Abstract
Four patients in one generation of a multiply consanguineous pedigree died with cardiomyopathy, cataracts, and lactic acidemia. Postmortem heart and skeletal muscle tissues from one patient were analyzed. A low (12% control) activity of NADH-CoQ reductase (complex I) in heart and normal activity in skeletal muscle mitochondria was found. Cultured skin fibroblasts obtained from two individuals in the pedigree showed elevated lactate to pyruvate ratios in the range of 2 to 3.5 times normal and decreased complex I + III activity (42 and 54% of control activities) in isolated mitochondria. Western blot analysis and enzymatic assay showed normal levels of CuZn-superoxide dismutase, but grossly elevated levels of the mitochondrial Mnsuperoxide dismutase. Southern blot analysis in heart muscle cells from the patient tested revealed multiple mitochondrial DNA deletions which indicate free oxygen radical damage. We hypothesize that a nuclear-encoded defect in the respiratory chain is responsible for excessive free oxygen radical production in these infants which contributes to the prenatal onset of cardiomyopathy and cataracts.
Similar content being viewed by others
Main
Cardiomyopathy with cataracts is listed by McKusick (Entry 212350)(1) as an autosomal recessive condition with the onset of symptoms at birth, childhood, or early adulthood. Cardiomyopathy with cataracts has been previously described in the literature in a number of patients(2–4). The early work by Sengerset al.(3) described seven affected patients in three unrelated sibships with congenital cataracts and mitochondrial myopathy of skeletal and heart muscle. Mitochondrial abnormalities accompanied by lipid and glycogen accumulation was found in both skeletal and heart muscle in these patients. In addition, Cruysberg et al.(2) described 12 patients in which hypertrophic cardiomyopathy was the predominant clinical feature. Although the cataracts were congenital, the onset of cardiomyopathy was variable and progressive. Several patients were affected in the neonatal period, resulting in premature death, commonly in adulthood. The presence of constitutive lactic acidemia with slight muscular exercise in surviving patients was evident. Valsson et al.(4) also described six cases in three Icelandic families that were remotely related, with cardiomyopathy, cataracts, and lactic acidosis.
The inheritance of isolated hypertrophic cardiomyopathy generally follows an autosomal dominant form of inheritance and has been attributed to defects of the β-myosin heavy-chain gene on chromosome 14(5, 6). In addition, linkage studies have localized hypertrophic cardiomyopathy loci on chromosome 1q3(7) and on chromosome 11(8). Furthermore, maternally inherited forms of cardiomyopathy transmitted by mtDNA have now been described, for example mutations at bp 9997 of mtDNA in the tRNAGly(9) and at bp 3260 of mtDNA in the tRNALeu(10, 11).
Hypertrophic cardiomyopathy in conjunction with chronic lactic acidemia and encephalopathy can often be attributed to an impairment of the respiratory chain(12). In these patients a malfunction of the mitochondrial respiratory chain, which is reflected by an elevated lactate to pyruvate ratio in cultured skin fibroblasts, has been demonstrated(12, 13).
This study investigates the etiology of cardiomyopathy and cataracts in a Pakistani-Canadian family. Here we show that an altered redox state in skin fibroblasts and an apparent defect in the function of mitochondrial NADH-CoQ reductase are associated with an excessive rate of free radical generation and an elevation of Mn-SOD concentration.
CASE REPORTS
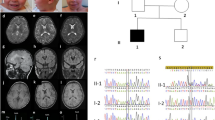
The pedigree (Fig. 1) illustrates that the offspring in this study were a result of two different consanguineous first cousin marriages. In the first family, one healthy child (V-1) and three children exhibiting cardiomyopathy and cataracts (V-2, V-3, and V-4) were produced. Another first cousin marriage within the same generation resulted in two healthy children (V-5 and V-6) and one affected child (V-7). Three of the four grandparents (III-2, III-3, and III-4) were consanguineous, as were two of the great grandparents (II-2 and II-4). In the present study one patient from each family (individuals V-7 and V-3) was investigated.
Patient V-7. The pregnancy was uneventful during gestation with the delivery of a female infant by elective cesarian section at 42 wk. The birth weight was 2.590 kg. An ECG, performed due to early cardiovascular instability, showed right ventricular hypertrophy, and an echocardiogram revealed pericardial effusion, hypertrophic cardiomyopathy, and poor ventricular function. On ophthalmic consultation, bilateral dense white cataracts were observed. The lactate/pyruvate ratio in the plasma was markedly elevated. The patient died at 12 d of age of cardiac failure. Tissue obtained at autopsy within 2 h after death (with consent) showed mitochondrial proliferation in heart muscle and normal skeletal muscle histology.
Patient V-3. The pregnancy was uncomplicated with delivery of a female infant by cesarian section at 39 wk of gestation. Birth weight was 2.705 kg. Congenital cataracts and cardiomyopathy were noted in the perinatal period. Blood lactate was elevated (11.6 mM), whereas blood pyruvate was normal or increased (0.170 mM) resulting in an abnormally high lactate/pyruvate ratio (68:1). The infant remained stable and was electively given carnitine. Computerized tomography scan was normal; an echocardiogram indicated poor left ventricular function and bilateral ventricular hypertrophy. Additional investigations revealed delayed visual evoked potentials and prolonged central conduction time of the right auditory brainstem response, but the EEG was normal. The patient was operated on for bilateral cataracts. The patient was readmitted to hospital at 5 mo of age because of an upper respiratory tract infection and poor feeding. The clinical condition deteriorated rapidly, and the patient died at 5 mo of age. Consent for autopsy was not given.
