Abstract
Three series of CNPAEK/copper phthalocyanine (CuPc), 2CNPAEK/CuPc and sulfated poly(aryl ether ketone) (SPAEK)/CuPc composites were prepared using a solution blending technique. The CNPAEKs/CuPc composites have a spherical shape with a diameter range of 1–5 μm, although 2CNPAEK/CuPc and SPAEK/CuPc composites have a spherical shape with a diameter ∼100 nm because SPAEK and 2CNPAEK exhibit stronger polarities than CNPAEK, resulting in better interphase interactions with metallophthalocyanine. The degree of dispersion and the size of the CuPc particles in the matrix decreases in the following order: SPAEK>2CNPAEK>CNPAEK. The SPAEK/CuPc25 composites exhibit better dielectric properties than either the CNPAEK/CuPc or 2CNPAEK/CuPc composites, with a dielectric constant >400 at 100 Hz and a dielectric loss <0.5.
Similar content being viewed by others
Introduction
High-dielectric-constant polymers are used for charge-storage devices, smart skins for drag reduction and microelectromechanical systems1, 2, 3, 4, 5, 6, 7 because of their features, such as a high electric breakdown field, a low dielectric loss, easy processing and others. However, it is difficult for one-component systems to achieve all of these properties. Conventional polymers are easy to process, but generally suffer from a low dielectric constant (<10). The composite approach, in which high-dielectric-constant particulates (most are ferroelectric ceramics) are added to a polymer matrix, has been used in the last several decades to raise the dielectric constant of polymer-based materials.8, 9, 10 However, the increased dielectric constant comes at the cost of losing flexibility and significantly increasing the elastic modulus because ceramic materials have a much higher elastic modulus than polymers.
Copper phthalocyanine oligomer11 (o-CuPc) has a very high dielectric constant (>10 000) due to the electron delocalization within the giant conjugated molecule.12 As an organic material, CuPc has a modulus comparable to that of the poly(vinylidene fluoride_trifluoroethylene). Therefore, a high dielectric constant can be achieved with this composite without increasing the material’s modulus. An all-organic composite of poly(vinylidene fluoride_trifluoroethylene)-co-poly(vinylidene fluoride_trifluoroethylene) and CuPc oligomers displays high electromechanical properties.13 Under a field of 13 V μm−1, a strain of around −2% can be induced and the elastic energy density can reach 0.13 J cm−3 while maintaining the composite film’s flexibility.
However, CuPc particles are susceptible to agglomeration within the polymer matrix (the size of CuPc particles is ∼1 mm) due to the incompatibility of CuPc with the polymer matrix, which reduces the breakdown field and increases the dielectric loss. In polymer composites, the compatibility between the filler and the polymer matrix can be enhanced by adding a dispersant,14 forming intermolecular hydrogen bonding,15, 16, 17 crosslinking18, 19 and grafting20, 21, 22, 23, 24, 25 and so on. The resulting products are fully functionalized nanopolymers and improve the compatibility of the two and three components, offer good dielectric and mechanical properties and increase the breakdown field as well. According to a theoretical model of these types of composites by Li,26 the interface exchange-coupling effect can result in a significant change in the local polarization level. By further reducing the size of CuPc, the dielectric properties can be dramatically improved because the exchange coupling exists only in the near-interface region. Furthermore, the content of the filler should also be taken into consideration because low loadings of the filler will reduce the amounts of voids/defects in the final composite and will result in improved mechanical properties.27
Poly(aryl ether ketone)s (PAEKs) are a class of important high-performance aromatic polymers that are used in the aerospace, electronic and nuclear fields.28 Their excellent mechanical, thermooxidative, electrical and chemical resistance properties make them candidates for advanced materials. In this study, PAEKs/metallophthalocyanine nanocomposites were prepared with the goal of further improving the dielectric and mechanical properties, increasing the breakdown field and improving the predigestion preparation method. Phthalocyanine and its oligomer have a strong tendency to form stacked assemblies due to its planar shape and aromatic nature. As a consequence, in the composite, these high-dielectric-constant oligomers strongly micro-aggregate due to weak interactions between the oligomers and the common polymer matrix (Figure 1a). The polymer matrix was modified before making the composite to obtain the nanometer-scale building blocks for the composites. In this study, cyano and sulfate group-modified PAEKs, cyanophenyl-substituted PAEK (CN-PAEK, 2CN-PAEK) and SPAEK (Scheme 1) were prepared in our laboratory to be used as the composite matrix. Introducing cyano and sulfate groups into the polymer matrix can not only increase the dielectric constant of the composites, but can also improve the interaction between the polymer and the metallophthalocyanine as well as destroy the stacked assemblies of metallophthalocyanine.
