
- ZEEP:
-
zero end-expiratory pressure
References
Avery ME, Mead J 1959 Surface properties in relation to atelectasis and hyaline membrane disease. Am J Dis Child 97: 517–523
Jackson JC, Standaert TA, Truog WE, Murphy JH, Palmer S, Chi EY, Woodrum DE, Watchko JF, Hodson WA 1985 Changes in lung volume and deflation stability in hyaline membrane disease. J Appl Physiol 59: 1783–1789
Richardson P, Bose CL, Carlstrom JR 1986 The functional residual capacity of infants with respiratory distress syndrome. Acta Paediatr Scand 75: 267–271
Bose CL, Lawson EE, Greene A, Mentz W, Friedman M 1986 Measurement of cardiopulmonary function in ventilated neonates with respiratory distress syndrome using rebreathing methodology. Pediatr Res 20: 316–320
Edberg KE, Sandberg K, Silberberg A, Ekström-Jodal B, Hjalmarson O 1991 Lung volume, gas mixing, and mechanics of breathing in mechanically ventilated very low birth weight infants with idiopathic respiratory distress syndrome. Pediatr Res 30: 496–500
Truog WE 1988 Lung volume measurements in neonates. Part of panel discussion I: technical and physiological considerations. In: Bhutani VK, Shaffer TH, Vidyasagar D (eds) Neonatal Pulmonary Function Testing: Physiological, Technical and Clinical Considerations. Perinatology Press, Ithaca, NY, pp 137–145
Gaultier C 1989 Lung volumes in neonates and infants. Eur Respir J 2( suppl 4): 130–134
McCann EM, Goldman SL, Brady JP 1987 Pulmonary function in the sick newborn infant. Pediatr Res 21: 313–325
Davis JM, Veness-Meehan K, Notter RH, Bhutani VK, Kendig JW, Shapiro DL 1988 Changes in pulmonary mechanics after the administration of surfactant to infants with respiratory distress syndrome. N Engl J Med 319: 476–479
Couser RJ, Ferrara TB, Ebert J, Hoekstra RE, Fangman JJ 1990 Effects of exogenous surfactant therapy on dynamic compliance during mechanical breathing in preterm infants with hyaline membrane disease. J Pediatr 116: 119–124
Dreizzen E, Migdal M, Praud JP, Saby MA, Chambille B, Dehan M, Gaultier C 1989 Passive total respiratory system compliance and gas exchange in newborns with hyaline membrane disease. Pediatr Pulmonol 6: 2–7
England SJ 1988 Current techniques for assessing pulmonary function in the newborn and infant: advantages and limitations. Pediatr Pulmonol 4: 48
Beardsmore CS, Stocks J, Helms P 1989 Elastic properties of the respiratory system in infants. Eur Respir J 2( suppl 4): 135–139
Motoyama EK 1990 Respiratory physiology in infants and children. In: Motoyama EK, Davis PJ (eds) Anesthesia for Infants and Children CV Mosby, St Louis, pp 11–76
Vilstrup CT, Gommers D, Bos JAH, Lachmann B, Werner O, Larsson A 1992 Natural surfactant instilled in premature lambs increases lung volume and improves ventilation homogeneity within five minutes. Pediatr Res 32: 595–599
Gommers D, Vilstrup CT, Bos JAH, Larsson A, Werner O, Hannappel E, Lachmann B 1993 Exogenous surfactant therapy increases static lung compliance, and cannot be assessed by measurements of dynamic compliance alone. Crit Care Med 21: 567–574
Thorsteinsson A, Larsson A, Jonmarker C, Werner O 1994 Pressure-volume relations of the respiratory system in healthy children. Am J Respir Crit Care Med 150: 421–430
Vilstrup CT, Björklund LJ, Larsson A, Lachmann B, Werner O 1992 Functional residual capacity and ventilation homogeneity in mechanically ventilated small neonates. J Appl Physiol 73: 276–283
Jonmarker C, Castor R, Drefeldt B, Werner O 1985 An analyzer for in-line measurement of expiratory sulfur hexafluoride concentration. Anesthesiology 63: 84–88
Fowler WS, Cornish ER Jr, Kety SS 1952 Lung function studies. VIII. Analysis of alveolar ventilation by pulmonary N2 clearance curves. J Clin Invest 31: 40–50