METHODS
All chemicals were the best grade available. Lucigenin(10,10′-dimethyl-9,9′-biacridinium dinitrate) was obtained from Sigma Chemical Co., St. Louis MO.
Biochemical Investigations of Autopsy Tissue
Mitochondria were prepared from liquid N2 frozen autopsy samples of skeletal and heart muscle by homogenizing the tissue in a buffer of sucrose(250 mM), EDTA (2 mM), and Tris-HCl (10 mM), pH 7.4. The homogenate was centrifuged at 1000 × g. The resulting supernatant was centrifuged at 10 000 × g to sediment the mitochondria. Activities of the respiratory chain complexes were measured as outlined by Applegarth et al.(14). Citrate synthase was measured as a marker enzyme by the method of Srere et al.(15).
Cultured Skin Fibroblast Investigations
Human skin fibroblasts were grown from explants of forearm skin biopsies(taken with informed consent). Culture medium was Eagle's α-minimal essential medium supplemented with 10% (vol/vol) FCS and 10.5 mM glucose.
Lactate to pyruvate ratios were determined as described previously(16) by determining the levels of lactate and pyruvate in the incubation medium of cultured fibroblasts after 1 h of incubation in Krebs phosphate buffer (pH 7.4) containing 1 mM glucose.
Respiratory chain enzyme activities. Cytochrome c oxidase (complex IV) and succinate cytochrome c reductase (complexes II and III) were determined from whole cells. Cytochrome c oxidase(complex IV) was measured as described by Glerum et al.(17) and succinate cytochrome c reductase(complexes II and III) by following the reduction of cytochrome c using the method of Fischer et al.(18). Mitochondria, isolated from cultured skin fibroblasts, were used to determine rotenonesensitive NADH-cytochrome c reductase activity (complexes I and III) following the method of Moreadith et al.(19). All required protein concentrations were assayed by the method of Lowry et al.(20).
ATP synthesis. The ability of fibroblast mitochondria to synthesize ATP was tested using digitonin-treated fibroblasts(21, 22). The cells (1 × 106) were trypsinized, pooled, pelleted by centrifugation at 500 × g for 5 min, and washed twice with sucrose medium (0.25 M sucrose, 5 mM Tris, 2 mM EDTA, pH 7.4). Finally, the cells were resuspended in media containing 150 mM KCl, 25 mM Tris-HCl, 2 mM EDTA, 10 mM KHPO4, 0.1% (wt/vol) BSA, 30 nM digitonin (pH 7.4) and divided into five aliquots. Digitonin was used to permeabilize the cells. In these aliquots various substrates: pyruvate (1 mM) plus malate (1 mM), isocitrate (5 mM) plus malate (1 mM), succinate (10 mM) plus rotenone (2.5 μg/mL), and ascorbate (10 mM) plus TMPD (0.1 mM) were added to test the efficiency of ATP production. The samples were incubated for 30 min at 37°C and the reaction was terminated by adding perchloric acid(final concentration 76 mM). The supernatants were assayed for ATP content using enzyme fluorometric methods(16, 29).
Western and Northern blot analysis
Western blotting was carried out as described by Glerum et al.(17) using mitochondria isolated from fibroblasts. Three different primary antibodies were used for Western blot analysis in this study; anti-bovine complex I antibody raised in rabbits (a gift from Dr. C. I. Ragan, Southampton, England), and two monospecific anti-SOD antibodies (Mn and CuZn; Calbiochem, San Diego, CA).
Northern blotting was carried out using total RNA prepared from cultured fibroblasts as described previously(21) and electrophoresed in 1% (wt/vol) agarose containing 1.8% (wt/vol) formaldehyde. The RNA was transferred to a Hybond-N+ support membrane (Amersham Corp., Arlington Heights, IL) and hybridized with 32P-labeled cDNA probes generated by random priming of fragments for the 75 000(23), 51 000(24), 24 000(25), 20 000 (a gift from Dr. S. Hyslop, Toronto, Canada), 14 000 (a gift from W. Chow, Toronto, Canada) subunits of complex I and Mn-SOD. Total RNA loading was monitored by comparison to mitochondrial EFTU transcript levels (mitochondrial translation factor, 1.7 kb; F. Merante personal communication). The mitochondrial elongation factor EFTU cDNA was isolated from a human kidney λgt10 phage library.
Southern Blot Analysis and Probe Labeling
Total genomic DNA was isolated from fibroblast cultures as outlined by Maniatis et al.(26). Ten micrograms of purified DNA was digested with appropriate restriction enzymes and electrophoresed through a 0.6% (wt/vol) agarose gel. The DNA was transferred onto a Hybond support nylon membrane by alkaline transfer and hybridized to a32 P-labeled mitochondrial DNA probe mixture. mtDNA probes were generated by combining equimolar concentrations of polymerase chain reaction products spanning the entire mtDNA genome. Prehybridization was performed in 40% (vol/vol) formamide, 1% (wt/vol) SDS, 5 × SSPE, 0.5% (wt/vol) skim milk powder, and 250 μg/mL denatured and sheared salmon sperm DNA for approximately 6 h at 42°C. Hybridization was performed in 40% (vol/vol) formamide, 5 × SSPE, 1% (wt/vol) SDS, 0.5% (wt/vol) skim milk powder, and 10% (wt/vol) dextran sulfate for 18 h at 42°C. Membrane was subsequently rinsed in 2 × SSPE, 0.1% (wt/vol) SDS and washed in the same buffer as follows: twice at room temperature for 15 min and once at 65°C for 1 h.