Experimental procedure
Materials
The o-CuPc was synthesized following the procedure reported by Venkatragavaraj et al.9p-Chloromethylstyrene (97%) was purchased from Aldrich and used without further purification. The polymers CN-PAEK, 2CN-PAEK and SPAEK were prepared in our laboratory according to the previously published protocols.29, 30, 31 The dimethyl sulfoxide was of analytical grade and was dried with CaH2 followed by distillation in vacuo prior to use.
Preparation of PAEK matrix composites
Composite films were prepared using the solution cast method. The PAEKs were dissolved in dimethyl sulfoxide before adding the desired amount of o-CuPc to the solution. The solution was ultrasonically stirred until the o-CuPc was dissolved. The solution was then poured onto a glass slide and was dried in air at 80 °C for 6 h and then under vacuum at 80 °C for 12 h. Finally, the composites were annealed at 140 °C in a vacuum for 12 h and were slowly cooled to room temperature. Here, four kinds of composites were prepared:
CNPAEK/CuPc: CNPAEK/CuPc composites, 25% weight ratio of CuPc;
2CNPAEK/CuPc: 2CNPAEK/CuPc composites, 25% weight ratio of CuPc;
SPAEK/CuPc25: SPAEK/CuPc composites, 25% weight ratio of CuPc;
SPAEK/CuPc40: SPAEK/CuPc composites, 40% weight ratio of CuPc.
Characterization
The glass transition temperatures (Tgs) were determined using a modulated differential scanning calorimeter (Model Mettler DSC821e, METTLER TOLEDO Co. Ltd, Zurich, Switzerland) instrument at a heating rate of 20 °C min−1 under a nitrogen flow of 100 ml min−1. The mechanical properties of the composites were measured at room temperature on a SHIMADZU (SHIMADZU Co. Ltd., Kyoto, Japan) AG-I 1KN at a strain rate of 10 mm min−1. The samples had dimensions of 20 mm span length and 4 mm width. Scanning electron microscopy (SEM) images were taken using a JEOL JSM-6700 F scanning electron microscope (JEOL Co. Ltd., Tokyo, Japan) and Iridium (IXRF Systems, IXRF Co. Ltd., Houston, TX, USA) software. The dielectric properties of the polymer films (diameter 10 mm and thickness 0.1 mm, coated with gold by vacuum evaporation) were obtained using an HP 4192 A impedance analyzer. The dielectric constant K of the film was calculated using the formula of a parallel plate capacitor:

where C is the capacitance of the metal-insulator-metal element,  is the vacuum dielectric permittivity, A is the area of the electrode and t is the thickness of the capacitor.
is the vacuum dielectric permittivity, A is the area of the electrode and t is the thickness of the capacitor.
Results and Discussion
Micromorphology of CNPAEK/CuPc and 2CNPAEK/CuPc composites
SEM was used to characterize the microstructures and morphology of the composites. The sample was broken in liquid nitrogen and its cross-section was analyzed by SEM to investigate the bulk morphology of the polymers.