Bouhuys A, Jönsson R, Lundin G 1958 Non-uniformity of pulmonary ventilation in chronic diffuse obstructive emphysema. Acta Med Scand 157: 29–46
Larsson A, Jonmarker C, Werner O 1988 Ventilation inhomogeneity during controlled ventilation. Which index should be used?. J Appl Physiol 65: 2030–2039
Thorsteinsson A, Jonmarker C, Larsson A, Werner O 1988 Pressure-volume curves of the total respiratory system in anesthetized and paralyzed children. Anesthesiology 69: A784( abstr)
Carlo WA 1988 Validation of the interrupter technique in normal and surfactant deficient lungs. In: Bhutani VK, Shaffer TH, Vidyasagar D (eds) Neonatal Pulmonary Function Testing: Physiological, Technical and Clinical considerations. Perinatology Press, Ithaca, NY, pp 35–45
Berglund G, Karlberg P 1956 Determination of functional residual capacity in newborn infants. Preliminary report. Acta Pediatr 45: 541–544
Krauss AN, Auld PAM 1970 Measurement of functional residual capacity in distressed neonates by helium rebreathing. J Pediatr 77: 228–232
Ronchetti R, Stocks J, Keith I, Godfrey S 1975 An analysis of a rebreathing method for measuring lung volume in premature infant. Pediatr Res 9: 797–802
Bryan MH, Hardie MJ, Reilly BJ, Swyer PR 1973 Pulmonary function studies during the first year of life in infants recovering from the respiratory distress syndrome. Pediatrics 52: 169–178
Sjöqvist BA, Sandberg K, Hjalmarsson O, Olsson T 1984 Calculation of lung volume in newborn infants by means of a computer-assisted nitrogen washout method. Pediatr Res 18: 1160–1164
Gerhardt T, Hehre D, Bancalari E, Watson H 1985 A simple method for measuring functional residual capacity by N2 washout in small animals and newborn infants. Pediatr Res 19: 1165–1169
Sivan Y, Deakers TW, Newth CJL 1990 An automated bedside method for measuring functional residual capacity by N2 washout in mechanically ventilated children. Pediatr Res 28: 446–450
Auld PAM, Nelson NM, Cherry RB, Rudolph AJ, Smith CA 1963 Measurement of thoracic gas volume in the newborn infant. J Clin Invest 42: 476–483
Jackson JC, Mackenzie AP, Chi EY, Standaert TA, Truog WE, Hodson WA 1990 Mechanisms for reduced total lung capacity at birth and during hyaline membrane disease in premature newborn monkeys. Am Rev Respir Dis 142: 413–419
Yu VYH, Rolfe P 1976 Effect of feeding on ventilation and respiratory mechanics in newborn infants. Arch Dis Child 51: 310–313
Gerhardt T, Bancalari E 1980 Chestwall compliance in full-term and premature infants. Acta Paediatr Scand 69: 359–364
Björklund L, Vilstrup CT, Larsson A, Werner O 1992 Pressure-volume relations and surfactant. Pediatr Res 31: A301( abstr)
Author information
Authors and Affiliations
Additional information
[Editor's note: We regret the delay in publication of this article, which occurred because of a clerical oversight.]
Supported by the Medical Faculty (4021-2 and 4421-4), University of Lund; AB AGA, Stockholm; The Swedish Society of Medicine; and the Swedish Medical Research Council, project B93-17X-09506-03A.
Rights and permissions
About this article
Cite this article
Vilstrup, C., Björklund, L., Werner, O. et al. Lung Volumes and Pressure-Volume Relations of the Respiratory System in Small Ventilated Neonates with Severe Respiratory Distress Syndrome. Pediatr Res 39, 127–133 (1996). https://doi.org/10.1203/00006450-199601000-00019
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1203/00006450-199601000-00019
This article is cited by
-
A practical guide to neonatal volume guarantee ventilation
Journal of Perinatology (2011)
-
Incomplete protection by prophylactic surfactant against the adverse effects of large lung inflations at birth in immature lambs
Intensive Care Medicine (2004)