Total genomic DNA from patient skeletal and heart muscle was isolated as described by Maniatis et al.(26), and Southern blot analysis was performed as outlined above. A human32 P-labeled D-loop probe was used for hybridization to mtDNA.
SOD Activity
The method used for the assay of SOD activity was a modification of an indirect, spectrophotometric, NBT inhibition assay(27). This assay uses xanthine-xanthine oxidase as a source of superoxide and the rate of NBT reduction by superoxide in the absence of SOD (either pure SOD or mitochondria containing SOD activity) was used as the reference rate (2.1± 0.01 nmol/min). A standard curve was generated from data obtained by adding increasing units of SOD to determine the linear response segment of the inhibition curve. When increasing amounts of mitochondria were added into the system, the rate of NBT reduction was progressively inhibited. The amount of the inhibition was subtracted from the reference rate indicating the activity of total SOD. Sodium cyanide (CuZn-SOD inhibitor) was added to ensure that this assay measured only Mn-SOD activity. The data were plotted as Mn-SOD activity (nmol/min) versus protein quantity (μg) using three replicate measurements.
Mn-SOD ELISA
The Mn-SOD ELISA kit (Bender MedSystems, Vienna, Austria) was used to quantify Mn-SOD levels in mitochondrial samples made from patients' cultured fibroblasts.
Lucigenin-Dependent Chemiluminescence
Lucigenin as a chemiluminogenic probe was prepared as 1 mM stock concentration in 50 mM KOH fresh daily. Experimental buffer was potassium phosphate, pH 10.5. Luminescence was monitored as an integrated rate using a luminometer (1253 Luminometer, Bio-Orbit Oy, Finland) with 15 μg of mitochondria and 50 μg of NADH which also served as a reducing agent for the lucigenin probe which is a prerequisite for lucigenin's reaction with O-2(28).
RESULTS
Biochemical investigation of autopsy tissue from patient V-7. Because of the marked proliferation of mitochondria seen in cardiac muscle at autopsy, mitochondrial enzymes involved in oxidative phosphorylation were analyzed. Respiratory chain activities from the patient's skeletal muscle mitochondria were comparable to control values (Table 1). In the heart mitochondria, however, the activities of NADH-cytochromec reductase (complexes I and III), succinate cytochrome c reductase (complexes II and III), NADH-CoQ reductase (complex I), and cytochrome c oxidase (complex IV) were all below control values, whereas succinate: ubiquinone oxidoreductase (complex II) activity was normal(Table 1).
Cultured skin fibroblast investigations. To follow up on the observations of mitochondrial hypertrophy and respiratory chain enzyme deficiency in cardiac muscle, cultured skin fibroblasts were analyzed to see if a similar defect could be demonstrated. Whole cell cytochrome c oxidase and succinate cytochrome c reductase activities were within the normal range. However, the lactate to pyruvate ratio was above normal in both cases, indicating a respiratory chain defect(29). Rotenone-sensitive NADH-cytochrome c reductase activity in mitochondria was found to be significantly decreased to 54 and 43% of control activity for patients V-7 and V-3, respectively, well out of the control range of activities (Table 2).
To assess whether this partial defect in the activity of complex I + III in fibroblast mitochondria had any overall affect on oxidative phosphorylation(12), respiratory chaindriven ATP-synthesis was measured in digitonin permeabilized fibroblasts. ATP production in this system was similar to control samples after the addition of pyruvate plus malate, isocitrate plus malate, succinate plus rotenone, or ascorbate plus TMPD as substrates (Table 3). Thus it seemed unlikely that simple lack of ATP production was responsible for the severe pathologic disturbance in these children in the face of a partial complex I defect.
Western blot analysis. Five different subunits (75-, 42-, 30-, 24-, and 20-kD proteins) of complex I were examined by Western blot analysis in an attempt to ascertain if there was any sign of an abnormality in the visualized subunits. There were no major differences in the mobility of the subunits between patient and control fibroblasts. However, the 42-, 30-, 24-, and 20-kD subunit proteins appeared to be more abundant in patient V-7 compared with patient V-3 and fibroblasts from either controls or patients with complex I deficiency (Fig. 2).
Western blot analysis of cultured skin fibroblast mitochondrial protein with anti-complex I antibody. Mitochondria (100 μg of protein) were isolated from skin fibroblast cultures, and the proteins run on a 10% polyacrylamide gel. Proteins were transferred onto a nitrocellulose support matrix and incubated with anti-complex I antibody. Immunoreactive proteins were visualized with a biotinylated IgG-avidin peroxidase system. Lane 1, control cultured skin fibroblasts; lane 2, patient V-7; lane 3, patient V-3; lanes 4 and 5, other patients with complex I deficiency without cardiomyopathy and cataracts.
Because both cardiomyopathy and cataract formation have been linked in both human and animal model systems with free radical damage, we decided to look for signs of a response to free radical overproduction. Western blotting techniques were used to measure the relative levels of mitochondrial Mn-SOD and cytoplasmic CuZn-SOD in patient fibroblasts. Both patients produced high levels of immunoreactive Mn-SOD (≈23-24 kD) compared with control fibroblasts mitochondria (Fig. 3A). Mn-SOD levels in fibroblast mitochondria from patient V-7, was 220% higher (densitometric data) than the milder affected cousin (patient V-3), and both were grossly elevated when compared with control. CuZn-SOD (≈32 kD) protein levels, however, were similar in patient and control fibroblasts (Fig. 3B).