The microstructures of the fractured surfaces of the CNPAEK/CuPc and 2CNPAEK/CuPc composites are shown in Figure 2. The SEM images show that the CNPAEK/CuPc composite samples have a spherical shape, with particulates that exhibit nonuniformly distributed diameters between 1 and 5 μm (Figures 2a and ). These spherical particles may be formed from o-CuPc aggregation. These micromorphologies indicate that the compatibility of the CNPAEK/CuPc system is unsatisfactory. Figure 2c shows a SEM micrograph of the 2CNPAEK/CuPc composites that contain spherical particulates. A well-defined spherical structure can be observed with higher magnification (Figure 2d) for the 80–100 nm particles. A certain amount of coupling agent is also distributed among the spherical particles. Compared with the CNPAEK/CuPc composites, the size of the 2CNPAEK/CuPc composite particles is smaller and more uniform, which is probably due to the stronger polarity of SPAEK and 2CNPAEK compared with CNPAEK, resulting in better interphase interactions with metallophthalocyanine.
Micromorphology of SPAEK/CuPc composites
Figure 3 shows the microstructures of the fractured surfaces of the SPAEK/CuPc composites at different concentrations (mass fraction) of CuPc oligomers. Figure 3b shows the SEM images of the SPAEK/CuPc25 nanocomposites with nanoparticles approximately 80–100 nm embedded in the polymer matrix. The size of the nanoparticles was quite similar and more uniform than the nanoparticles in the CNPAEK and 2CNPAEK composites (Figure 2). The SPAEK/CuPc composites showed good interphase interaction, which is based on the spontaneous and strong chemisorptions of SPAEK onto the o-CuPc surfaces due to strong electrostatic and complexation forces. The adsorption of some SPAEK molecules drives the gradual division of the initially large o-CuPc microaggregates into much smaller particles that are stabilized by the charged SPAEK layer, leading to highly dispersed colloidal nanoparticles with a very narrow size distribution (Figure 4b). These nanoparticles form nanocomposites (Figure 4c) when formed in films. The size of the nanoparticles in SPAEK/CuPc40 (see Figure 3d) is more uniform than in the SPAEK/CuPc25 composites.
The SPAEK/CuPc25 composite films were created by the hot-pressing method at a temperature above the glass transition temperature of these composites, but not high enough to cause the disintegration of the materials. After decreasing the temperature under stable pressure, the sample was broken in liquid nitrogen, and its cross-section was analyzed by SEM. Figure 4 shows the microstructures of the SPAEK/CuPc25 nanocomposites pre- (Figure 4a) and post-autoclaving (Figure 4b). After hot pressing, the spherical particle morphology disappeared, as shown in Figure 4b. Some particles aggregated together, losing the microstructural morphology, while the majority of particles were pressed into a plane, especially on the edge of composite films. Thus, we believe that the spherical particles of SPAEK/CuPc25 composite are enveloped by SPAEK. The molecular chains of SPAEK move and adhere when the composite films are autoclaved above the glass transition temperature. After decreasing the temperature under stable pressure, the movements of the SPAEK molecular chains are frozen, resulting in the morphology shown in Figure 4b.
Dielectric properties of composites
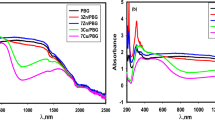
The dielectric properties of the composites with different polymer matrices were studied using samples with a parallel plate capacitor configuration. Figure 5 shows the dielectric constant (K) and loss (D) as a function of frequency (f) for the CNPAEK/CuPc and 2CNPAEK/CuPc composites, ranging from 100 Hz to 1 MHz at room temperature. The dielectric constant of the 2CNPAEK/CuPc composite is more than 150 (100 Hz), ∼40 times that of the 2CNPAEK, while the dielectric constant of the CNPAEK/CuPc composite does not reach 50 (100 Hz), which is only 10 times that of the CNPAEK, and shows a higher dielectric loss than 2CNPAEK/CuPc. The dielectric properties are consistent with the morphology of these composites.
Figure 6 shows the dielectric constant (K) and loss (D) as a function of frequency (f) for the composites as well as SPAEK, ranging from 100 Hz to 1 MHz at room temperature. The dielectric constant of composites increased. For example, the dielectric constant of SPAEK/CuPc25 is more than 450 (100 Hz), which is ∼40 times that of the SPAEK, while the dielectric constant of SPAEK/CuPc40 is close to 800 (100 Hz), or ∼70 times that of SPAEK. The dielectric constant of the composites is enhanced by the presence of CuPc oligomers because they facilitate the displacement of electrons under electric fields through the highly conjugated π-bonds within the entire sheet-like molecule, resulting in a high dielectric response.