Western blot analysis of SOD in cultured skin fibroblasts or fibroblast mitochondria. (A) 100 μg of mitochondrial protein were isolated from skin fibroblast cultures, and the proteins were run on a 10% polyacrylamide gel. Proteins were transferred onto a nitrocellulose support matrix and incubated with anti-Mn-SOD. Immunoreactive proteins were visualized with anti-sheep IgG alkaline phosphatase and NBT-BCIP color development reaction. Lane 1, low molecular weight standard; lane 2, control; lane 3, patient V-7; lane 4, patient V-3. (B) Western blot was performed using 100 μg of sonicated fibroblasts and CuZn-anti-SOD as a primary antibody. Lane 1, patient V-3; lane 2, patient V-7; lane 3, control; lane 4, low molecular weight standard.
Northern blot analysis. To establish whether transcript levels for complex I subunits or SOD were altered in these fibroblasts, Northern blot analysis was used to investigate the mRNA levels for the 75-, 51-, 24-, 20-, and 14-kD subunits. The transript levels of these complex I subunits were comparable in patient and control fibroblasts (data not shown). Northern blot analysis was also used to study Mn-SOD mRNA levels in these patients. Two transcripts for Mn-SOD are visible in the mRNA from most tissues, one at 4 kb and the other at 1 kb(37). Examination by densitometry of both transcripts revealed that Mn-SOD mRNA was increased 330% in patient V-7 and 220% in patient V-3 compared with control. Total RNA loading was equal monitored by comparison to a mitochondrial EFTU (mitochondrial translation factor, 1.7 kb) transcript (Fig. 4).
Northern blotting of total RNA from cultured skin fibroblasts using a 32P-labeled cDNA probe for Mn-SOD. Total RNA loading was monitored by use of mitochondrial EFTU (mitochondrial translation factor, 1.7 kb) probe. The 1- and 4-kb bands reflecting the mRNA species for Mn-SOD are indicated in the figure. Lane 1, patient V-3; lane 2, patient V-7; and lane 3, control.
Southern blot analysis. Respiratory chain defects can be accompanied by deletion or duplication of the mitochondrial genome so to ascertain the size and status of the mitochondrial genome in cultured fibroblasts from patient V-7, Southern blot analysis was performed with a variety of restriction endonucleases to both linearize and digest mitochondrial DNA into fragments. In patient V-7 DNA, digestion withBam HI and PvuII resulted in a single band (16.5 kb) consistent with linearized mitochondrial DNA(30). Digestion with EcoRI, HindIII, KpnI, andPst I yielded the expected restriction patterns(31) (Fig. 5). In contrast, DNA prepared from heart muscle showed a ladder of bands of lowerMr, in addition to the normal 16.5-kb mtDNA band suggesting deleted mtDNA (Fig. 6). An identical result was obtained by ethidium bromide-stained agarose gel electrophoresis (data not shown). mtDNA from six other cardiac and skeletal muscle samples obtained at autopsy showed only the normal 16.5-kb mtDNA band, even though two patients died of heart failure (results not shown).
Southern blot analysis of cultured fibroblast mitochondrial DNA from patient V-7. Ten micrograms of total fibroblast DNA were digested to completion with restriction endonucleases, electrophoresed through a 0.6% agarose gel, and transferred onto a Hybond N+ membrane. The membrane was subsequently hybridized with a mixture of 32P-labeled mitochondrial sequences. Each lane contained genomic DNA digested with the following restriction enzyme: lane 1, BamHI; lane 2,Eco RI; lane 3, HindIII; lane 4, KpnI; lane 5,Pst I; and lane 6, PvuII.
Southern blot analysis of skeletal and heart muscle mitochondrial DNA from patient V-7. Ten micrograms of total skeletal or cardiac muscle DNA were digested with PvuII, and Southern blot analysis was performed as outlined in the text. A human 32P-labeled D-loop probe was used to recognize mitochondrial DNA. Lane 1 and 2, control skeletal muscle; lane 3, patient V-7, skeletal muscle; and lane 4, patient V-7, cardiac muscle DNA.
SOD activity. The activity and quantity of mitochondrial Mn-SOD were assayed to obtain evidence for free oxygen radical overproduction. The activity of SOD in mitochondrial membrane preparations calculated from inhibition of NBT assay system was cyanide insensitive. This indicated that the assay was appropriate for the measurement of Mn-SOD. It is likely that CuZn-SOD was washed off during the preparation of mitochondrial samples. This finding was supported by Western blot analysis, as there was no detectable immunoreactive CuZn-SOD in mitochondrial preparations (data not shown). Both patients' fibroblast mitochondria had higher Mn-SOD activity compared with control values. For example, using 15 μg of mitochondrial protein the Mn-SOD activity of patient V-7 was 0.8 nmol/min, whereas that of patient V-3 was 0.48 nmol/min which were, 3.6- and 2.2-fold higher than the control value(0.22 nmol/min), respectively (Fig. 7).