As a semiconductor material, the conductivity of the o-CuPc can vary over a broad range, which significantly affects the composite dielectric property. The enhanced dielectric constant of SPAEK/CuPc25 composites is likely caused by the interfacial effects due to the nanoparticle size, as observed in the SEM micrographs. Although the content of o-CuPc in SPAEK/CuPc25, CNPAEK/CuPc and 2CNPAEK/CuPc composites are the same, the SPAEK/CuPc25 exhibits a higher dielectric constant in most frequency ranges, which is probably caused by the stronger interface effect due to the much smaller particle size, as observed in the SEM micrographs. In a recent publication, Li26 showed that the interface exchange-coupling effect can lead to a very significant enhancement in the dielectric response in composites such as polyvinylidene difluoride, which have very large differences in the dielectric constant between the two components.
The dielectric constant decreases with increasing frequency for the composites, which is expected based on previous results. These observations indicate that more o-CuPc dipoles and charge carriers within the composites fail to keep up with the electric field of the increasing frequency. The AC-conductivity exhibited at higher frequencies will increase with frequency and thus decrease charge storing capability, which may contribute to the observed relaxation as well. The dielectric constant of the SPAEK/CuPc composites increases more significantly with decreasing frequency compared with that of the CNPAEK/CuPc and 2CNPAEK/CuPc composites, that is, the SPAEK–CuPc composites exhibit stronger dielectric dispersion at low frequency (100–1000 Hz). The low-frequency dielectric dispersion seems to originate from the Maxwell–Wagner–Sillars polarization mechanism, which exists in composites with large differences in both the dielectric constant and conductivity between its two components. Figure 6b shows the frequency dependence of the dielectric loss tangent in the experimental frequency range. The peaks could be related to the interfacial polarization relaxation effects, which correspond to the relaxation of K-value (Figure 6a).
CuPc oligomers suffer from a high dielectric loss due to the long-range intermolecular hopping of electrons. In composites, the copolymer matrix provides insulation layers to significantly reduce the dielectric loss of the o-CuPc. If the o-CuPc is dispersed in the polymer matrix as a single molecule, a much lower dielectric loss will be expected. However, o-CuPc aggregation is still inevitable in composites, which explains its higher dielectric loss with respect to the copolymer matrix. The loss of the 2CNPAEK/CuPc composite is lower than that of the CNPAEK/CuPc, which is attributed to the improved dispersion of the o-CuPc in the former composite. Although SPAEK/CuPc composites show better dispersion, the loss of SPAEK/CuPc is still higher than that in the 2CNPAEK/CuPc and CNPAEK/CuPc composites, most likely due to the high loss of matrix in the former composite.
Mechanical properties of SPAEK/CuPc composites
The mechanical properties of the SPAEK/CuPc nanocomposites are shown in Table 1. The SPAEK/CuPc15 and SPAEK/CuPc25 composites have tensile strengths of 70.7 and 69.6 MPa, respectively, and tensile moduli of 2.5 and 3.0 GPa, respectively, which are much higher than those of pure SPAEK (with a tensile strength of 49.8 MPa and a tensile modulus of 1.6 GPa). The composite with a CuPc mass fraction of 0.40 had a tensile strength of 34.3 MPa, which is lower than that of SAPEK. The elongation at break of SPAEK/CuPc15, SPAEK/CuPc25 and SPAEK/CuPc40 were 8.9, 5.7 and 2%, respectively, which were lower than the SPAEK (39.8%). When the o-CuPc content was low, the tensile strength of these composites increased because of the enhancement effect of o-CuPc on the SAPEK matrix. As the o-CuPc content in composites further increased, the tensile strength of the composites decreased due to the dilution effect of o-CuPc on the SPAEK matrix. The tensile modulus increased and the elongation at break decreased in composites with an increased o-CuPc content.
Conclusions
CNPAEK/CuPc, 2CNPAEK/CuPc and SPAEK/CuPc composites were prepared using a simple solution blend method to increase the breakdown field, improve the dielectric and mechanical properties and aid the predigestion preparation method. The CuPc particles in the composites have a spherical shape with different diameter ranges depending on the composite. The size of the CuPc particles in the matrix decreased in the following order: SPAEK>2CNPAEK>CNPAEK. Consequently, the SPAEK/CuPc composite exhibits better dielectric properties than the CNPAEK/CuPc and 2CNPAEK/CuPc composites.

The structure of modified PAEKs.
References
Bai, Y., Cheng, Z.Y., Bharti, V., Xu, H. & Zhang, Q.M. High-dielectric-constant ceramic-powder polymer composites. Appl. Phys. Lett. 76, 3804–3806 (2000).
Xu, H., Bai, Y., Bharti, V. & Cheng, Z.Y. High dielectric constant composites based on metallophthalocyanine oligomer and poly(vinylidene fluoride-trifluoroethylene) copolymer. J. Appl. Polym. Sci. 82, 70–75 (2001).
Arbatti, M., Shan, X.B. & Cheng, Z.Y. Ceramic–polymer composites with high dielectric constant. Adv. Mater. 19, 1369–1372 (2007).
Shen, Y., Lin, Y.H., Li, M. & Nan, C.W. High dielectric performance of polymer composite films induced by a percolating interparticle barrier layer. Adv. Mater. 19, 1418–1422 (2007).
Shankar, R., Ghosh, T.K. & Spontak, R.J. Electroactive nanostructured polymers as tunable actuators. Adv. Mater. 19, 2218–2223 (2007).
Zhang, S.H., Huang, C., Klein, R.J., Xia, F., Zhang, Q.M. & Cheng, Z.Y. High performance electroactive polymers and nano-composites for artificial muscles. J. Intell. Mater. Syst. Struct. 18, 133–145 (2007).
Gao, Y., Xu, H.P., Bai, J.B. & Dang, Z.M. Fabrication and characteristics of organic semiconductor nanoparticles of copper phthalocyanine oligomers. J. Colloid. Interf. Sci. 322, 491–496 (2008).
Li, J.Y. & Rao, N. Dramatically enhanced effective electrostriction in ferroelectric polymeric composites. Appl. Phys. Lett. 81, 1860–1862 (2002).
Venkatragavaraj, E., Satish, B., Vinod, P.R. & Vijaya, M.S. Piezoelectric properties of ferroelectric PZT–polymer composites. J. Phys. D: Appl. Phys. 34, 487–492 (2001).
Rao, Y., Yue, J. & Wong, C.P. High K polymer-ceramic nano-composite development, characterization, and modeling for embedded capacitor RF application. electronic components and technology conference, proceedings. IEEE Proc. 51, 1408–1412 (2001).
Nalwa, H.S., Dalton, L. & Vasudevan, P. Dielectric properties of copper-phthalocyanine polymer. Eur. Polym. J. 21, 943–947 (1985).
Pohl, H.A. Superdielectrics Polymers. IEEE Trans. Electr. Insul. EI-21, 683–692 (1986).
Zhang, Q.M., Li, H., Poh, M., Xu, H., Cheng, Z.Y., Xia, F. & Huang, C. An all-organic composite actuator material with a high dielectric constant. Nature 419, 284–287 (2002).
Gasa, J., Wang, H.B., DeSousa, R. & Tasaki, K. Fabrication and characterization of fullerene–Nafion composite membranes. Polymer 48, 4438–4448 (2007).
Liu, G.Y., Yang, X.L. & Wang, Y.M. Silica/poly(N,N′-methylenebisacrylamide) composite materials by encapsulation based on a hydrogen-bonding interaction. Polymer 48, 4385–4392 (2007).
Joshi, S.S. & Mebel, A.M. Computational modeling of biodegradable blends of starch amylose and poly-propylene carbonate. Polymer 48, 3893–3901 (2007).