Mn-SOD ELISA. The quantity of Mn-SOD as measured by ELISA, was 3.2 ± 0.07 (3) ng/mL in patient V-7 and 1.9 ± 0.4 (3) ng/mL in patient V-3. Two different control fibroblast mitochondrial samples were used to measure Mn-SOD levels in normal fibroblast mitochondria, and the values were 0.5 ± 0.004 (3) ng/mL, 0.7 ± 0.10 (3) ng/mL. The results were expressed as mean ± SD with the number of independent observations shown in parentheses. In mitochondria from three patients with other forms of complex I deficiency, patients 2, 5, and 6 respectively fromFig. 2, the levels of Mn-SOD as determined by ELISA were 0.84 ± 0.12, 0.74 ± 0.16, and 1.17 ± 0.18 ng/mL. Patient 2 is 5-y-old and has asymptomatic lactic acidemia after having had severe neonatal lactic acidosis, whereas patients 5 and 6 both had Leigh disease, death occurring at 8 y and 6 mo of age, respectively. The residual activities of patient's complex I + III measurements in fibroblast mitochondria were 52, 33, and 26%, respectively, of control values for patients 2, 4, and 5.
Citrate synthase activity was measured in the same samples as a marker enzyme reflecting the quality of the mitochondrial preparations. The values were at the same range in all samples (between 72 and 90 nmol/min mg.) indicating that there were no major differences in the purity of the mitochondrial membrane preparations.
Lucigenin-dependent chemiluminescence. Because of elevated Mn-SOD quantity and activity superoxide induced lucigenin chemiluminescence was used to analyze the rate of superoxide production in patients' skin fibroblast mitochondria. Lucigenin as a luminometric probe measures superoxide production in the sample if a reducing agent is added in the assay system(28). NADH addition served both to prime mitochondrial electron transport and to reduce lucigenin so that any superoxide produced from the respiratory chain would result in luminescence. The steady state of superoxide production in patient V-7 and patient V-3 was 38% and 86% of control, respectively (Fig. 8).
Lucigenin-dependent chemiluminescence catalyzed by 15μg of mitochondria isolated from skin fibroblast cultures from patient V-7, V-3, and control. The reaction conditions were described in“Methods.” Lucigenin-induced chemiluminescence was measured in 20 intervals of 1 s each using three to five replicate measurements. Results were expressed as chemiluminescence (counts/s) vs time (s).
DISCUSSION
The syndrome of hypertrophic cardiomyopathy, cataracts, and lactic acidosis occurring in these families shows a pattern of inheritance which is compatible with an autosomal recessive disorder(2–4, 32). Although Sengers et al.(32) postulated a mitochondrial etiology for this syndrome, there was little direct evidence to support this view, except ultrastructural changes and the distribution of mitochondria.
This study provides evidence that the defect in these patients with hypertrophic cardiomyopathy, cataracts, and lactic acidosis was located in the mitochondrial respiratory chain. There was observable mitochondrial proliferation in the heart muscle in one patient's autopsy specimen (patient V-7) accompanied by high citrate synthase and decreased respiratory chain activities. Particularly NADH-CoQ reductase activity (complex I) was only 12% of control value in heart muscle mitochondria. In cultured skin fibroblasts from both affected patients, complex I deficiency was also demonstrated; the lactate to pyruvate ratio was elevated, and the NADH cytochrome c reductase (complexes I and III) activity was below normal with increased activities of complexes I and III. The rate of ATP production with NAD-linked substrates was similar in mitochondria from the patients and control fibroblasts, suggesting that in skin fibroblasts the impairment of electron transport was not severe enough to adversely affect oxidative phosphorylation in all tissues. However, the impairment was significant enough to alter the redox state both in blood and in cultured skin fibroblasts, so it is conceivable that such an impairment would also influence the rate at which electrons are misdirected into forming superoxide. Normal ATP production in the presence of a complex I respiratory chain defect has been shown previously in fibroblasts(12). This is because in fibroblast mitochondria cytochrome oxidase rather than complex I is ratelimiting for the respiratory chain(17). It is likely, therefore, in these mitochondria that the impairment in the passage of electrons down the respiratory chain is overcome by the subsequent redox change, allowing passage of electrons at a normal rate albeit at a new redox equilibrium. Another possibility is that ATP synthesis is sustained by accelerated but less efficient electron transport.
Southern blot analysis revealed multiple mitochondrial DNA deletions in heart muscle from the patient tested (patient V-7). mtDNA from control cardiac tissue obtained at autopsy and patient skeletal muscle obtained at autopsy did not show deletions, making it unlikely that the heart muscle mtDNA deletions were a postmortem artifact. The deleted mtDNA ladder seen in the heart of patient V-7 is similar to that seen in muscle from patients with an autosomally dominant inherited condition of myopathy and cardiomyopathy(10). The reduced cardiac activity of succinate cytochrome c reductase (20% of normal) observed in heart but not in fibroblasts is perhaps indicative of a further drop in mitochondrial respiratory chain activities associated with deleted mtDNA. Katsumataet al.(33) indicated that extensive fragmentation of mtDNA was associated with damage by oxygen radicals.
Further investigation showed that patient V-7 skin fibroblasts demonstrated a very high level of immunoreactive Mn-SOD. The Mn-SOD level of patient V-3 was also elevated relative to control fibroblasts. Increased levels of this enzyme is a strong indicator that excessive superoxide production had occurred in the patients' skin fibroblasts as this enzyme is known to be inducible by excess oxygen radicals(34, 35). In addition, there is evidence for the involvement of reactive oxygen species in the mechanism of cataracts(36), the other major manifestation of these patients.
Mn-SOD enzyme activity was higher in patient V-7 and V-3 compared with control values. This correlates well with the quantitative differences in Mn-SOD in fibroblast mitochondrial samples, i.e. the greater the quantity of the Mn-SOD enzyme the higher Mn-SOD enzyme activity. Three patients with complex I defects, two of them with much lower activities than the patients in our consanguineous pedigree demonstrated elevations of Mn-SOD but not to the same extent as seen with cardiomyothy and cataracts. Luminometric data showed that in patient V-7 and V-3 the respiratory chain superoxide production was quenched by the high activity of Mn-SOD compared with control. It was not possible to ascertain whether the superoxide production from the respiratory chain was abnormal in patients' fibroblast mitochondria because of the considerable quenching imposed by increased Mn-SOD activity. Superoxide itself is a rather poor reactive oxygen species, being unable to cause lipid peroxidation or hydroxylation of DNA(37). However, when excess superoxide is reacted upon by Mn-SOD, large amounts of intramitochondrial H2O2 are produced, leading to the generation of hydroxyl radical which can cause both lipid peroxidation and DNA hydroxylation. The production of such damaging reactive oxygen species by the action of copper or iron ions on H2O2 by the Fenton reaction would provide a mechanism for the unusual pathology in these patients. Increased generation of hydroxyl radicals is known to induce cataract formation, cardiomyopathy, and premature aging in certain rat strains(38).
The evidence therefore suggests that a recessive mutation affecting one of the subunits of complex I leads to partial impedance of electron transport in fibroblast mitochondria with concomitant misdirection of electrons into superoxide production. Further metabolism of the superoxide to H2O2 and hydroxyl radicals resulting from the superinduction of Mn-SOD then causes hydroxylation and breakage of mtDNA in the heart with subsequent mitochondrial and cardiac hypertrophy, and in the eye the hydroxylation and disruption of lens proteins causes cataract formation. The absence of a complex I defect in muscle mitochondria may result from the presence of tissue-specific forms of the complex expressed in heart and skeletal muscle, which is the case for complex IV(39), and suspected to be the case for heart and skeletal muscle with complex I(40, 41). The deleterious effect of reactive oxygen species on cardiac metabolism may give rise to the patients' cardiac symptoms and premature death. A similar process may affect the lens of the eye which is known to be susceptible to cataract formation when reactive oxygen species are abundant(38, 42). Alternatively, in skin fibroblasts elevated Mn-SOD can reduce superoxide production, but in the heart with its high oxidative rate, removal of superoxide by Mn-SOD may not be sufficient to confer any protective effect. This is supported by Southern blot analysis revealing that mtDNA was deleted in the heart but not in skin fibroblasts. If these children had lived longer further damage to the CNS may have become apparent in addition to the abnormal brain stem responses already demonstrated.
This study suggests that the syndrome of hypertrophic cardiomyopathy, cataracts, and lactic acidosis is caused by an abnormality in the mitochondrial respiratory chain (complex I deficiency). Which one of the 30 or more nuclear-encoded complex I components is responsible for the observed complex I defect is not discernable at this time. The function of many of these subunits is obscure ouside of those that contain an iron sulfur center or a flavin prosthetic group(13, 23, 24). Although it is known that the mitochondrial respiratory chain, especially complex I, is the main source of superoxide in nonphagocytic cells, the exact site of its production within the complex is not known(42). Oxygen is thought to become reduced to superoxide by one electron transfer through interaction with ubisemiquinone or some other intermediate within complex I. Mn-SOD, an enzyme localized in the mitochondria, converts superoxide to hydrogen peroxide and these two together can form even more damaging oxygen species such as the hydroxyl radical and singlet oxygen(43). An imbalance between reactive oxygen species and antioxidants may lead to damage affecting several types of biological molecules, including DNA, lipids, proteins and carbohydrates(44). It is known that impairment of complex I activity by simply blocking electron transport with inhibitors causes increased production of superoxide(45). The response in cases of complex I deficiency detectable in skin fibroblasts seems to be an increase in Mn-SOD activity, a response that is greatly exaggerated in this family with cardiomyopathy plus cataracts. It is of interest that about one third of patients with autosomal recessive complex I deficiency develop cardiomyopathy. Whether this same defect is present in all cases of cardiomyopathy and cataracts is a subject for future investigation. Certainly a respiratory chain defect affecting the redox state of the pyridine nucleotides would account for the exercise intolerance observed in older patients and the abnormal appearance of mitochondria(3), whereas the overproduction of free radicals would account for the cataracts and cardiomyopathy.
Abbreviations
- BCIP:
-
5-bromo-4-chloro-3-indoyl phosphatep-toluidine salt
- NBT:
-
p-nitro blue tetrazolium chloride
- SOD:
-
superoxide dismutase
- TMPD:
-
N′N′N′N′-tetramethyl phenylene diamine
References
McKusick VA 1992 Mendelian inheritance in man. In: Catalogs of Autosomal Dominant, Autosomal Recessive, and X-Linked Phenotypes, 10th Ed. Johns Hopkins University Press, Baltimore
Cruysberg JRM, Sengers RCA, Pinckers A, Kubat K, van Haelst UJGM 1986 Features of a syndrome with congenital cataract and hypertrophic cardiomyopathy. Am J Ophthalol 102: 740–749.