Tian, M., Gao, Y., Liu, Y., Liao, Y.L., Xu, R.W., Hedin, N.E. & Fong, H. Bis-GMA/TEGDMA dental composites reinforced with electrospun nylon 6 nanocomposite nanofibers containing highly aligned fibrillar silicate single crystals. Polymer 48, 2720–2728 (2007).
Zheng, Q.B., Xue, Q.Z., Yan, K.Y., Gao, X.L., Li, Q. & Hao, L.Z. Effect of chemisorption on the interfacial bonding characteristics of carbon nanotube–polymer composites. Polymer 49, 800–808 (2008).
Zhang, Q.M., Huang, C., Levstik, A. & Bobnar, V. Distinctive contributions from organic filler and relaxorlike polymer matrix to dielectric response of CuPc-P(VDF-TrFE-CFE) composite. Phys. Rev. Lett. 92, 0476041–0476044 (2004).
Wang, J.W., Bao, H.M., Yang, C.Z. & Shen, Q.D. Microstructure and dielectric properties of P(VDF-TrFE-CFE) with partially grafted copper phthalocyanine oligomer. Macromolecule 38, 2247–2252 (2005).
Wang, J.W., Yang, C.Z. & Shen, Q.D. High dielectric constant composite of P(VDF-TrFE) with grafted copper phthalocyanine oligomer. Macromolecule 37, 2294–2298 (2004).
Wang, Y., Wang, F., Li, S.Q., Xiao, J. & Wang, J.W. PVDF based all-organic composite with high dielectric constant. Polymer Bull. 60, 647–655 (2008).
Wang, Y., Wang, F., Li, S.Q., Xiao, J., Shen, Q.D. & Wang, J.W. A large enhancement in dielectric properties of poly(vinylidene fluoride)based all-organic nanocomposite. Polymer 50, 679–684 (2009).
Huang, C. & Zhang, Q.M. Fully functionalized high dielectric constant nanophase polymers with high electromechanical response. Adv. Mater. 17, 1153–1158 (2005).
Huang, C. & Zhang, Q.M. Enhanced dielectric and electromechanical responses in high dielectric constant all-polymer percolative composites. Adv. Funct. Mater. 14, 501–506 (2004).
Li, J.Y. Exchange coupling in P(VDF-TrFE) copolymer based all-organic composites with giant electrostriction. Phys. Rev. Lett. 90, 2176011–2176014 (2003).
Dai, K., Xu, X.B. & Li, Z.M. Electrically conductive carbon black (CB) filled in situ microfibrillar poly(ethylene terephthalate) (PET)/polyethylene (PE) composite with a selective CB distribution. Polymer 48, 849–859 (2007).
Searle, O.B. & Pfeiffer, R.H. Victrex® poly(ethersulfone) (PES) and Victrex® poly(etheretherketone) (PEEK). Polym. Eng. and Sci. 25, 474–476 (1985).
Zhang, Y., Ren, D., Guan, S., Na, Y., Wang, G. & Jiang, Z. Synthesis and characterization of novel cyano-functionalized poly(aryl ether ketone)s. E-Polymer 083 (2009).
Zhang, Y., Sun, X., Niu, Y., Xu, R., Wang, G. & Jiang, Z. Synthesis and characterization of novel poly(aryl ether ketone)s with metallophthalocyanine pendant unit from a new bisphenol containing dicyanophenyl side group. Polymer 47, 1569–1574 (2006).
Wang, F., Chen, T.L. & Xu, J.P. Sodium sulfonate-functionalized poly(ether ether ketone)s. Macromol. Chem. Phys. 199, 1421–1426 (1998).
Acknowledgements
We thank the National Science Foundation of China (no. 5 08 03 025) for its financial support.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Yang, X., Wang, Q., Zhang, Y. et al. The microstructure and dielectric properties of modified poly(aryl ether ketone)/metallophthalocyanine composites. Polym J 44, 1042–1047 (2012). https://doi.org/10.1038/pj.2012.50
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/pj.2012.50
Keywords
This article is cited by
-
Improvement in dielectric properties of the three-phase GN–BaTiO3–PEK nanocomposites with and without silane coupling agent
Journal of Materials Science: Materials in Electronics (2021)