Sengers RCA, ter Haar BGA, Trijbels JMF, Willems JL, Daniels O, Stadhouders AM 1975 Congenital cataract and mitochondrial myopathy of skeletal and heart muscle associated with lactic acidosis after exercise. J Pediatr 86: 873–880.
Valsson J, Laxdal T, Jonsson A, Kristjansson K, Helgason H 1988 Congenital cardiomyopathy and cataracts with lactic acidosis. Am J Cardiol 61: 193–194.
Geisterfer AA-T, Kass S, Tanigawa G, Vosberg H-P, McKenna W, Seidman CE, Seidman JG 1990 A molecular basis for familial hypertrophic cardiomyopathy: a/b cardiac myosin heavy chain gene missense mutation. Cell 62: 999–1006.
Tanigawa G, Jarcho JA, Kass S, Solomon SD, Bosberg H-P, Seidman JG, Seidman CE 1990 A molecular basis for familial hypertrophic cardiomyopathy: An a/b cardiac myosin heavy chain hybrid gene. Cell 62: 991–998.
Watkins H, MacRae C, Thierfelder L, Chou Y-H, Frenneaux M, McKenna W, Seidman JG, et al 1993 A disease locus for familial hypertrophic cardiomyopathy maps to chromosome 1q3. Nature Genet 3: 331–337.
Carrier L, Hengstenberg C, Beckmann JS, Guicheney P, Dufour C, Bercovici J, Dausse E, et al 1993 Mapping of a novel gene for familial hypertrophic cardiomyopathy to chromosome 11. Nature Genet 4: 311–313.
Merante F, Tein I, Benson L, Robinson BH 1994 Maternally inherited hypertrophic cardiomyopathy due to a novel T-to-C transition at nucleotide 9997 in the mitochondrial tRNAGlycine gene. Am J Hum Genet 55: 437–446.
Zeviani M, Servidei S, Gellera C, Bartini E, Di Mauro S, DiDonato S 1989 An autosomal dominant disorder with multiple deletions of mitochondrial DNA starting at the D-loop region. Nature 339: 309–311.
Sweeney MG, Brockington M, Weston MJ, Morgan-Hughes JA, Harding AE 1993 Mitochondrial DNA transfer RNA mutation Leu(uur) A-G3260: a second family with myopathy and cardiomyopathy. Q J Med 86: 435–438.
Robinson BH, Glerum DM, Chow W, Petrova-Benedict R, Lightowlers R, Capaldi R 1990 The use of skin fibroblast cultures in the detection of respiratory chain defects in patients with lacticacidemia. Pediatr Res 28: 549–555.
Robinson BH 1993 Lacticacidemia. Biochim Biophys Acta 1182: 231–244.
Applegarth DA, Tong T, Clarke LA 1994 Stability of frozen muscle used for mitochondrial enzyme assays. Eur J Pediatr 153: 142
Srere PA, Brazil H, Gonen L 1963 The citrate condensing enzyme of pigeon breast muscle and moth flight muscle. Acta Chem Scand 17: 5129–5134.
Robinson BH, MacKay N, Goodyer P, Lancaster G 1985 Defective intramitochondrial NADH oxidation in skin fibroblasts from an infant with fatal neonatal lacticacidemia. Am J Hum Genet 37: 938–946.
Glerum M, Yanamura W, Capaldi R, Robinson BH 1988 Characterisation of cytochrome oxidase mutants in human fibroblasts. FEBS Lett 236: 100–104.
Fischer JC, Ruitenbeek W, Stadhouders AM, Trijbels JMF, Sengers RCA, Janssen AJM, Veerkamp JH 1985 Investigation of mitochondria metabolism in small human skeletal muscle biopsy specimens. Improvement of preparation procedure. Clin Chim Acta 145: 89–94.
Moreadith RW, Batshaw ML, Ohnishi T, Kerr D, Knowx B, Jackson D, Hruba R, Olson J, Reynafarje B, Lehninger AL 1984 Deficiency of the iron-sulfur clusters of mitochondrial reduced nicotinamide-adenine dinucleotide-ubiquinone oxidoreductase (complex I) in an infant with congenital lactic acidosis. J Clin Invest 74: 685–697.
Lowry OH, Rosenbrough NJ, Farr AL, Randall RJ 1951 Protein measurement with the folin phenol reagent. J Biol Chem 193: 265–275.
Robinson BH, Oei J, Sherwood WG, Applegarth D, Wong L, Haworth J, Goodyer P, Casey R, Zaleski LA 1984 The molecular basis for the two different clinical presentations of classical pyruvate carboxylase deficiency. Am J Hum Genet 36: 283–294.
Wanders RJA, Ruiter JPN, Wijburg FA 1993 Studies on mitochondrial oxidative phosphorylation in permeabilized human skin fibroblasts: application to mitochondrial encephalomyopathies. Biochim Biophys Acta 1181: 219–222.
Chow W, Ragan I, Robinson BH 1991 Determination of the cDNA sequence for the human mitochondrial 75 kDa Fe-S protein of NADH-coenzyme Q reductase. Eur J Biochem 201: 547–550.
Pilkington SJ, Walker JE 1989 Mitochondrial NADH-ubiquinone reductase: Complementary DNA sequences of import precursors of the bovine and human 24 kDa subunit. Biochemistry 28: 3257–3264.
Ali ST, Duncan AMV, Schappert K, Heng HHQ, Tsui LC, Chow W, Robinson BH 1993 Chromosomal localization of the human gene encoding the 51-kDa subunit of mitochondrial complex I (NDUFV1) to 11q13. Genomics 18: 435–439.
Maniatis T, Fritsch ER, Sambrook J 1989 Molecular Cloning: A Laboratory Manual. Cold Spring Harbor Laboratory, Cold Spring Harbor, NY
Spitz DR Oberley LW 1989 An assay for superoxide dismutase activity in mammalian tissue homogenates. Anal Biochem 179: 8–18.
Faulkner K, Fridovich I 1993 Luminol and lucigenin as detectors for O-2. Free Radic Biol Med 15: 447–451.
Robinson BH, Ward J, Goodyer P, Beaudet A 1986 Respiratory chain defects in the mitochondria of cultured skin fibroblasts from three patients with lacticacidemia. J Clin Invest 77: 1422–1427.
Anderson S, Bankier AT, Barrell BG, de Bruijn MH, Coulson AR, Drouin J, Eperon IC, Nierlich DP, Roe BA, Sanger F, Schreier PH, Smith AJ, Staden R, Young IG 1981 Sequence and organization of human mitochondrial genome. Nature 290: 457–465.
Wallace DC, Lott MT, Torroni A, Shoffner JM 1991 Report of the committee on human mitochondrial DNA. Cytogenet Cell Genet 58: 1203–1123.
Sengers RCA, Stadhouders AM, van Lakwijk-Vondrovicova E, Kubat K, Ruitenbeek W 1985 Hypertrophic cardiomyopathy associated with a mitochondrial myopathy of voluntary muscles and congenital cataract. Br Heart J 54: 543–547.
Katsumata K, Hayakawa M, Tanaka M, Sugiyama S, Ozawa T 1994 Fragmentation of human heart mitochondrial DNA associated with premature aging. Biochem Biophys Res Commun 202: 102–110.
Janssen YMW, Marsh JP, Absher MP, Gabrielson E, Borm PJA, Driscoll K, Mossman BT 1994 Oxidant stress responses in human pleural mesothelial cells exposed to asbestos. Am J Crit Care Med 149: 795–802.
Shull S, Heintz NH, Periasamy M, Manohar M, Janssen YMW, Marsh JP, Mossman BT 1991 Differential regulation of antioxidant enzymes in response to oxidants. J Biol Chem 266: 24398–24403.
Bhuyan DK, Bhuyan KC 1994 Assessment of oxidative stress to eye in animal model for cataract. Methods Enzymol 233: 630–639.
Hockenbery DM, Oltvai ZN, Yin X-M, Milliman CL, Korsemeyer SJ 1993 Bcl-2 functions in an antioxidant pathway to prevent apoptosis. Cell 75: 241–245.
Salganik RI, Solovyova NA, Dikalov SI, Grishaeva ON, Semenova LA, Popovsky AV 1994 Inherited enhancement of hydroxyl radical generation and lipid peroxidation in the S strain rats results in DNA rearrangements, degenerative diseases, and premature aging. Biochem Biophys Res Commun 199: 726–733.
Capaldi RA 1990 Structure and assembly of cytochrome c oxidase. Arch Biochem Biophys 280: 252–262.
Watmough NJ, Birch-Machin MA, Bindoff LA, Aynsely-Green A, Simpson K, Ragan CI, Sherratt HSA, Turnbull DM 1989 Tissue-specific defects of complex I of the mitochondrial respiratory chain. Biochem Biophys Res Commun 160: 623–627.
Clay VJ, Ragan CI 1988 Evidence for the existence of tissue-specific isoenzymes of mitochondrial NADH dehydrogenase. Biochem Biophys Res Commun 157: 1423–1428.
Hennet T, Richter C, Peterhans E 1993 Tumor necrosis factor-alpha induces superoxide anion generation in mitochondria of L929 cells. Biochem J 289: 587–592.
Hassan MH, Fridovich I 1979 Intracellular production of superoxide radical and of hydrogen peroxide by redox active compounds. Arch Biochem Biophys 196: 385–395.
Sies H 1991 Oxidative Stress: Oxidants and Antioxidants. Academic Press, London
Flint DH, Tuminello JF, Emptage MH 1993 The inactivation of Fe-S cluster containing hydrolyases by superoxide. J Biol Chem 268: 22369–22376.
Author information
Authors and Affiliations
Additional information
Supported by the Networks of Centres of Exellence Programme of the Canadian Government, the National Foundation the March of Dimes, and the Emil Aaltonen Foundation.
Rights and permissions
About this article
Cite this article
Pitkänen, S., Merante, F., McLeod, D. et al. Familial Cardiomyopathy with Cataracts and Lactic Acidosis: A Defect in Complex I (NADH-Dehydrogenase) of the Mitochondria Respiratory Chain. Pediatr Res 39, 513–521 (1996). https://doi.org/10.1203/00006450-199603000-00021
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1203/00006450-199603000-00021
This article is cited by
-
Mitochondria in the human heart
Journal of Bioenergetics and Biomembranes (2009)
-
Dietary oxysterols induce in vivo toxicity of coronary endothelial and smooth muscle cells
European Journal of Nutrition (2005)
-
Sengers disease: A rare association of hypertrophic cardiomyopathy and congenital cataracts
The Indian Journal of Pediatrics (2004)
-
The genetics and pathology of oxidative phosphorylation
Nature Reviews Genetics (2001)
-
Metabolic and Molecular Bases of Menkes Disease and Occipital Horn Syndrome
Pediatric and Developmental Pathology (1998)